Chinese Journal of Tissue Engineering Research ›› 2013, Vol. 17 ›› Issue (41): 7303-7309.doi: 10.3969/j.issn.2095-4344.2013.41.018
Previous Articles Next Articles
Growth factors in the construction of tissue-engineered meniscus
Chen Song, Fu Pei-liang, Cong Rui-jun, Wu Yu-li
- Department of Joint Surgery, Shanghai Changzheng Hospital Affiliated to the Second Military Medical University, Shanghai 200003, China
-
Received:2013-04-11Revised:2013-08-09Online:2013-10-08Published:2013-11-01 -
Contact:Wu Yu-li, Associate professor, Department of Joint Surgery, Shanghai Changzheng Hospital Affiliated to the Second Military Medical University, Shanghai 200003, China wuyuli6019@189.cn -
About author:Chen Song★, Studying for master’s degree, Department of Joint Surgery, Shanghai Changzheng Hospital Affiliated to the Second Military Medical University, Shanghai 200003, China chensongchinese@sohu.com Fu Pei-liang, M.D., Attending physician, Department of Joint Surgery, Shanghai Changzheng Hospital Affiliated to the Second Military Medical University, Shanghai 200003, China fupeiliang@163.com Chen Song and Fu Pei-liang contributed equally to this paper. -
Supported by:the National Natural Science Foundation of China, No. 81000798*
CLC Number:
Cite this article
Chen Song, Fu Pei-liang, Cong Rui-jun, Wu Yu-li. Growth factors in the construction of tissue-engineered meniscus[J]. Chinese Journal of Tissue Engineering Research, 2013, 17(41): 7303-7309.
share this article
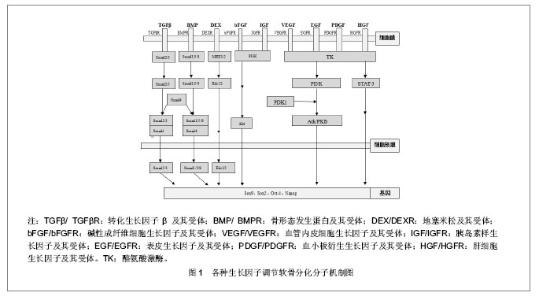
目前软骨组织工程用于种子细胞增殖和分化的生长因子主要有转化生长因子β、骨形态发生蛋白、地塞米松、碱性成纤维细胞生长因子、胰岛素样生长因子、软骨调节素、血管内皮细胞生长因子、 表皮生长因子、肝细胞生长因子、血小板衍生生长因子、软骨素酶-ABC、维生素C等。 2.1 转化生长因子β (transforming growth factor-β, TGF-β) 转化生长因子β的生物学活性非常广泛,对多种细胞的生长和分化起调节作用。一般来说,转化生长因子β对间充质起源的细胞起刺激作用,而对上皮或神经外胚层来源的细胞起抑制作用。Pangborn 等[9]对半月板细胞进行体外单层培养,向培养基中加入了各种生长因子,结果显示转化生长因子β1形成细胞外基质的能力最强。转化生长因子β1不仅能刺激细胞外基质合成,还能下调基质金属蛋白2(matrix metalloproteinase2,MMP-2)和膜型金属蛋白酶1(membrane type1-metalloprotease,MT1)等细胞外基质降解酶[10],抑制间充质干细胞向终末肥大软骨细胞分化[11]。杨玥等[12]探讨转化生长因子β2调节骨髓间充质干细胞增殖和向软骨细胞方向分化的作用时,得出结论:转化生长因子β2通过促进软骨特异性基质如Ⅱ型胶原及蛋白多糖的合成,从而发挥软骨诱导作用。目前认为转化生长因子β1是诱导间充质干细胞向软骨细胞分化的主要细胞因子,能够促进软骨细胞分泌 Aggrecan 和Ⅱ型胶原,并保持软骨细胞表型稳定,在间充质干细胞向软骨细胞分化过程中起重要作用[13]。转化生长因子β调节软骨分化的分子机制为:转化生长因子β与其Ⅱ型受体(TGFβRⅡ)结合使其磷酸化,磷酸化的TGFβRⅡ激活下游信号分子Smad2/3/4,磷酸化的Smad2/3/4被转移到细胞核中调节软骨相关基因Sox-9的表达,最终调节细胞的成软骨分化能力[14],见图1。"
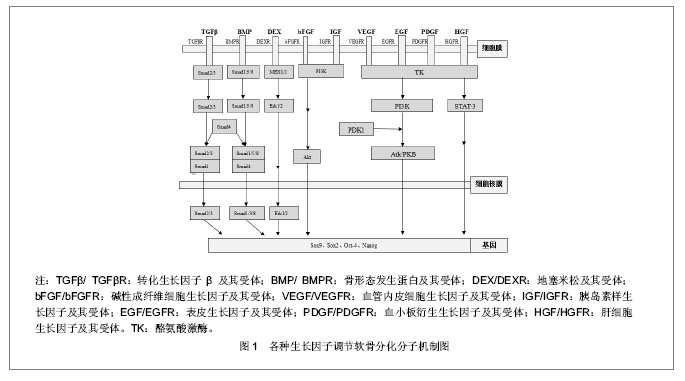

2.2 骨形态发生蛋白(bone morphogenetic protein,BMP) 骨形态发生蛋白家族是转化生长因子β超家族的亚科,这些因子在调节细胞生长、间充质分化和组织再生及重塑方面发挥重要作用,能够诱导动物或人体间充质细胞分化为骨、软骨、韧带、肌腱和神经。迄今为止已发现20多种骨形态发生蛋白[15-16],但骨形态发生蛋白2是目前已知的骨形态发生蛋白中成骨能力最强的细胞因子,近年来研究表明其亦可诱导间充质干细胞向软骨分化,促进关节软骨细胞的增殖和软骨特异性细胞外基质的合成[17-18]。除骨形态发生蛋白2可以诱导细胞成软骨外,骨形态发生蛋白4/6/7亦可以影响细胞成软骨分化,使Ⅱ型胶原纤维和蛋白多糖产生增加[19]。Sekiya等[20]比较了骨形态发生蛋白2,4,6在体外促进骨髓间充质干细胞向软骨分化的能力,结果发现骨形态发生蛋白2的作用最有效。目前在半月板组织工程中,骨形态发生蛋白复合支架材料及重建病毒骨形态发生蛋白质粒转染间充质干细胞从而让骨形态发生蛋白持续高表达的研究较多,结果显示转染的间充质干细胞可持续稳定分泌骨形态发生蛋白2[21]。骨形态发生蛋白2调节软骨分化的机制为:骨形态发生蛋白2与其Ⅱ型受体(BMPRⅡ)结合使其磷酸化,磷酸化的BMPRⅡ激活下游信号分子Smad1/5/8,磷酸化的Smad1/5/8被转移到细胞核中调节软骨相关基因的表达,最终调节细胞的成软骨分化能力,见图1。 2.3 地塞米松(dexamethasone, DEX) 地塞米松具有抗炎、抗内毒素、抑制免疫、抗休克及增强应激反应等药理作用。除此之外,在组织工程中,地塞米松还可作为细胞因子诱导细胞向软骨细胞分化[22]。Nishimura等[23]将滑膜细胞在含有转化生长因子β1和地塞米松培养基中进行,结果显示滑膜细胞可分化为软骨细胞。随后,Shirasawa等[24]在此基础上,将骨形态发生蛋白2加入上述培养基中,诱导培养滑膜间充质干细胞,结果显示软骨细胞外基质产量显著增加。芮云峰等[25]认为转化生长因子β1、骨形态发生蛋白2和地塞米松三联组合是诱导细胞向软骨细胞分化最为有效的组合形式。地塞米松调节软骨分化的机制为:地塞米松对转化生长因子β1诱导关节软骨细胞的生长有抑制作用,但却可以增加细胞外基质的表达,具体分子机制不太明确,可能与Erk(转化生长因子β信号通路调节分子)偶联激动蛋白的失活有关,从而最终对细胞的成软骨起调节作用[26]。 2.4 碱性成纤维细胞生长因子(basic fabroblast growth factor,bFGF) 成纤维细胞生长因子为软骨源性细胞因子,具有促进软骨细胞增殖、分化、基质形成及增强滑膜间充质干细胞成软骨分化的能力[27]。Kim等[28]在滑膜间充质干细胞单层扩增培养和诱导培养时加入碱性成纤维细胞生长因子,结果显示actin表达增加,细胞增殖明显;Ⅱ型胶原和X型胶原的mRNA及蛋白表达水平均升高,表明诱导前加入碱性成纤维细胞生长因子能促进滑膜间充质干细胞的增殖和成软骨分化。张经纬等[29]将碱性成纤维细胞生长因子基因转染到半月板纤维软骨细胞上,结果证实碱性成纤维细胞生长因子基因转染能促进半月板纤维软骨细胞的增殖和胶原合成能力。叶川等[30]研究显示碱性成纤维细胞生长因子、转化生长因子β1及胰岛素样生长因子1均可用于体外扩增人胚半月板细胞,其中胰岛素样生长因子1/转化生长因子β1联用或碱性成纤维细胞生长因子/胰岛素样生长因子1联用后效果更佳。碱性成纤维细胞生长因子增强细胞向软骨分化能力的分子机制为:碱性成纤维细胞生长因子与其受体结合后,激活下游的信号分子PI3K,活化的PI3K作用于Akt使其磷酸化,磷酸化的Akt被转运到细胞核内调节Oct-4、Sox-2、Nanog等基因的表达,从而调节细胞的成软骨分化[31]。 2.5 胰岛素样生长因子(insulin-like growth factor, IGF) 胰岛素样生长因子是一类多功能细胞增殖调控因子,包括胰岛素样生长因子1和胰岛素样生长因子2两种。在细胞的分化、增殖、个体的生长发育中具有重要的促进作用。胰岛素样生长因子1具有很强的合成代谢刺激作用,可促进软骨细胞的有丝分裂、Ⅱ型胶原及Aggrecan 合成,促进间充质干细胞向软骨分化,有利于软骨发生及软骨细胞沉淀。Fukumoto等[32]研究表明在人体软骨内短时间增加胰岛素样生长因子1,可增加局部软骨下骨间充质干细胞和软骨细胞的修复能力。胰岛素样生长因子与其受体结合后,可以增加细胞中蛋白聚糖的含量,从而调节细胞的成软骨能力,其具体调节机制类似于碱性成纤维细胞生长因 子[33]。 2.6 软骨调节素(chondromodulin, ChM) 软骨调节素是一族软骨源性生长因子,也是一种血管内皮细胞生长抑制剂。软骨调节素分为软骨调节素Ⅰ、软骨调节素Ⅱ、软骨调节素Ⅲ 3种,其中软骨调节素Ⅰ是家族中效应最强的一种软骨特异性生长因子。软骨调节素Ⅰ表达的下降是软骨骨化和退变的重要原因。Klinger等[34]研究表明软骨调节素Ⅰ可以维持软骨细胞表型,促进透明样基质的表达,并且具有抑制软骨内成骨的作用。邢双春等[35]在研究软骨调节素Ⅰ和结缔组织生长因子在大鼠骨髓间充质干细胞、透明软骨细胞及弹性软骨细胞中的差异表达时发现,透明软骨细胞和弹性软骨细胞中高表达软骨调节素Ⅰ蛋白,软骨调节素高表达结缔组织生长因子蛋白,说明软骨调节素Ⅰ可能在成熟的软骨细胞中发挥着重要作用。 2.7 血管内皮生长因子(vascular epithelial growth factor, VEGF) 血管内皮生长因子又称血管通透因子或促血管因子,是一种糖蛋白。它能特异性地作用于血管内皮细胞,具有维持血管正常状态和完整性、提高血管通透性、促血管生成的作用。Gerber等[36]给小鼠注射血管内皮生长因子受体抗体后,发现血管长入几乎停止,小梁骨及肥大软骨区扩展性损害,破骨细胞的分化和趋化能力下降,导致对终末软骨细胞的吞噬作用下降;如解除血管内皮生长因子抗体,则骨吸收、肥大软骨细胞吸收及骺板生长结构均正常。这说明血管内皮生长因子介导的血管增生是决定骨骺板形态发生,从而进行软骨改建的基本信号。血管内皮生长因子在软骨成骨过程中协调着软骨细胞的消长、破骨细胞的功能、细胞外基质的改建、血管增生及骨形成之间的关系,对软骨内成骨及骨折愈合过程中血管增生亦发挥着重要作用[37]。刘傥等[38]在观察兔骨髓间充质干细胞成骨诱导分化过程中血管内皮生长因子和骨形态发生蛋白2的表达时发现,骨髓间充质干细胞在成骨诱导过程中,骨形态发生蛋白2和血管内皮生长因子表达均先增强后减弱,血管内皮生长因子表达可能对血管生成有促进作用。血管内皮生长因子调节软骨的分子机制为:血管内皮生长因子与其受体结合后,使下游的信号分子酪氨酸激酶(Tyrosine Kinase,TK)磷酸化,磷酸化的酪氨酸激酶作用于PI3K使其磷酸化,活化的PI3K再使Akt/PKB磷酸化,随后磷酸化的Akt/PKB被转运到细胞核内调节Sox-9、RUNX-2等基因的表达,从而调节细胞的成软骨分化[39]。 2.8 表皮生长因子(epidermal growth factor, EGF) 表皮生长因子是一种多功能的生长因子,在体内体外都对多种组织细胞有强烈的促分裂作用,促进生成大量的成骨细胞、抑制破骨细胞。Tigli等[40]将小鼠软骨细胞置于壳聚糖-RGD和壳聚糖-表皮生长因子支架上进行培养,结果显示只有壳聚糖-表皮生长因子支架能够促进细胞增殖,说明表皮生长因子具有促细胞成软骨的作用。表皮生长因子与转化生长因子β1具有相同的受体,两者协同可以促进细胞的增殖,有助于组织工程半月板的血管化[41]。李静等[42]在观察碱性成纤维细胞生长因子、表皮生长因子和血小板源性生长因子对小鼠骨髓间充质干细胞体外增殖和成骨分化的影响时发现,联合使用碱性成纤维细胞生长因子、表皮生长因子和血小板源性生长因子可促进小鼠骨髓间充质干细胞的体外增殖和干性的维持,并且有利于其向成骨细胞的分化。表皮生长因子调节软骨的分子机制与血管内皮生长因子相似。 2.9 肝细胞生长因子(hepatocyte growth factor, HGF) 肝细胞生长因子是一个多效应生长因子,也是很强的促肝细胞增殖因子,不仅能刺激肝细胞的再生,其在软骨分化中也起到重要作用。Hidaka等[43]用腺病毒载体编码的肝细胞生长因子转染半月板细胞,结果显示被转染的半月板细胞形成血管能力较强。另外,肝细胞生长因子与血小板衍生生长因子联合应用,可以趋化半月板细胞向损伤部位迁移并产生细胞外基质[41]。张诚等[44]分别把肝细胞生长因子-骨髓间充质干细胞组和其他对照组即未转染细胞组、绿色荧光蛋白-骨髓间充质干细胞组、Ad-肝细胞生长因子对照组、假伤组移植到背部深Ⅱ度烧伤的大鼠上。结果显示,肝细胞生长因子-骨髓间充质干细胞组的再生表皮明显厚于其他组,并可见真皮钉脚下伸,创面中肝细胞生长因子的阳性表达位于真皮层,表达强度明显强于其他组,说明经肝细胞生长因子转染的骨髓间充质干细胞移植对烧伤创面愈合存在一定的促进作用。肝细胞生长因子调节软骨分化的机制为:HGF-α/β亚单位形成二聚体,与细胞表面cMET-RTK偶联受体结合后使其磷酸化,磷酸化的TK作用于胞浆中STAT-3信号分子使其磷酸化,STAT-3再被转移到细胞核中调节软骨相关基因的表达,从而实现对软骨形成的调节作用[45]。 2.10 血小板源性生长因子(platelet-derived growth factor,PDGF) 血小板源性生长因子是一种促进生长的内生性蛋白质,在伤口愈合过程中,血小板源性生长因子可由多种细胞来释放,而且血小板源性生长因子分成AA/AB/BB三种具生理活性形式,其中BB型式更能促进纤维母细胞的生长,所以促进与伤口修复有关的细胞化学触觉补强及细胞增殖,加强肉芽组织的形成,促进伤口愈合并缩短愈合时间。血小板源性生长因子在关节修复方面的应用也取得了巨大的进展。Iwata等[46]用含有碱性成纤维细胞生长因子、胰岛素样生长因子1、表皮生长因子、血小板源性生长因子及转化生长因子β的培养基进行软骨细胞扩增培养时,结果显示软骨细胞数量是对照组的3倍。Murphy等[47]用转化生长因子β1、碱性成纤维细胞生长因子及血小板源性生长因子的培养基进行肋骨软骨分化时,结果显示细胞能够产生大量的黏多糖及Ⅱ型胶原。林旺等[48]观察在体外培养条件下胰岛素样生长因子1、血小板源性生长因子对人退变髓核细胞生物学活性的影响时发现胰岛素样生长因子1促进细胞合成Ⅰ型胶原,血小板源性生长因子抑制细胞合成Ⅰ型胶原。结果证实胰岛素样生长因子1、血小板源性生长因子均可通过促进细胞增殖、促进细胞合成Ⅱ型胶原和聚集蛋白聚糖,从而提高人退变髓核细胞的生物学活性。血小板源性生长因子调节软骨分化的分子机制与血管内皮生长因子相似[49]。 2.11 软骨素酶ABC(Chondroitinase ABC,C-ABC) 软骨素酶ABC作为一种生化刺激因子已被运用于软骨组织工程中。软骨素酶ABC可降解硫酸软骨素A、硫酸软骨素B(硫酸皮肤素)和硫酸软骨素C,通过β消除机制作用于二糖重复单元之间的β1-4糖苷上并将其切断。Natoli等[50]将软骨素酶ABC运用到关节软骨组织工程中时发现,软骨素酶ABC可以增加软骨的抗拉特性及糖胺多糖的含量。Huey等[51]也得到了相似的结论。但是,软骨素酶ABC在半月板组织工程中的运用,尤其是和其他生长因子的联合运用还有待进一步的研究。 2.12 维生素C(vitamin C,Vit C) 维生素C能使细胞分泌的基质和胶原纤维有机的组成与排列,构成特殊的环境,有利于内、外源活性因子的传输,在局部有选择的储存和排泄活性物质。细胞基质和胶原纤维有机的组成和排列使细胞与细胞间密切接触,利于细胞发生分泌与旁分泌作用,利于细胞发生各种转化,其本身不能是细胞发生转化[52]。于宁等[53]将含碱性成纤维细胞生长因子和维生素C的羊膜载体复合膜负载骨髓间充质干细胞植入兔创面,探讨其对真皮新生和重建速度的影响以及促进表皮修复速度的最佳时期,研究显示添加骨髓间充质干细胞、碱性成纤维细胞生长因子和维生素C的羊膜载体复合膜植入全层皮肤缺损后,能加速真皮的修复重建,并且在真皮修复全层皮肤过程中表皮有新生和重建的最佳时期。"

| [1] Buma P, Ramrattan NN, van Tienen TG,et al. Tissue engineering of the meniscus. Biomaterials. 2004;25(9): 1523-1532.
[2] Bosch U.Stage-based treatment by partial meniscectomy, meniscal refixation and transplantation. Zentralbl Chir. 2005; 130(4):314-320.
[3] Koski JA, Ibarra C, Rodeo SA,et al. Meniscal injury and repair: clinical status. Orthop Clin North Am. 2000;31(3):419-436.
[4] Jones BA, Pei M. Synovium-derived stem cells: a tissue-specific stem cell for cartilage engineering and regeneration.Tissue Eng Part B Rev. 2012;18(4):301-311.
[5] Rath E, Richmond JC, Yassir W,et al. Meniscal allograft transplantation. Two- to eight-year results. Am J Sports Med. 2001;29(4):410-414.
[6] 符培亮,张雷,吴海山,等. 腺病毒介导BMP-2/7共表达转染兔滑膜MSCs体外向纤维软骨样细胞分化的研究[J]. 中国修复重建外科杂志, 2013,27(3):345-352.
[7] De Bari C, Dell'Accio F, Tylzanowski P,et al. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001 ;44(8):1928-1942.
[8] 赵文君,邢国胜,于顺禄. 滑膜间充质干细胞及其在组织工程中的应用[J]. 中国修复重建外科杂志,2011,25(12):1504-1507.
[9] Pangborn CA, Athanasiou KA. Growth factors and fibrochondrocytes in scaffolds. J Orthop Res. 2005;23(5): 1184-1190.
[10] Liu Y, Li JM, Hu YG.Transplantation of gene-modified nucleus pulposus cells reverses rabbit intervertebral disc degeneration.Chin Med J (Engl). 2011;124(16):2431-2437.
[11] Pagnotto MR, Wang Z, Karpie JC,et al. Adeno-associated viral gene transfer of transforming growth factor-beta1 to human mesenchymal stem cells improves cartilage repair. Gene Ther. 2007 ;14(10):804-813.
[12] 杨玥, 薛同圆, 田京. 软骨形成过程中的转化生长因子β[J]. 中国组织工程研究与临床康复,2011,15(46):8714-8717.
[13] Goessler UR, Bugert P, Bieback K,et al. In-vitro analysis of the expression of TGFbeta -superfamily-members during chondrogenic differentiation of mesenchymal stem cells and chondrocytes during dedifferentiation in cell culture. Cell Mol Biol Lett. 2005;10(2):345-362.
[14] Jin G, Westphalen CB, Hayakawa Y,et al. Progastrin Stimulates Colonic Cell Proliferation via CCKBR- and β-Arrestin-Dependent Suppression of BMP2. Gastroenterology. 2013. pii: S0016-5085(13)01085-8.
[15] McIntosh CJ, Lawrence S, Smith P,et al. Active immunization against the proregions of GDF9 or BMP15 alters ovulation rate and litter size in mice. Reproduction. 2012;143(2): 195-201.
[16] Kaneda A, Fujita T, Anai M,et al. Activation of Bmp2-Smad1 signal and its regulation by coordinated alteration of H3K27 trimethylation in Ras-induced senescence. PLoS Genet. 2011;7(11):e1002359.
[17] Shirasawa S, Sekiya I, Sakaguchi Y,et al. In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: optimal condition and comparison with bone marrow-derived cells. J Cell Biochem. 2006;97(1):84-97.
[18] Horie M, Sekiya I, Muneta T,et al. Intra-articular Injected synovial stem cells differentiate into meniscal cells directly and promote meniscal regeneration without mobilization to distant organs in rat massive meniscal defect. Stem Cells. 2009;27(4):878-887.
[19] Miljkovic ND, Cooper GM, Marra KG. Chondrogenesis, bone morphogenetic protein-4 and mesenchymal stem cells. Osteoarthritis Cartilage. 2008;16(10):1121-1130.
[20] Sekiya I, Larson BL, Vuoristo JT,et al. Comparison of effect of BMP-2, -4, and -6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell Tissue Res. 2005;320(2):269-276.
[21] 孙立,田晓滨,胡如印,等. pIRES-骨形态发生蛋白2质粒转染大鼠骨髓间充质干细胞后的持续表达[J]. 中国组织工程研究, 2012, 16(27):5017-5021.
[22] 周小锐,刘世清,贺斌,等. 地塞米松可影响兔髓核细胞增殖及Ⅱ型胶原的表达[J].中国组织工程研究,2012,16(11):1915-1918.
[23] Nishimura K, Solchaga LA, Caplan AI, et al. Chondroprogenitor cells of synovial tissue. Arthritis Rheum. 1999;42(12):2631-2637.
[24] Shirasawa S, Sekiya I, Sakaguchi Y,et al. In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: optimal condition and comparison with bone marrow-derived cells. J Cell Biochem. 2006;97(1): 84-97
[25] 芮云峰,王友.人滑膜间充质干细胞的研究现状[J].中华关节外科 杂志:电子版,2007,1(1):75-78.
[26] Shintani N, Hunziker EB. Differential effects of dexamethasone on the chondrogenesis of mesenchymal stromal cells: influence of microenvironment, tissue origin and growth factor. Eur Cell Mater. 2011;22:302-319.
[27] 赵文君,邢国胜,于顺禄. 滑膜间充质干细胞及其在组织工程中的应用[J]. 中国修复重建外科杂志, 2011,25(12):1504-1507.
[28] Kim JH, Lee MC, Seong SC,et al. Enhanced proliferation and chondrogenic differentiation of human synovium-derived stem cells expanded with basic fibroblast growth factor. Tissue Eng Part A. 2011;17(7-8):991-1002.
[29] 张经纬,曾炳芳,赵金忠. 碱性成纤维细胞生长因子转染对半月板纤维软骨细胞增殖、细胞周期及胶原合成影响的研究[J]. 中国修复重建外科杂志,2006,20(12):1253-1256.
[30] 叶川,邓展生,李宝军,等. 三种生长因子对人胚半月板细胞增殖及细胞表型的影响[J]. 中国修复重建外科杂志,2007,21(10): 1137-1141.
[31] Cheng T, Yang C, Weber N,et al. Fibroblast growth factor 2 enhances the kinetics of mesenchymal stem cell chondrogenesis. Biochem Biophys Res Commun. 2012; 426(4): 544-550.
[32] Fukumoto T, Sperling JW, Sanyal A,et al. Combined effects of insulin-like growth factor-1 and transforming growth factor-beta1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthritis Cartilage. 2003; 11(1):55-64.
[33] Patil AS, Sable RB, Kothari RM. Role of insulin-like growth factors (IGFs), their receptors and genetic regulation in the chondrogenesis and growth of the mandibular condylar cartilage. J Cell Physiol. 2012;227(5):1796-1804.
[34] Klinger P, Surmann-Schmitt C, Brem M,et al. Chondromodulin 1 stabilizes the chondrocyte phenotype and inhibits endochondral ossification of porcine cartilage repair tissue. Arthritis Rheum. 2011;63(9):2721-2731.
[35] 邢双春,翟立杰,王志强,等. 软骨调节素Ⅰ和结缔组织生长因子蛋白在骨髓间充质干细胞和软骨细胞中的差异表达[J]. 中国组织工程研究与临床康复, 2009,13(41):8085-8089.
[36] Gerber HP, Vu TH, Ryan AM,et al. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation.Nat Med. 1999;5(6):623-628.
[37] 侯锐,毛天球.生长因子在组织工程中的应用[J]. 国外医学:生物医学工程分册,2002,25(5):219-223.
[38] 刘傥,张湘生,张庆,等.骨髓间充质干细胞成骨分化中血管内皮生长因子和骨形态发生蛋白2的表达[J]. 中国组织工程研究, 2012, 16(36):6651-6657.
[39] Studer D, Millan C, Öztürk E,et al. Molecular and biophysical mechanisms regulating hypertrophic differentiation in chondrocytes and mesenchymal stem cells. Eur Cell Mater. 2012;24:118-135.
[40] Ti?li RS, Gümü?derelio?lu M. Evaluation of RGD- or EGF-immobilized chitosan scaffolds for chondrogenic activity. Int J Biol Macromol. 2008;43(2):121-128.
[41] 钱赫亮. 运动性半月板损伤及组织工程半月板的应用[J]. 中国组织工程研究与临床康复, 2011,15(11):2044-2046.
[42] 李静,闵丽姗,李栋立,等. 碱性成纤维生长因子、表皮生长因子和血小板衍生因子对小鼠骨髓间充质干细胞成骨分化的影响[J]. 中华实验外科杂志, 2013,30(1):122-124.
[43] Hidaka C, Ibarra C, Hannafin JA,et al. Formation of vascularized meniscal tissue by combining gene therapy with tissue engineering.Tissue Eng. 2002;8(1):93-105.
[44] 张诚,哈小琴,刘毅. 肝细胞生长因子基因转染骨髓间充质干细胞异体移植修复烧伤创面[J]. 中国组织工程研究与临床康复, 2008,12(51):10134-10138.
[45] Amano O, Koshimizu U, Nakamura T,et al. Enhancement by hepatocyte growth factor of bone and cartilage formation during embryonic mouse mandibular development in vitro. Arch Oral Biol. 1999;44(11):935-946.
[46] Iwata J, Tung L, Urata M,et al. Fibroblast growth factor 9 (FGF9)-pituitary homeobox 2 (PITX2) pathway mediates transforming growth factor β (TGFβ) signaling to regulate cell proliferation in palatal mesenchyme during mouse palatogenesis. J Biol Chem. 2012;287(4):2353-2363.
[47] Murphy MK, Huey DJ, Reimer AJ,et al. Enhancing post-expansion chondrogenic potential of costochondral cells in self-assembled neocartilage. PLoS One. 2013;8(2): e56983.
[48] 林旺,刘寿坤,王盈盈,等. 胰岛素样生长因子1及血小板源性生长因子对人退变髓核细胞生物学活性的影响[J]. 中国组织工程研究,2012,16(2):223-236.
[49] Ataliotis P. Platelet-derived growth factor A modulates limb chondrogenesis both in vivo and in vitro. Mech Dev. 2000; 94(1-2):13-24.
[50] Natoli RM, Revell CM, Athanasiou KA. Chondroitinase ABC treatment results in greater tensile properties of self-assembled tissue-engineered articular cartilage.Tissue Eng Part A. 2009;15(10):3119-3128.
[51] Huey DJ, Athanasiou KA. Maturational growth of self-assembled, functional menisci as a result of TGF-β1 and enzymatic chondroitinase-ABC stimulation. Biomaterials. 2011;32(8):2052-2058.
[52] 张伊,韦登明,尹向旭,等. 关节软骨缺损的组织工程修复[J]. 中国组织工程研究与临床康复, 2011,15(33):6227-6230.
[53] 于宁,翟希,辛畅泰. 含bFGF和维生素C的羊膜载体复合膜负载BMSCs修复兔创面的实验研究[J]. 中国修复重建外科杂志, 2008,22(12):1495-1500. |
| [1] | Tan Xinfang, Guo Yanxing, Qin Xiaofei, Zhang Binqing, Zhao Dongliang, Pan Kunkun, Li Yuzhuo, Chen Haoyu. Effect of uniaxial fatigue exercise on patellofemoral cartilage injury in a rabbit [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(在线): 1-6. |
| [2] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [3] | Wu Cong, Jia Quanzhong, Liu Lun. Relationship between transforming growth factor beta1 expression and chondrocyte migration in adult articular cartilage after fragmentation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1167-1172. |
| [4] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [5] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [6] | Xu Kuishuai, Zhang Liang, Chen Jinli, Ren Zhongkai, Zhao Xia, Li Tianyu, Yu Tengbo. Effect of force line changes on lower limb joints after medial open wedge high tibial osteotomy [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 821-826. |
| [7] | Liu Dongcheng, Zhao Jijun, Zhou Zihong, Wu Zhaofeng, Yu Yinghao, Chen Yuhao, Feng Dehong. Comparison of different reference methods for force line correction in open wedge high tibial osteotomy [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 827-831. |
| [8] | Li Jie, Zhang Haitao, Chen Jinlun, Ye Pengcheng, Zhang Hua, Zhou Bengen, Zhao Changqing, Sun Youqiang, Chen Jianfa, Xiang Xiaobing, Zeng Yirong. Anterior cruciate ligament rupture and patellofemoral joint stability before sagittal and axial measurement using MRI [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 969-972. |
| [9] | Lin Xuchen, Zhu Hainian, Wang Zengshun, Qi Tengmin, Liu Limin, Suonan Angxiu. Effect of xanthohumol on inflammatory factors and articular cartilage in a mouse mode of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 676-681. |
| [10] | Xu Jing, Yan Yongmin, Cai Mengjie . miR-373 inhibits hepatic stellate cell activation by downregulating transforming growth factor beta type II receptor [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 756-761. |
| [11] | Xu Lei, Han Xiaoqiang, Zhang Jintao, Sun Haibiao. Hyaluronic acid around articular chondrocytes: production, transformation and function characteristics [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 768-773. |
| [12] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [13] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [14] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [15] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||