Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (21): 3329-3335.doi: 10.3969/j.issn.2095-4344.1761
Previous Articles Next Articles
MicroRNA-20 regulates directional differentiation of stem cells from human exfoliated deciduous teeth
Deng Xuan1, Yang Fang1, Tang Xiqing1, Zhao Yan2
- 1Department of Stomatology, the Second Hospital of University of South China, Hengyang 421001, Hunan Province, China; 2Department of Stomatology, People’s Hospital of Sanya, Sanya 572000, Hainan Province, China
-
Revised:2019-03-08Online:2019-07-28Published:2019-07-28 -
About author:Deng Xuan, Master, Attending physician, Department of Stomatology, the Second Hospital of University of South China, Hengyang 421001, Hunan Province, China -
Supported by:the Natural Science Foundation of Hainan Province, No. 814390 (to ZY)
CLC Number:
Cite this article
Deng Xuan, Yang Fang, Tang Xiqing, Zhao Yan. MicroRNA-20 regulates directional differentiation of stem cells from human exfoliated deciduous teeth[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(21): 3329-3335.
share this article
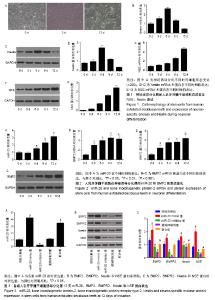
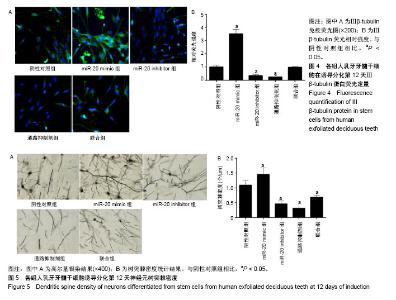
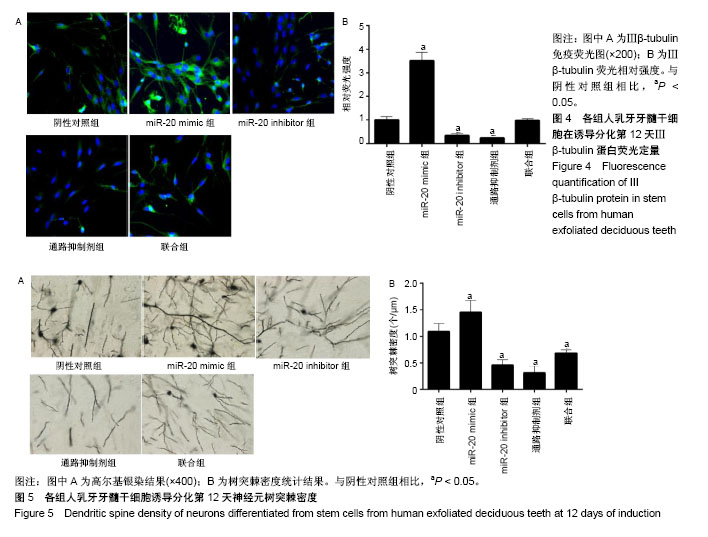
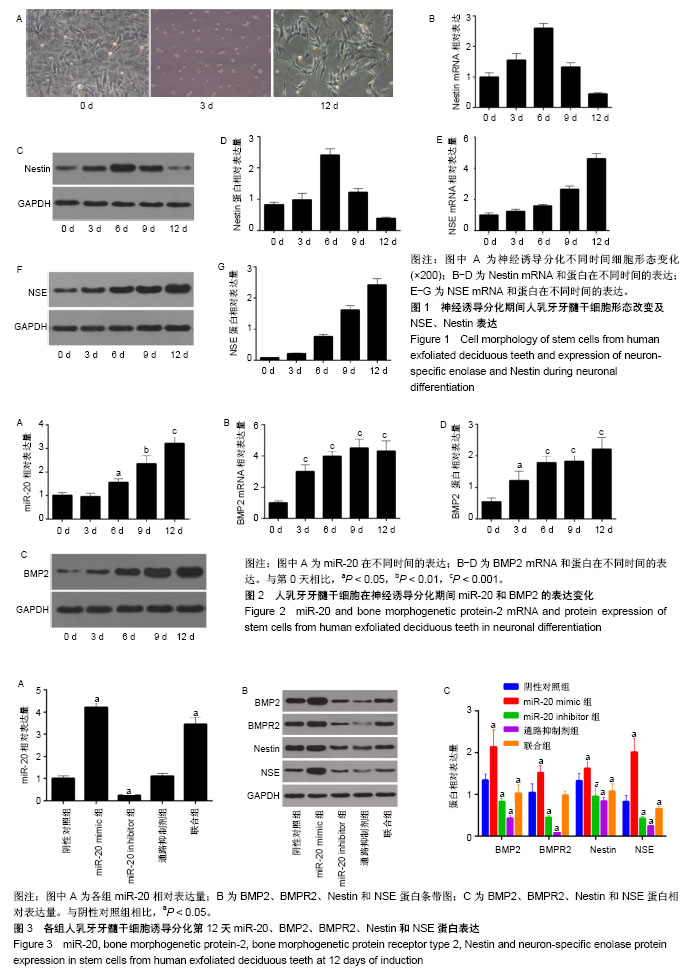
2.1 人乳牙牙髓干细胞向神经元诱导分化的形态变化及标志物表达 人乳牙牙髓干细胞体积较小、轮廓清晰,大多数为梭形或多角形,少部分为卵圆形,高表达Vinmentin、CD44和CD29。在神经分化诱导培养基中培养第3天,细胞胞体收缩,变为神经球样且成团生长。在神经分化诱导培养基中培养第12天,突起成网状且相互连接,核大而圆,位于细胞中央,为神经元样细胞,见图1A。Nestin表达在分化期间先升高后降低,在诱导第6天表达最高,见图1B-D。NSE蛋白及mRNA表达水平随着培养时间延长而上调,见图1E-G。 2.2 人乳牙牙髓干细胞中miR-20和BMP2的表达水平 在神经诱导分化第3天后miR-20表达逐步上调,BMP2 mRNA和蛋白表达随着培养时间延长逐渐上调且在培养第9天其表达趋于平缓,见图2。总体而言,二者表达呈时间依赖性上升。 2.3 miR-20通过骨形态发生蛋白信号通路诱导人乳牙牙髓干细胞分化为神经元 Nestin和NSE分别为神经干细胞和神经元的特异标志物。在诱导分化第12天,Real-time PCR检测显示:与阴性对照组相比,miR-20 mimic组和联合组miR-20表达显著上调,miR-20 inhibitor组miR-20表达显著下调,通路抑制剂组miR-20表达无明显变化。Western blot检测结果发现:与阴性对照组相比,miR-20 mimic组BMP2、BMPR2、Nestin和NSE蛋白表达显著上调,miR-20 inhibitor组和通路抑制剂组BMP2、BMPR2、Nestin和NSE蛋白表达显著下调。此外,联合组对上述因子的诱导效果能被BMP通路抑制剂抵消,见图3。 2.4 免疫荧光检测各组细胞的神经元早期标志物Ⅲβ-tubulin表达 在诱导分化第12天,Ⅲβ-tubulin表达几乎贯穿整个神经元。与阴性对照组相比,miR-20 mimic组Ⅲβ-tubulin表达显著上调,但miR-20 inhibitor组和通路抑制剂组Ⅲβ-tubulin蛋白水平明显下调。联合组骨形态发生蛋白通路抑制剂能中和miR-20对Ⅲβ-tubulin生成的诱导,见图4。 2.5 各组高尔基染色结果 为评定神经元发育及神经传递功能,通过高尔基染色观察各组人乳牙牙髓干细胞诱导的神经元树突棘密度。与阴性对照组相比,miR-20 mimic组单位面积树突棘数目显著增加,但miR-20 inhibitor组和通路抑制剂组树突棘稀疏。联合组骨形态发生蛋白通路抑制剂能中和miR-20对于神经元树突棘的诱导,见图5。"
| [1] Shimojima C, Takeuchi H, Jin S, et al. Conditioned Medium from the Stem Cells of Human Exfoliated Deciduous Teeth Ameliorates Experimental Autoimmune Encephalomyelitis. J Immunol. 2016; 196(10): 4164-4171.[2] 项海东,李浩渤,陈惠珍. 脂多糖调控Notch信号通路作用于人牙髓干细胞增殖、分化、凋亡的实验研究[J]. 中国免疫学杂志, 2017, 33(10): 1483-1486. [3] Miura M, Gronthos S, Zhao M, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100(10): 5807-5812.[4] Majumdar D, Kanafi M, Bhonde R, et al. Differential Neuronal Plasticity of Dental Pulp Stem Cells From Exfoliated Deciduous and Permanent Teeth Towards Dopaminergic Neurons. J Cell Physiol. 2016;231(9):2048-2063.[5] Su WT, Wu PS, Huang TY. Osteogenic differentiation of stem cells from human exfoliated deciduous teeth on poly(ε-caprolactone) nanofibers containing strontium phosphate. Mater Sci Eng C Mater Biol Appl. 2015;46:427-434.[6] Nicola FDC, Marques MR, Odorcyk F, et al. Neuroprotector effect of stem cells from human exfoliated deciduous teeth transplanted after traumatic spinal cord injury involves inhibition of early neuronal apoptosis. Brain Res. 2017;1663:95-105.[7] 郑琼丹,邢泉,廖维立,等. Notch信号通路参与BMP-6对人牙髓细胞增殖分化作用的研究[J]. 中华老年口腔医学杂志, 2015,13(2):65-69.[8] Zhang F, Song J, Zhang H, et al. Wnt and BMP Signaling Crosstalk in Regulating Dental Stem Cells: Implications in Dental Tissue Engineering. Genes Dis. 2016;3(4):263-276.[9] Hegarty SV, Sullivan AM, O'Keeffe GW. BMP2 and GDF5 induce neuronal differentiation through a Smad dependant pathway in a model of human midbrain dopaminergic neurons. Mol Cell Neurosci. 2013;56: 263-271. [10] 白广超,金宏亮,雷堃,等. BMP-7诱导大鼠骨髓间充质干细胞向神经元细胞分化的初步研究[J].安徽医科大学学报, 2018,53(2):190-195.[11] 赵红斌,甄英丽,田蓉,等. Notch和BMP信号通路介导红景天苷促进小鼠骨髓间充质干细胞向神经元细胞定向分化研究[J]. 中草药, 2016, 47(13):2294-2300.[12] Sun DG, Xin BC, Wu D, et al. miR-140-5p-mediated regulation of the proliferation and differentiation of human dental pulp stem cells occurs through the lipopolysaccharide/toll-like receptor 4 signaling pathway. Eur J Oral Sci. 2017;125(6):419-425.[13] Zhang JF, Fu WM, He ML, et al. MiRNA-20a promotes osteogenic differentiation of human mesenchymal stem cells by co-regulating BMP signaling. RNA Biol. 2011;8(5):829-838.[14] 杨倩娟,刘文佳,常文悦,等. miR-20a对人炎症牙周膜干细胞成骨分化能力的影响[J]. 实用口腔医学杂志, 2016, 32(2):186-189.[15] 杨鑫,李思洁,赵玮. Wnt信号通路在调控牙髓干细胞多向分化及炎症损伤修复中的作用[J]. 国际口腔医学杂志, 2018, 45(3):286-290.[16] 马子洋,郭晓霞. 牙髓干细胞在再生医学中的应用研究与进展[J]. 中国组织工程研究, 2016, 20(19):2872-2878.[17] Bagher Z, Azami M, Ebrahimi-Barough S, et al. Differentiation of Wharton's Jelly-Derived Mesenchymal Stem Cells into Motor Neuron-Like Cells on Three-Dimensional Collagen-Grafted Nanofibers. Mol Neurobiol. 2016;53(4):2397-2408.[18] Song M, Jue SS, Cho YA, et al. Comparison of the effects of human dental pulp stem cells and human bone marrow-derived mesenchymal stem cells on ischemic human astrocytes in vitro. J Neurosci Res. 2015; 93(6):973-983.[19] 张凤兰,杨璐军,胡炜彦,等.人参皂苷Rg3对体外培养小鼠神经干细胞分化的影响[J]. 中国组织工程研究, 2018, 22(13):2098-2103.[20] 康湘萍,陈超,梁超,等.大鼠骨髓间充质干细胞培养、鉴定及神经样细胞分化[J]. 中国老年学杂志, 2018, 38(1):10-14.[21] Martin EC, Qureshi AT, Dasa V, et al. MicroRNA regulation of stem cell differentiation and diseases of the bone and adipose tissue: Perspectives on miRNA biogenesis and cellular transcriptome. Biochimie. 2016;124: 98-111.[22] 张浩,康焱,马元琛,等.脂肪源性干细胞成骨分化过程中成骨相关miRNA的表达模式[J]. 中国组织工程研究, 2011,15(23):4247-4250.[23] Chen JF, Yang YM, Dong XX, et al. MicroRNA-20a Promotes Osteogenic Differentiation of C3H/10T1/2 Cells through Regulating CKIP-1 Expression. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2017; 25(1):214-220.[24] Kunimatsu R, Nakajima K, Awada T, et al. Comparative characterization of stem cells from human exfoliated deciduous teeth, dental pulp, and bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2018;501(1):193-198.[25] Jovanovic VM, Salti A, Tilleman H, et al. BMP/SMAD Pathway Promotes Neurogenesis of Midbrain Dopaminergic Neurons In Vivo and in Human Induced Pluripotent and Neural Stem Cells. J Neurosci. 2018;38(7): 1662-1676.[26] Jin J, Tilve S, Huang Z, et al.Effect of chondroitin sulfate proteoglycans on neuronal cell adhesion, spreading and neurite growth in culture.Neural Regen Res. 2018;13(2):289-297. [27] Xu L, Long J, Shi C, et al. Effect of leukocyte inhibitory factor on neuron differentiation from human induced pluripotent stem cell-derived neural precursor cells. Int J Mol Med. 2018;41(4): 2037-2049. |
| [1] | Jiang Tao, Ma Lei, Li Zhiqiang, Shou Xi, Duan Mingjun, Wu Shuo, Ma Chuang, Wei Qin. Platelet-derived growth factor BB induces bone marrow mesenchymal stem cells to differentiate into vascular endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3937-3942. |
| [2] | Chen Yang, Huang Denggao, Gao Yuanhui, Wang Shunlan, Cao Hui, Zheng Linlin, He Haowei, Luo Siqin, Xiao Jingchuan, Zhang Yingai, Zhang Shufang. Low-intensity pulsed ultrasound promotes the proliferation and adhesion of human adipose-derived mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3949-3955. |
| [3] | Zhang Lishu, Liu Anqi, He Xiaoning, Jin Yan, Li Bei, Jin Fang. Alpl gene affects the therapeutic effect of bone marrow mesenchymal stem cells on ulcerative colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3970-3975. |
| [4] | Ruan Guangping, Yao Xiang, Liu-Gao Miyang, Cai Xuemin, Li Zian, Pang Rongqing, Wang Jinxiang, Pan Xinghua. Umbilical cord mesenchymal stem cell transplantation for traumatic systemic inflammatory response syndrome in tree shrews [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3994-4000. |
| [5] | Chen Lei, Zheng Rui, Jie Yongsheng, Qi Hui, Sun Lei, Shu Xiong. In vitro evaluation of adipose-derived stromal vascular fraction combined with osteochondral integrated scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3487-3492. |
| [6] | Wei Qin, Zhang Xue, Ma Lei, Li Zhiqiang, Shou Xi, Duan Mingjun, Wu Shuo, Jia Qiyu, Ma Chuang. Platelet-derived growth factor-BB induces the differentiation of rat bone marrow mesenchymal stem cells into osteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 2953-2957. |
| [7] | Guo Zhibin, Wu Chunfang, Liu Zihong, Zhang Yuying, Chi Bojing, Wang Bao, Ma Chao, Zhang Guobin, Tian Faming. Simvastatin stimulates osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 2963-2968. |
| [8] | Li Congcong, Yao Nan, Huang Dane, Song Min, Peng Sha, Li Anan, Lu Chao, Liu Wengang. Identification and chondrogenic differentiation of human infrapatellar fat pad derived stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 2976-2981. |
| [9] | Gao Yuanhui, Xiang Yang, Cao Hui, Wang Shunlan, Zheng Linlin, He Haowei, Zhang Yingai, Zhang Shufang, Huang Denggao. Comparison of biological characteristics of adipose derived mesenchymal stem cells in Wuzhishan inbreed miniature pigs aged two different months [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 2988-2993. |
| [10] | Cao Yang, Zhang Junping, Peng Li, Ding Yi, Li Guanghui. Isolation and culture of rabbit aortic endothelial cells and biological characteristics [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 3000-3003. |
| [11] | Dai Min, Wang Shuai, Zhang Nini, Huang Guilin, Yu Limei, Hu Xiaohua, Yi Jie, Yao Li, Zhang Ligang. Biological characteristics of hypoxic preconditioned human amniotic mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 3004-3008. |
| [12] | Qin Yanchun, Rong Zhen, Jiang Ruiyuan, Fu Bin, Hong Xiaohua, Mo Chunmei. Chinese medicine compound preparation inhibits proliferation of CD133+ liver cancer stem cells and the expression of stemness transcription factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 3016-3023. |
| [13] | Dai Yaling, Chen Lewen, He Xiaojun, Lin Huawei, Jia Weiwei, Chen Lidian, Tao Jing, Liu Weilin. Construction of miR-146b overexpression lentiviral vector and the effect on the proliferation of hippocampal neural stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 3024-3030. |
| [14] | Chen Jie, Liao Chengcheng, Chen Zhiwei, Wang Yan. Bladder cancer stem cell markers and related signaling pathways: antibody targeted therapy [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 3090-3096. |
| [15] | Zhang Wei, Cui Shuaishuai, Zhou Zhichao, Hu Xiaohua, Yang Xiaohong. MicroRNA regulates histone deacetylase in the treatment of bone-related diseases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(17): 2767-2774. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||