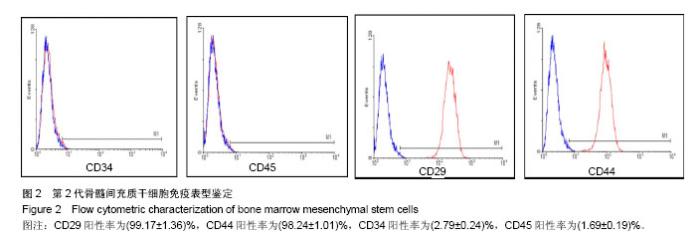
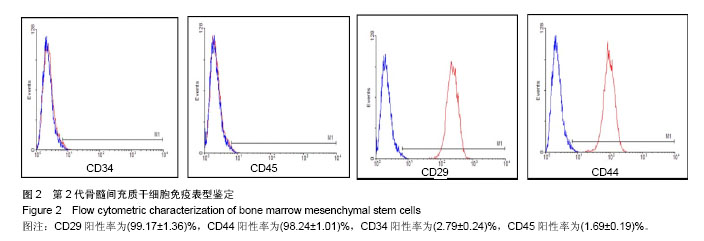
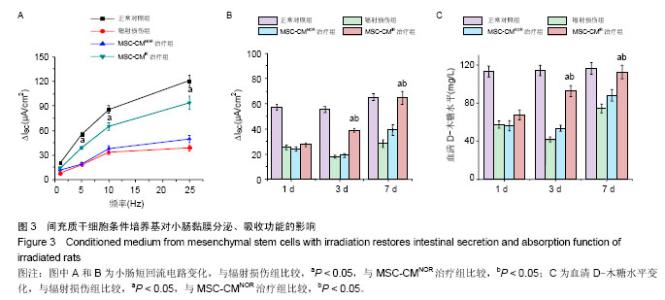
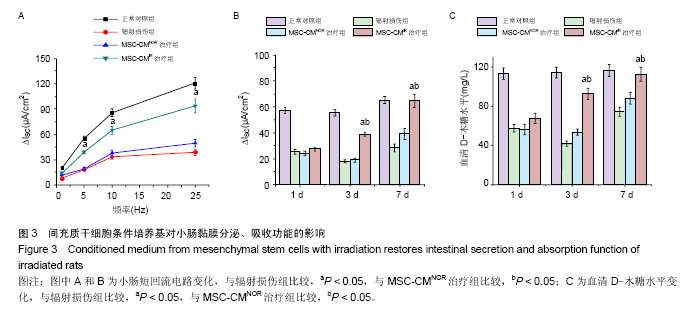
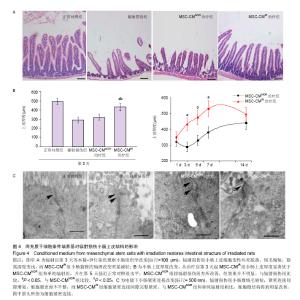
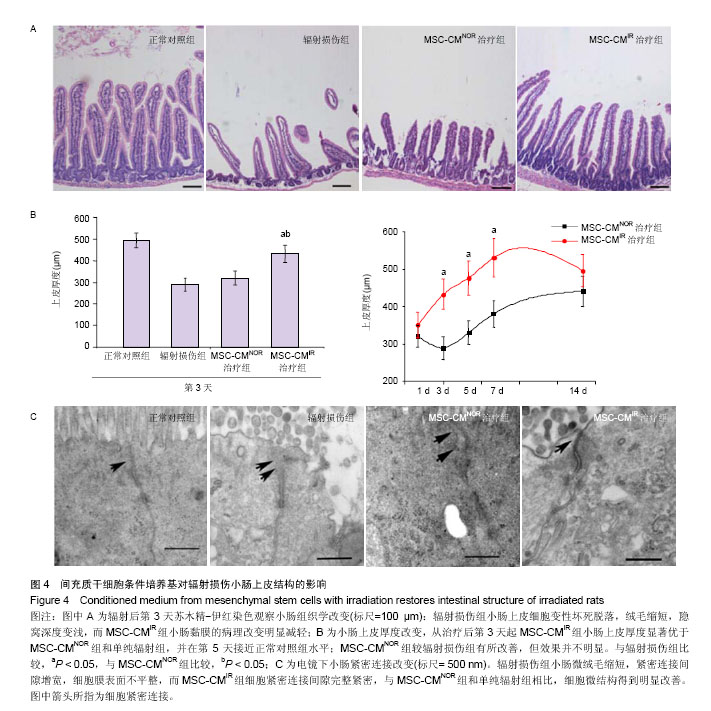
| [1]Sémont A, Mouiseddine M, François A, et al. Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ. 2010;17(6):952-961.
[2]Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10(6):709-716.
[3]Marx J. Cancer research. Mutant stem cells may seed cancer. Science. 2003;301(5638):1308-1310.
[4]Menasché P. Stem cells for clinical use in cardiovascular medicine: current limitations and future perspectives. Thromb Haemost. 2005;94(4):697-701.
[5]Sell S. On the stem cell origin of cancer. Am J Pathol. 2010; 176(6):2584-2494.
[6]Khubutiya MS, Vagabov AV, Temnov AA, et al. Paracrine mechanisms of proliferative, anti-apoptotic and anti-inflammatory effects of mesenchymal stromal cells in models of acute organ injury. Cytotherapy. 2014;16(5): 579-585.
[7]van Poll D, Parekkadan B, Cho CH, et al. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 2008;47(5): 1634-1643.
[8]Gao Z, Zhang Q, Han Y, et al. Mesenchymal stromal cell-conditioned medium prevents radiation-induced small intestine injury in mice. Cytotherapy. 2012;14(3):267-273.
[9]Chang YH, Lin LM, Lou CW, et al. Bone marrow transplantation rescues intestinal mucosa after whole body radiation via paracrine mechanisms. Radiother Oncol. 2012; 105(3):371-377.
[10]da Silva AF, Silva K, Reis LA, et al. Bone Marrow-Derived Mesenchymal Stem Cells and Their Conditioned Medium Attenuate Fibrosis in an Irreversible Model of Unilateral Ureteral Obstruction. Cell Transplant. 2015. [Epub ahead of print]
[11]Duijvestein M, Wildenberg ME, Welling MM, et al. Pretreatment with interferon-γ enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells. 2011;29(10):1549-1558.
[12]Hou Y, Ryu CH, Jun JA, et al. IL-8 enhances the angiogenic potential of human bone marrow mesenchymal stem cells by increasing vascular endothelial growth factor. Cell Biol Int. 2014;38(9):1050-1059.
[13]Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009;4(1):102-106.
[14]Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008; 2(2):141-150.
[15]Gunn WG, Conley A, Deininger L, et al. A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells. 2006;24(4):986-991.
[16]Ko IK, Kim BG, Awadallah A, et al. Targeting improves MSC treatment of inflammatory bowel disease. Mol Ther. 2010; 18(7):1365-1372.
[17]Cui X, Chen J, Zacharek A, et al. Nitric oxide donor upregulation of stromal cell-derived factor-1/chemokine (CXC motif) receptor 4 enhances bone marrow stromal cell migration into ischemic brain after stroke. Stem Cells. 2007; 25(11):2777-2785.
[18]Xun CQ, Thompson JS, Jennings CD, et al. Effect of total body irradiation, busulfan-cyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in H-2-incompatible transplanted SCID mice. Blood. 1994;83(8):2360-2367.
[19]Hill GR, Crawford JM, Cooke KR, et al. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90(8):3204-3213.
[20]Gonzalez-Rey E, Anderson P, González MA, et al. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58(7):929-939.
[21]Polchert D, Sobinsky J, Douglas G, et al. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008; 38(6):1745-1755. |