Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (13): 2009-2014.doi: 10.3969/j.issn.2095-4344.2017.13.008
Previous Articles Next Articles
Effects of recombinant adenovirus vector expressing human lactoferrin on proliferation and apoptosis of cervical cancer stem-like cells
Zhang Xia, Du Zhi-xiang, Wang Na, Meng Ya-li
- First Hospital of Hebei Medical University, Shijiazhuang 050031, Hebei Province, China
-
Revised:2017-03-22Online:2017-05-08Published:2017-06-09 -
Contact:Meng Ya-li, Chief physician, the First Hospital of Hebei Medical University, Shijiazhuang 050031, Hebei Province, China -
About author:Zhang Xia, Master, Attending physician, the First Hospital of Hebei Medical University, Shijiazhuang 050031, Hebei Province, China -
Supported by:the Key Project of Medical Science Research in Hebei Province in 2014, No. ZD20140325
CLC Number:
Cite this article
Zhang Xia, Du Zhi-xiang, Wang Na, Meng Ya-li. Effects of recombinant adenovirus vector expressing human lactoferrin on proliferation and apoptosis of cervical cancer stem-like cells[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(13): 2009-2014.
share this article
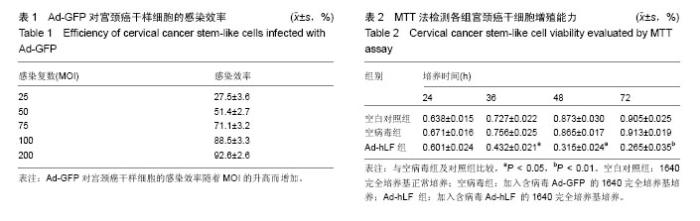
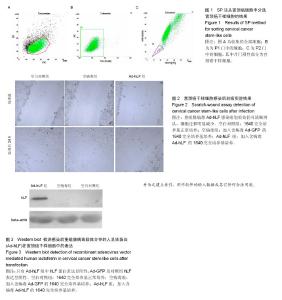
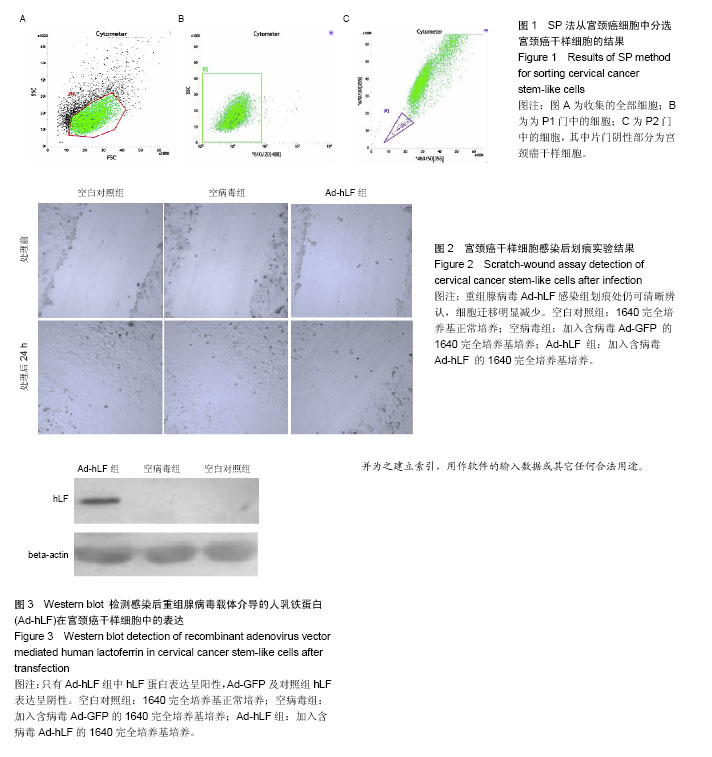
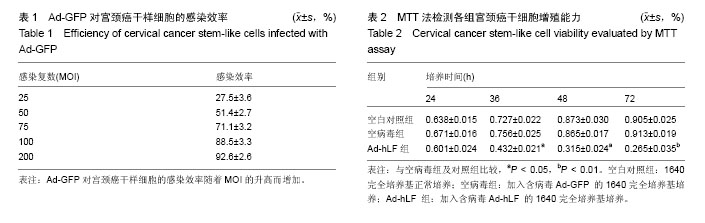
2.1 SP法从宫颈癌细胞中分选宫颈癌干样细胞的鉴定结果 在宫颈癌原代细胞中采用流式细胞仪进行分析,采用Hoechst 33342荧光染料进行染色,可见多数细胞聚集在图的中央及偏右部分,为非SP细胞,将其他部分对Hoechst 33342荧光染料显示阴性结果的细胞分选出来即为宫颈癌干样细胞,见图1。 2.2 Ad-GFP对宫颈癌干样细胞的感染效率 由表1可见,Ad-GFP对宫颈癌干样细胞的感染效率随着MOI的升高而增加,但在显微镜下观察到当MOI=200时,细胞状态较差,虽然感染率较高,但出现细胞脱落不贴壁的状况,所以采用MOI=100在后续实验中感染细胞。 2.3 MTT法检测各组宫颈癌干样细胞增殖能力 由表2可见Ad-hLF感染宫颈癌干样细胞后,培养24 h检测时各组细胞增殖未见显著性差异,在36,48,72 h检测结果显示Ad-hLF组宫颈癌干样细胞的增殖情况受到明显抑制,比空白对照组及空病毒组明显降低(P < 0.05,P < 0.01),而对照组与空病毒组相比宫颈癌干样细胞增殖情况均无明显差异。 2.4 宫颈癌干样细胞凋亡情况 Annexin V-FITC/PI染色后,荧光显微镜下可以观察到细胞发出荧光信号,计算出200个细胞视野中阳性细胞数,空白对照组中宫颈癌干样细胞凋亡率为(20.11±3.25)%,空病毒组中宫颈癌干样细胞凋亡率为(23.43±2.55)%,Ad-hLF组中宫颈癌干样细胞凋亡率为(55.57±3.41)%。与对照组及空病毒组相比,Ad-hLF组阳性细胞数明显增多,宫颈癌干样细胞的凋亡数量明显增加(P < 0.05,P < 0.01),而对照组与空病毒组相比宫颈癌干样细胞凋亡情况差异无显著性 意义。 2.5 宫颈癌干样细胞感染后划痕实验结果 从图2可见,细胞划痕实验后经过24 h培养,空白对照组及空病毒组细胞增殖明显增加,划痕处可见细胞迁移,基本能使划痕处汇合,而重组腺病毒Ad-hLF感染组划痕处仍可清晰辨认,细胞迁移明显减少。 2.6 Western blot检测Ad-hLF感染后乳铁蛋白在宫颈癌干样细胞中的表达 由图3可见,对照组及空病毒GFP感染组均未见乳铁蛋白的表达,而重组腺病毒Ad-hLF感染组可见乳铁蛋白在宫颈癌干样细胞中的表达显著升高(P < 0.01),其中空白对照组中乳铁蛋白相对灰度值为8.8±3.1,空病毒组灰度值为30.9±1.4,Ad-hLF组为110.2±2.4,3组比较差异有显著性意义(P < 0.01)。"
| [1] Goodman MT, McDuffie K, Hemandez BY, et al. The influence of multiple human papilloma virus stypes on the risk of genotype-concordant incident infections of theanus andcervix: the Hawaii HPV cohort study. J Infect Dis. 2011;203(3): 335-340.[2] Sukasem C, Pairoj W, Saekang N, et al. Molecular epidemiology of human papillomavirus genotype in women with high-grade squamous intraepithelial lesion and cervical cancer:will a quadrivalent vaccine be necessary in Thailand? J Med Viro. 2011;83(1):119-126.[3] Zhang D, Zhang Q, Zhou L, et al. Comparison of prevalence, viral load, physical status and expression ofhuman papillomavirus-16, -18 and -58 in esophageal and cervica lcancer: a case-controls tudy. BMC Cancer. 2010;10:650.[4] Nicolás-Párraga S, Gandini C, Pimenoff VN, et al. HPV16 variants distribution in invasive cancers of the cervix, vulva, vagina, penis, and anus. Cancer Med. 2016;5(10):2909- 2919.[5] Li H, Anuwongcharoen N, Malik AA, et al. Roles of d-Amino Acids on the Bioactivity of Host Defense Peptides. Int J Mol Sci. 2016;17(7):34-36.[6] Liang YK, Zeng, Xiao YS, et al. MCAM/CD146 promotes tamoxifen resistance in breast cancer cells through induction of epithelial-mesenchymal transition, decreased ERá expression and AKT activation. Cancer Lett. 2016.[7] Garcia-Montoya IA, Cendon TS, Arevalo-Gallegos S, et al. Lactoferrin a multiple bioactive protein: an overview. Biochim Biophys Acta. 2012;1820(3):226-236.[8] Luo G, Zhou Y, Yi W, et al. Lactotransferrin expression is downregulated and affects the mitogen-activated protein kinase pathway in gastric cancer. Oncol Lett. 2015;9(5): 2409-2413.[9] Mariller C, Hardivill S, Hoedt E, et al. Proteomic approach to the identification of novel delta-lactoferrin target genes: characterization of DcpS,an mRNA scavenger decapping enzyme. Biochimie. 2009;91(1):109-122. [10] Morad SA, Cabot MC. Tamoxifen regulation of sphingolipid metabolism-Therapeutic implications. Biochim Biophys Acta. 2015;1851(9):1134-1145.[11] Li H, Anuwongcharoen N, Malik AA,et al. Roles of d-amino acids on the bioactivity of host defense peptides. Int J Mol Sci. 2016;17(7):E1023.[12] Yamada Y, Sato R, Kobayashi S, et al. The antiproliferative effect of bovine lactoferrin on canine mammary gland tumor cells. J Vet Med Sci. 2008;70(5):443-448.[13] Suda H, Sekine K, Takasuka N, et al. Prevention of colon carcinogenesis and carcinoma metastasis by orally administered bovine lactoferrin in animals. Biofactors. 2000; 12(1-4):83-88.[14] Iigo M, Kuhara T, UshidaY et al. Inhibitory effects of bovine lactoferrin on colon carcinoma 26 lung metastasis in mice. Clin Exp Metastasis. 1999;17(1):35-40.[15] Parkin DM, Bray F, Ferlay J, et al. Global Cancer Statistics, 2002. CA Cancer J Clin. 2005;55:74-79.[16] Shi H, Li W. Inhibitory effects of human lactoferrin on U14 cervical carcinoma through upregulation of the immune response. Oncol Lett. 2014;7(3):820-826.[17] Vorland LH. Lactoferrin: a multifunctional glycoprotein. APMIS. 1999;107:971.[18] van der Strate BWA, Beljaars L, Molema G, et al. Antiviral activities of lactoferrin. Antivir Res. 2001;52:225-228.[19] 刘自明,严律南.细菌内同源重组法制备腺病毒介导 TK 基因对肝癌细胞的杀伤作用[J].四川大学学报,2007,38(2):205-208. [20] Chen GX, Zhang S, He XH, et al. Clinical utility of recombinant adenoviral human p53 gene therapy: current perspectives. Onco Targets Ther. 2014;7:1901-1909. [21] Putzer BM, Bramson JL, Addison CL, et al. Combination therapy with interleukin-2 and wild-type p53 expressed by adenoviral vectoes potentiates tumor regression in amuring model of breast cancer. Hum Gene Ther. 1998;9(5):707-718. [22] 韩德民,黄志刚,张伟,等.重组人 p53 腺病毒注射液治疗喉癌的Ⅰ期临床实验及追踪观察[J].中华医学杂志,2003,83(23): 2029-2032. [23] Bai J, Li J, Mao Q, et al. Construction of a single lentiviral vector containing tetracycline-inducible Alb-uPA for transduction of uPA expression in murine hepatocytes. PLoS One. 20133;8(4):e61412. [24] 李萍,王德华.ABCE1基因沉默对人宫颈癌XB1702细胞生物学特性的影响[J].中国组织工程研究,2015,19(37):5972-5977.[25] Yu Z, Jiang Q, Liu J, et al. A simplified system for generating recombinant E3-deleted canine adenovirus-2. Plasmid. 2015; 77:1-6. [26] Ng P, Parks RJ, Cummaings DT, et al. An enhanced system for construction of adenoviral vectors by the two-plasmid rescue method. Hum Gene Ther. 2000;11(5):693-699. [27] Hong JS, Engler JA. Domains required for assembly of adenovirus type 2 fiber trimers. J Virol. 1996;70:7071-7078. [28] 韩玉刚,李建凡.动物生物反应器的研究现状和进展[J].国外畜牧科技,2002,29(1):30-33. [29] McEcoy TG, Sreenan J M. The efficiency of production, centrifugation and transfer of one and two-cell bovine ova in a gene transfer program. Theriogenology. 1990;33:819-828. [30] Halter R. Strategies to express factor Ⅷ gene constructs in the ovine mammary gland. Theriogenologr. 1993;39:137-149.[31] Roy K, Patel YS, Kanwar RK, et al. Biodegradable Eri silk nanoparticles as a delivery vehicle for bovine lactoferrin against MDA-MB-231 and MCF-7 breast cancer cells. Int J Nanomedicine. 2015;11:25-44. [32] Sohrabi SM, Niazi A, Chahardoli M, et al. In silico investigation of lactoferrin protein characterizations for the prediction of anti-microbial properties. Mol Biol Res Commun. 2014;3(2):85-100.[33] Jones EM, Smart A, Bloomberg G, et al. Lactoferricin a new antimicrobial peptide. Appl Bacteriol. 1994;77(2):208-214. [34] Barber MF, Kronenberg Z, Yandell M, et al. Antimicrobial functions of lactoferrin promote genetic conflicts in ancient primates and modern humans. PLoS Genet. 2016;12(5): e1006063. [35] Zhang H, Feng X, Liu W, et al. Underling mechanisms for LTF inactivation and its functional analysis in nasopharyngeal carcinoma cell lines. J Cell Biochem. 2011;112(7):1832-1843.[36] Li WY, Li QW, Han ZS, et al. Growth suppression effects of recombinant adenovirus expressing human lactoferrin on cervical cancer in vitro and in vivo. Cancer Biother Radiopharm. 2011;26(4):477-483.[37] Fujita K, Matsuda E, Sekine K, et al. Lactoferrin modifies apoptosis-related gene expression in the colon of the azoxymethane-treated rat. Cancer Lett. 2004;213(1):21-29.[38] Sakai T, Banno Y, Kato Y, et al. Pepsin-digestedbovine lactofemninduces cell death with JNK/SAPK activation in oral cancer cells. Japoptotic Pharmacol Sci. 2005;98(1):41-48.[39] Wang J, Li Q, Ou Y, et al. Inhibition of tumor growth by recombinant adenovirus containing human lactoferrin through inducing tunlor cell apoptosis in mice bearing EMT6 breast cancer. Arch Pharm Res. 2011;34(6):987-995. [40] Lee SH, Park SW, Pyo CW, et al. Requirement of the JNK-associated Bcl.2 pathway for human lactoferrin-induced apoptosis in the Jurkat leukemia T cell line. Biochimie. 2009; 91(1):102-108. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [3] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [4] | Yang Sidi, Wang Qian, Xu Nuo, Wang Ronghan, Jin Chuanqi, Lu Ying, Dong Ming. Biodentine enhances the proliferation and differentiation of osteoblasts through upregulating bone morphogenetic protein-2 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 516-520. |
| [5] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [6] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [7] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [8] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [9] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [10] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [11] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [12] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [13] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [14] | Feng Dongfei, He Hongxu, Xie Qi, Zhang Lili, Zhou Hui, Li Wei. Selection of key genes related to biological functions and regulation pathway in periodontal reconstruction [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(2): 253-259. |
| [15] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2600-2606. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||