Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (29): 4629-4636.doi: 10.3969/j.issn.2095-4344.1799
Previous Articles Next Articles
Astragaloside-incubated adipose-derived stem cells for the treatment of diabetic nephropathy
Gao Junli1, 2, Zhu Yunjie1, Liu Guoxiang1, Wang Weiwei1, Zhang Jinyuan1
- 1Department of Nephrology, the 455th Hospital of PLA, Shanghai 201203, China; 2Department of Nephrology, Jinshan Branch, Shanghai Sixth People’s Hospital, Shanghai 201500, China
-
Revised:2019-03-20Online:2019-10-18Published:2019-10-18 -
Contact:Wang Weiwei, MD, Associate chief physician, Department of Nephrology, the 455th Hospital of PLA, Shanghai 201203, China -
About author:Gao Junli, Master, Department of Nephrology, the 455th Hospital of PLA, Shanghai 201203, China; Department of Nephrology, Jinshan Branch, Shanghai Sixth People’s Hospital, Shanghai 201500, China -
Supported by:Shanghai Advanced Chinese and Western Medicine Talents Training Program, No. ZY3-RCPY-4-2039 (to WWW); Medical Science and Technology Innovation Project of the Nanjing Military Region, No. 14ZX05 (to WWW)
CLC Number:
Cite this article
Gao Junli, Zhu Yunjie, Liu Guoxiang, Wang Weiwei, Zhang Jinyuan. Astragaloside-incubated adipose-derived stem cells for the treatment of diabetic nephropathy[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(29): 4629-4636.
share this article
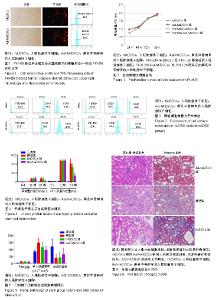
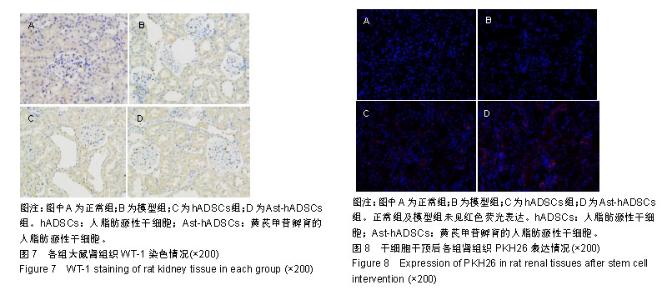
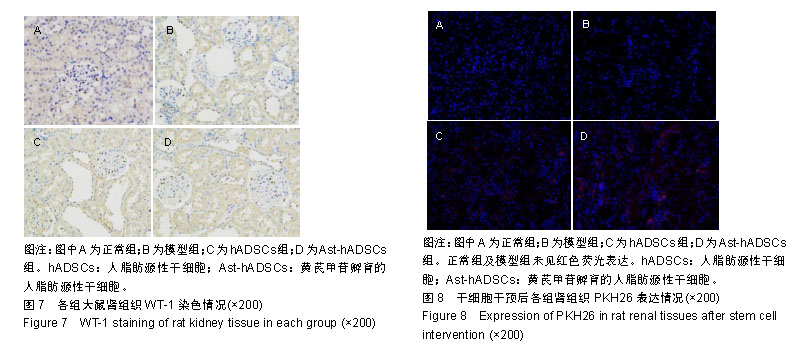
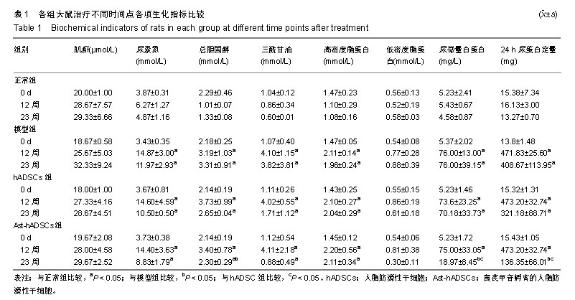
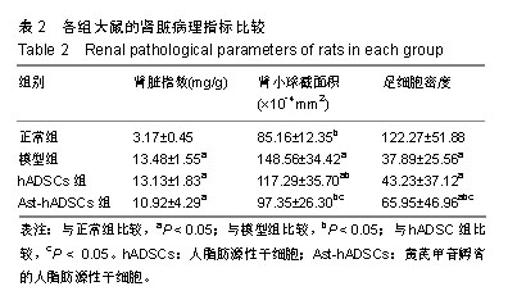
2.1 PKH26标记后的细胞形态及标记率 光镜下观察贴壁生长的hADSCs及Ast-hADSCs两组细胞,细胞呈梭形、成纤维细胞样,漩涡状、放射性生长。荧光显微镜下可观察到两组细胞的胞膜及胞浆发出红色的荧光。流式细胞仪上机检测分析显示:hADSCs组与Ast-hADSCs组细胞PKH26膜染色的阳性标记率分别为99.4%比99.6%,见图1。 2.2 黄芪甲苷对hADSC增殖及细胞表型的影响 2.2.1 CCK8检测细胞的增殖情况 随着孵育时间的增加,各组细胞的增殖逐渐增加,当细胞孵育72 h后,细胞的增殖速度减缓;与hADSCs组及PKH26+hADSCs组比较,Ast-hADSCs组及PKH26+Ast-hADSCs组细胞的增殖增加,24 h及48 h差异有统计学意义(P < 0.05),但72 h及96 h差异无统计学意义(P > 0.05),黄芪甲苷可增加细胞的增殖能力;hADSCs组与PKH26+hADSCs组细胞各时间点的增殖差异无统计学意义(P > 0.05);Ast-hADSCs组及PKH26+Ast-hADSCs组细胞各时间点增殖差异无统计学意义(P > 0.05),即经PKH26膜染标记后并不影响细胞的增殖,见图2。 2.2.2 细胞免疫表型检测 PKH26标记后进行流式细胞仪上机分析,结果显示:hADSCs及Ast-hADSCs组细胞表面分子的阳性标记率分别为CD29(99.5%比99.6%)、CD44 (99.9%比99.7%)、CD34(0.38%比0.61%)、CD45(0.36%比0.19%),见图3。 2.3 动物实验结果 2.3.1 实验动物数量分析 在链脲佐菌素注射后第2天模型组死亡1只大鼠,可能是因为血糖骤然升高,大鼠不适应死亡,未做补充实验。共20只大鼠进入结果分析。 2.3.2 各组大鼠血清肌酐、尿素氮浓度比较 0 d时、造模后12周末及实验结束时(23周),各组血清肌酐水平总体均数相等(P > 0.05)。0 d时,各组尿素氮水平总体均数相等(P > 0.05);造模后12周末,与正常组比较,其他3组尿素氮水平均上升(P < 0.05);实验结束时(23周),造模的3组大鼠血尿素氮水平仍高于正常组(P < 0.05),与模型组比较,hADSCs组及Ast-hADSCs组尿素氮水平均下降,与hADSCs组比较,Ast-hADSCs组尿素氮水平下降更显著,但差异均无显著性意义(P > 0.05)。见表1。 2.3.3 各组大鼠血脂水平比较 0 d时,各组总胆固醇、三酰甘油、高密度脂蛋白及低密度脂蛋白水平的总体均数相等(P > 0.05);造模后12周末,与正常组比较,其他3组总胆固醇、三酰甘油、高密度脂蛋白均显著上升(P < 0.05);实验结束时(23周),造模的3组大鼠以上3项血脂指标仍高于正常组(P < 0.05),与模型组比较:hADSCs组及Ast-hADSCs组三酰甘油水平下降及高密度脂蛋白水平升高,但差异无统计学意义(P > 0.05),Ast-hADSCs组总胆固醇水平显著下降 (P < 0.05),hADSCs组总胆固醇水平下降但差异无统计学意义(P > 0.05), hADSCs组与Ast-hADSCs组比较各指标差异无统计学意义(P > 0.05)。3个时间点各组低密度脂蛋白水平差异无统计学意义(P > 0.05)。见表1。 2.3.4 各组尿微量白蛋白及尿蛋白定量水平比较 0 d时,各组尿微量白蛋白及尿蛋白定量水平的总体均数相等(P > 0.05);造模后12周末,与正常组比较,其他3组上述2项指标均显著上升(P < 0.05);实验结束时(23周),模型组及hADSCs组大鼠尿微量白蛋白及尿蛋白定量水平仍高于正常组(P < 0.05),Ast-hADSCs组数值虽仍高于正常组但无统计学差异(P > 0.05),模型组与hADSCs组比较差异无显著性意义(P > 0.05),而Ast-hADSCs组尿微量白蛋白及尿蛋白定量水平较模型组及hADSCs组显著降低(P < 0.05)。见表1及图4。 2.3.5 各组肾脏病理指标比较 与正常组比较,其他3组肾脏指数水平显著升高(P < 0.01),提示造模的3组大鼠肾脏肥大;与模型组比较,干细胞干预两组肾脏指数水平均降低,尤其是Ast-hADSCs组降低更改显著,但组间比较差异均无统计学意义(P > 0.05),提示干细胞干预两组大鼠肾脏肥大较模型组减轻;而黄芪甲苷孵育的hADSCs可更好的改善糖尿病肾病模型大鼠的肾损伤。见表2及图5。 2.3.6 各组肾脏病理变化 正常组大鼠肾组织结构清晰正常;模型组大鼠肾小球基底膜增厚,系膜基质增加,肾小管空泡样变性,血管内膜增厚,部分大鼠出现轻度肾间质纤维化;分别予hADSCs及Ast-hADSCs干预后,肾小球病变程度减轻,间质纤维化明显好转,Ast-hADSCs组减轻尤为明显。见图6。 2.3.7 各组肾小球截面积比较 与正常组比较,其他3组的肾小球截面积显著增加(P < 0.01);与模型组比较,hADSCs组及Ast-hADSCs组的肾小球截面积显著下降(P < 0.01);与hADSCs组比较,Ast-hADSCs组肾小球截面积缩小(P < 0.05)。见表2。 2.3.8 各组足细胞密度变化 与正常组比较,其他3组的足细胞密度显著降低(P < 0.01);与模型组比较,hADSCs组足细胞密度增加但无统计学差异(P > 0.05),Ast-hADSCs组的足细胞密度增加(P < 0.05);与hADSCs组比较,Ast-hADSCs组足细胞密度增加(P < 0.05)。见表2及图7。 2.3.9 干细胞在肾组织的分布情况 激光共聚焦显示PKH26标记的hADSCs及Ast-hADSCs在大鼠肾组织有少量表达,且红色荧光与肾脏细胞呈明显融合状态,正常组及模型组均未见红色荧光表达。见图8。"

| [1]Collins AJ, Foley RN, Chavers B, et al.'United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States.Am J Kidney Dis. 2012; 59(1 Suppl 1):A7, e1-420.[2]Toth-Manikowski S, Atta MG.Diabetic Kidney Disease: Pathophysiology and Therapeutic Targets.J Diabetes Res. 2015;2015:697010.[3]Saran R, Li Y, Robinson B, et al.US Renal Data System 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States.Am J Kidney Dis. 2015;66(1 Suppl 1):Svii, S1-305.[4]Checheri?? IA, Turcu F, Dragomirescu RF, et al.Chronic complications in hemodialysis: correlations with primary renal disease.Rom J Morphol Embryol. 2010;51(1):21-26.[5]Stoumpos S, Jardine AG, Mark PB.Cardiovascular morbidity and mortality after kidney transplantation.Transpl Int. 2015;28(1):10-21.[6]杜俊文,吴韬,张坤,等. 骨髓间充质干细胞移植改善糖尿病肾病大鼠血糖及尿总蛋白[J]. 中国组织工程研究, 2016,20(6):855-860.[7]何文涓,何晓升,袁志坚,等. 黄芪甲苷对兔脂肪来源的间充质干细胞体外增殖和细胞周期的影响[J]. 中国生化药物杂志, 011,32(6):466-468.[8]王葳,姜燕,王巍巍,等. 黄芪甲苷对脂肪源性干细胞体外生物学行为的影响[J]. 中国药理学通报,2013,29(2):220-224.[9]王葳,姜燕,王巍巍,等.黄芪甲苷孵育脂肪源性干细胞对顺铂诱导肾小管上皮细胞凋亡的影响[J]. 中国组织工程研究, 2014,18(28):4498-4503.[10]姜燕,王葳,李泽争,等. 黄芪甲苷孵育的脂肪源性干细胞对顺铂诱导的急性肾损伤小鼠的保护作用[J]. 中国中西医结合肾病杂志,2014,15(2):114-117.[11]高俊丽,朱赟洁,王巍巍,等. 左肾切除术联合链脲佐菌素腹腔注射诱导糖尿病肾病大鼠模型的建立[J]. 临床肾脏病杂志, 2018,18(05):306-311.[12]Coombe L, Kadri A, Martinez JF, et al. Current approaches in regenerative medicine for the treatment of diabetes: introducing CRISPR/CAS9 technology and the case for non-embryonic stem cell therapy. Am J Stem Cells. 2018;7(5):104-113.[13]Chandra V, G S, Phadnis S, et al.Generation of pancreatic hormone-expressing islet-like cell aggregates from murine adipose tissue-derived stem cells.Stem Cells. 2009;27(8):1941-1953.[14]Kajiyama H, Hamazaki TS, Tokuhara M, et al.Pdx1-transfected adipose tissue-derived stem cells differentiate into insulin-producing cells in vivo and reduce hyperglycemia in diabetic mice.Int J Dev Biol. 2010;54(4):699-705.[15]Kim D, Dressler GR.Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia.J Am Soc Nephrol. 2005;16(12):3527-3534.[16]Narayanan K, Schumacher KM, Tasnim F, et al.Human embryonic stem cells differentiate into functional renal proximal tubular-like cells.Kidney Int. 2013;83(4):593-603.[17]Song B, Smink AM, Jones CV, et al.The directed differentiation of human iPS cells into kidney podocytes.PLoS One. 2012;7(9):e46453.[18]Lam AQ, Freedman BS, Morizane R, et al.Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers.J Am Soc Nephrol. 2014;25(6):1211-1225.[19]Ezquer ME, Ezquer FE, Arango-Rodriguez ML, et al. MSC transplantation:a promising therapeutic strategy to manage the onset and progression of diabetic nephropathy. Biol Res. 2012;45(3): 289-296.[20]Ezquer FE, Ezquer ME, Parrau DB, et al. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice.Biol Blood Marrow Transplant. 2008;14(6):631-640.[21]Wu S, Li L, Wang G, et al.Ultrasound-targeted stromal cell-derived factor-1-loaded microbubble destruction promotes mesenchymal stem cell homing to kidneys in diabetic nephropathy rats.Int J Nanomedicine. 2014;9:5639-5651. [22]Nagaishi K, Mizue Y, Chikenji T, et al.Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes.Sci Rep. 2016;6:34842.[23]Ezquer F, Giraud-Billoud M, Carpio D, et al. Proregenerative Microenvironment Triggered by Donor Mesenchymal Stem Cells Preserves Renal Function and Structure in Mice with Severe Diabetes Mellitus.Biomed Res Int. 2015;2015:164703.[24]Hamza AH, Al-Bishri WM, Damiati LA, et al. Mesenchymal stem cells: a future experimental exploration for recession of diabetic nephropathy. Renal Failure. 2017;39(1):67-76.[25]Lenoir N. Europe confronts the embryonic stem cell research challenge. Science.2000;287(5457):1425-1427.[26]Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663-676.[27]Ben-David U, Benvenisty N.The tumorigenicity of human embryonic and induced pluripotent stem cells.Nat Rev Cancer. 2011;11(4):268-277.[28]Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies.Tissue Eng. 2001;7(2): 211-228.[29]Panés J, García-Olmo D, Van Assche G, et al.Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn's disease: a phase 3 randomised, double-blind controlled trial.Lancet. 2016;388(10051):1281-1290.[30]Henry TD, Pepine CJ, Lambert CR, et al. The Athena trials: Autologous adipose-derived regenerative cells for refractory chronic myocardial ischemia with left ventricular dysfunction.Catheter Cardiovasc Interv. 2017;89(2):169-177.[31]Merceron C, Portron S, Masson M, et al. TThe effect of two- and three-dimensional cell culture on the chondrogenic potential of human adipose-derived mesenchymal stem cells after subcutaneous transplantation with an injectable hydrogel.Cell Transplant.2011; 20(10):1575-1588.[32]Aksu AE, Rubin JP, Dudas JR, et al. Role of gender and anatomical region on induction of osteogenic differentiation of human adipose-derived stem cells. Ann Plast Surg. 2008;60(3):306-322.[33]Wosnitza M, Hemmrich K, Groger A, et al. Plasticity of human adipose stem cells to perform adipogenic and endothelial differentiation. Differentiation. 2007;75(1):12-23.[34]Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013; 15(6):641-648.[35]Zack-Williams SD, Butler PE, Kalaskar DM.Current progress in use of adipose derived stem cells in peripheral nerve regeneration.World J Stem Cells. 2015;7(1):51-64.[36]Baer PC, Geiger H. Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity.Stem Cells Int. 2012;2012:812693.[37]Lee RH, Kim B, Choi I, et al.Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue.Cell Physiol Biochem. 2004;14(4-6):311-324.[38]Sheykhhasan M, Qomi RT, Ghiasi M.Fibrin Scaffolds Designing in order to Human Adipose-derived Mesenchymal Stem Cells Differentiation to Chondrocytes in the Presence of TGF-β3.Int J Stem Cells. 2015;8(2):219-227.[39]Mundra V, Gerling IC, Mahato RI. Mesenchymal Stem Cell-Based Therapy. Molecular Pharmaceutics. 2012;10(1):77-89.[40]Valina C, Pinkernell K, Song YH, et al.Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007;28(21):2667-2677.[41]Zhang DZ, Gai LY, Liu HW, et al.Transplantation of autologous adipose-derived stem cells ameliorates cardiac function in rabbits with myocardial infarction.Chin Med J (Engl). 2007;120(4):300-307. |
| [1] | Zeng Zhen, Hu Jingwei, Li Xuan, Tang Linmei, Huang Zhiqiang, Li Mingxing. Quantitative analysis of renal blood flow perfusion using contrast-enhanced ultrasound in rats with hemorrhagic shock during resuscitation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1201-1206. |
| [2] | Sun Weixing, Zhao Yongchao, Zhao Ranzun. Mesenchymal stem cell transplantation in the treatment of myocardial infarction: problems, crux and new breakthrough [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 3103-3109. |
| [3] | Chen Xiao, Guo Zhi, Chen Lina, Liu Xuanyong, Zhang Yihuizhi, Li Xumian, Wang Yueqiao, Wei Liya, Xie Jing, Lin Li. Factors affecting the mobilization and collection of autologous peripheral blood hematopoietic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 2958-2962. |
| [4] | Qian Nannan, Zhang Qian, Yang Rui, Ao Jun, Zhang Tao. Mesenchymal stem cells in the treatment of spinal cord injury: cell therapy and combination of new drugs and biomaterials [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(13): 2114-2120. |
| [5] | Cao Linlin, Ding Kaiyang, Song Hao, Wu Guolin, Hu Maogui, Fan Dandan, Zhou Chenyang, Wang Cuicui, Feng Yuanyuan. Efficacy and influencing factors of autologous hematopoietic stem cell transplantation in the treatment of malignant lymphoma [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(13): 1993-1998. |
| [6] | Zhang Wenjian, Ma Lingfu, Wang Zhimin, Mo Wenjian, Zhou Ruiqing. Muscle mass evaluation and influencing factors of sarcopenia in allogeneic hematopoietic stem cell transplantation patients [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(13): 1999-2004. |
| [7] | Chen Ganghong, Zeng Chaoming, Chen Ziming, Liao Junxing, Ma Yuanchen, Zheng Qiujian . Intra-articular injection of optimal concentration of bone marrow mesenchymal stem cells for treating rabbit cartilage defects [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(7): 996-1001. |
| [8] | Wang Guoyu, Cheng Zhijian, Yang Baohui, Li Haopeng, He Xijing. Olfactory ensheathing cell transplantation promotes the ultrastructure repair at the lesion site of rat models of spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(5): 699-703. |
| [9] | Shang Qingqing, Zhou Jianye. Combination of hyaluronic acid hydrogel and bone marrow mesenchymal stem cells promotes cardiac function after myocardial infarction in rats (II) [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(34): 5559-5563. |
| [10] | Zhang Suping, Sun Ling, Wan Dingming, Cao Weijie, Li Li, Liu Changfeng, Liu Yufeng, Wang Dao, Guo Rong, Jiang Zhongxing, Xie Xinsheng. Effectiveness of unrelated peripheral blood stem cell transplantation in the treatment of severe aplastic anemia [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(31): 4994-5001. |
| [11] |
Hou Xiaolin, Liang Jun, Yang Cheng, Cui Meihua.
Co-transplantation of adipose mesenchymal stem cells and endothelial progenitor cells in ulcerative colitis mice [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(25): 3981-3987. |
| [12] | Yang Ying, Yan Nan, Tian Wei, Han Cao, Zhang Xiaoyan, Zheng Xin, Liu Shidan, Zhang Shuo, Wang Zhengdong. Transplantation of bone marrow mesenchymal stem cells for denervated muscular atrophy [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(25): 4000-4005. |
| [13] |
Feng Yuan, Han Zhiqi, Zhou Nuo.
Endothelial progenitor cells promote vasculogenesis and osteogenesis in the repair of bone defects [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(25): 4046-4053. |
| [14] | Liu Yuetong, Wang Qin, Yang Ye, Zhu Jun, Abulikemu•Tuerdi. Effects of 1,25(OH)2D3 on microRNA-130b and transforming growth factor beta 1 in renal tissue of rat models of diabetic nephropathy [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(2): 248-253. |
| [15] | Yu Mingchuan, Zhao Jinbing, Yin Ming, Yin Changchang. Potentials of pericytes in orthopedics [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(19): 3078-3083. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||