Chinese Journal of Tissue Engineering Research ›› 2018, Vol. 22 ›› Issue (33): 5309-5314.doi: 10.3969/j.issn.2095-4344.0665
Previous Articles Next Articles
Bone defect repair with bone morphogenetic protein 14 transfected adipose-derived stem cells seeded onto a silk fibroin/hydroxyapatite scaffold
Ding Tao1, Tang Feng-lin1, Lu Miao1, Gu San-jun2, Wang Jian-bing2, Liu Hao2
- 1Department of Orthopedics, Wuxi People’s Hospital, Wuxi 214000, Jiangsu Province, China; 2Department of Orthopedics, Wuxi 9th People’s Hospital, Wuxi 214000, Jiangsu Province, China
-
Revised:2018-07-22Online:2018-11-28Published:2018-11-28 -
Contact:Liu Hao, Master candidate, Physician, Department of Orthopedics, Wuxi 9th People’s Hospital, Wuxi 214000, Jiangsu Province, China -
About author:Ding Tao, MD, Associate chief physician, Department of Orthopedics, Wuxi People’s Hospital, Wuxi 214000, Jiangsu Province, China -
Supported by:the Scientific Development Foundation of Nanjing Medical University, No. 2016NJMUZD069
CLC Number:
Cite this article
Ding Tao, Tang Feng-lin, Lu Miao, Gu San-jun, Wang Jian-bing, Liu Hao. Bone defect repair with bone morphogenetic protein 14 transfected adipose-derived stem cells seeded onto a silk fibroin/hydroxyapatite scaffold[J]. Chinese Journal of Tissue Engineering Research, 2018, 22(33): 5309-5314.
share this article
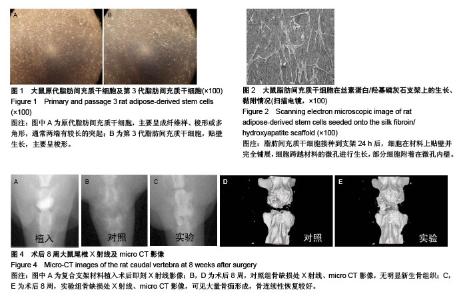
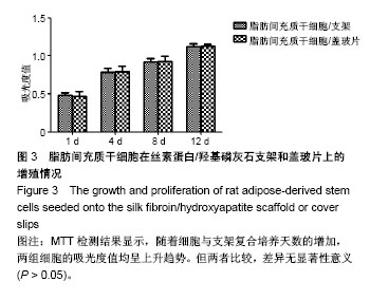
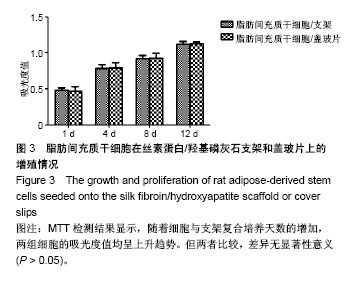
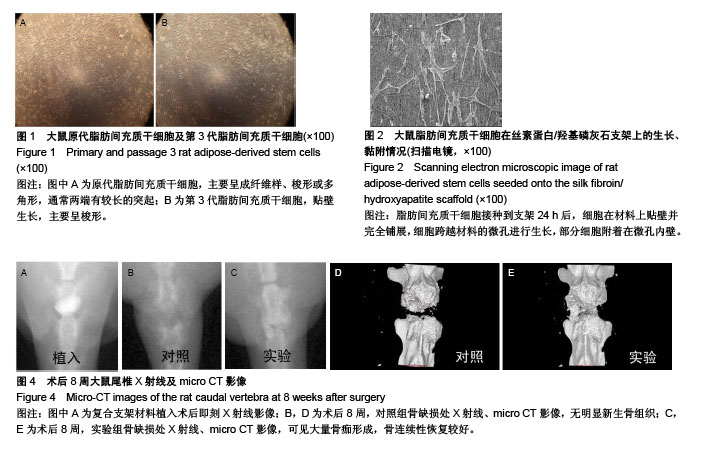
2.1 大鼠ADSCs的生长情况及鉴定结果 接种培养48 h后,细胞大部分已完全伸展,主要呈成纤维样、梭形或多角形,通常两端有较长的突起。培养9 d后,细胞汇合成单层,融合近90%。传代细胞呈克隆样生长,培养2 h即可贴壁,以类成纤维样细胞为主。随传代次数增多,逐渐以梭形细胞为主,见图1。流式细胞仪检测细胞表面抗原:CD44、CD90、CD105呈高表达,而CD34、CD68呈低表达。 2.2 转染效率及基因表达 转染后48 h,倒置相差显微镜观察细胞生长状态良好,形态未发生明显变化。荧光显微镜下可见细胞内有绿色荧光,根据EGFP表达率统计,转染效率为(73.1±3.1)%。RT-PCR检测结果显示,转染后的ADSCs在1 488 bp处明显有BMP14 mRNA特异性条带,而空白转染的ADSCs未检测到BMP14表达。 2.3 扫描电镜观察大鼠ADSCs在SF/HA支架上的生长、黏附情况 细胞接种到支架24 h后,可见细胞在材料上贴壁并完全铺展,细胞跨越材料的微孔进行生长,部分细胞附着在微孔内壁。细胞与支架复合72 h后,细胞数量逐渐增多,贴附于材料表面及孔内,细胞伸出的伪足紧紧贴附于材料表面,见图2。 2.4 MTT法检测大鼠ADSCs在SF/HA支架上的增殖情况 随着细胞、支架复合培养天数的增加,两组细胞的吸光度值均呈上升趋势,但两组吸光度值比较,差异无显著性意义(P > 0.05),见图3。该结果表明,BMP14诱导的ADSCs在SF/HA支架上生长增殖良好,支架材料对细胞具有良好的生物相容性。"
| [1] Giannoudis PV, Gudipati S, Harwood P, et al. Long bone non-unions treated with the diamond concept: a case series of 64 patients. Injury. 2015;46 Suppl 8:S48-54. [2] Dumic-Cule I, Pecina M, Jelic M, et al. Biological aspects of segmental bone defects management. Int Orthop. 2015; 39(5):1005-1011. [3] Carreira AC, Zambuzzi WF, Rossi MC, et al. Bone Morphogenetic Proteins: Promising Molecules for Bone Healing, Bioengineering, and Regenerative Medicine. Vitam Horm. 2015;99:293-322. [4] Curtin CM, Tierney EG, McSorley K, et al. Combinatorial gene therapy accelerates bone regeneration: non-viral dual delivery of VEGF and BMP2 in a collagen-nanohydroxyapatite scaffold. Adv Healthc Mater. 2015;4(2):223-227. [5] Liu F, Ferreira E, Porter RM, et al. Rapid and reliable healing of critical size bone defects with genetically modified sheep muscle. Eur Cell Mater. 2015;30:118-130. [6] Helary C, Desimone MF. Recent advances in biomaterials for tissue engineering and controlled drug delivery. Curr Pharm Biotechnol. 2015;16(7):635-645. [7] Beck-Broichsitter BE, Werk AN, Smeets R, et al. Targeting gene expression during the early bone healing period in the mandible: A base for bone tissue engineering. J Craniomaxillofac Surg. 2015;43(8):1452-1460. [8] Guan J, Zhang J, Li H, et al. Human Urine Derived Stem Cells in Combination with β-TCP Can Be Applied for Bone Regeneration. PLoS One. 2015;10(5):e0125253. [9] Wan W, Zhang S, Ge L, et al. Layer-by-layer paper-stacking nanofibrous membranes to deliver adipose-derived stem cells for bone regeneration. Int J Nanomedicine. 2015;10: 1273-1290. [10] Bolander J, Ji W, Geris L, et al. The combined mechanism of bone morphogenetic protein- and calcium phosphate-induced skeletal tissue formation by human periosteum derived cells. Eur Cell Mater. 2016;31:11-25. [11] Bolander J, Ji W, Leijten J, et al. Healing of a Large Long-Bone Defect through Serum-Free In Vitro Priming of Human Periosteum-Derived Cells. Stem Cell Reports. 2017; 8(3):758-772. [12] Li CJ, Madhu V, Balian G, et al. Cross-Talk Between VEGF and BMP-6 Pathways Accelerates Osteogenic Differentiation of Human Adipose-Derived Stem Cells. J Cell Physiol. 2015; 230(11):2671-2682. [13] Wang L, Zhang YG, Wang XM, et al. Naringin protects human adipose-derived mesenchymal stem cells against hydrogen peroxide-induced inhibition of osteogenic differentiation. Chem Biol Interact. 2015;242:255-261. [14] Abagnale G, Steger M, Nguyen VH, et al. Surface topography enhances differentiation of mesenchymal stem cells towards osteogenic and adipogenic lineages. Biomaterials. 2015;61: 316-326. [15] Di Benedetto A, Brunetti G, Posa F, et al. Osteogenic differentiation of mesenchymal stem cells from dental bud: Role of integrins and cadherins. Stem Cell Res. 2015;15(3): 618-628. [16] Wöltje M, Böbel M, Heiland M, et al. Purmorphamine and oxysterols accelerate and promote osteogenic differentiation of mesenchymal stem cells in vitro. In Vivo. 2015;29(2): 247-254. [17] Chung JE, Park JH, Yun JW, et al. Cultured Human Periosteum-Derived Cells Can Differentiate into Osteoblasts in a Perioxisome Proliferator-Activated Receptor Gamma-Mediated Fashion via Bone Morphogenetic Protein signaling. Int J Med Sci. 2016;13(11):806-818. [18] Kim YK, Nakata H, Yamamoto M, et al. Osteogenic Potential of Mouse Periosteum-Derived Cells Sorted for CD90 In Vitro and In Vivo. Stem Cells Transl Med. 2016;5(2):227-234. [19] Sung IY, Park BC, Hah YS, et al. FOXO1 Is Involved in the Effects of Cigarette Smoke Extract on Osteoblastic Differentiation of Cultured Human Periosteum-derived Cells. Int J Med Sci. 2015;12(11):881-890. [20] Bobinac D, Spanjol J, Marinovi? M, et al. Expression of bone morphogenetic proteins, cartilage-derived morphogenetic proteins and related receptors in normal and osteoarthritic human articular cartilage. Coll Antropol. 2008;32 Suppl 2: 83-87. [21] Jin J, Wang J, Huang J, et al. Transplantation of human placenta-derived mesenchymal stem cells in a silk fibroin/hydroxyapatite scaffold improves bone repair in rabbits. J Biosci Bioeng. 2014;118(5):593-598. [22] Sinlapabodin S, Amornsudthiwat P, Damrongsakkul S, et al. An axial distribution of seeding, proliferation, and osteogenic differentiation of MC3T3-E1 cells and rat bone marrow-derived mesenchymal stem cells across a 3D Thai silk fibroin/gelatin/hydroxyapatite scaffold in a perfusion bioreactor. Mater Sci Eng C Mater Biol Appl. 2016;58: 960-970. [23] Lane JM, Sandhu HS. Current approaches to experimental bone grafting. Orthop Clin North Am. 1987;18(2):213-225. [24] Dos Santos Pereira R, Boos FB, Gorla LF, et al. Maxillary Sinus Elevation Surgery with ChronOS and Autogenous Bone Graft: Analysis of Histometric and Volumetric Changes. Int J Periodontics Restorative Dent. 2016;36(6):885-892. [25] Lareau CR, Deren ME, Fantry A, et al. Does autogenous bone graft work? A logistic regression analysis of data from 159 papers in the foot and ankle literature. Foot Ankle Surg. 2015;21(3):150-159. [26] Nauth A, Lane J, Watson JT, et al. Bone Graft Substitution and Augmentation. J Orthop Trauma. 2015;29 Suppl 12: S34-38. [27] Durst A, Clibbon J, Davis B. Distal tibial fractures are a poorly recognised complication with fibula free flaps. Ann R Coll Surg Engl. 2015;97(6):409-413. [28] Lonie S, Herle P, Paddle A, et al. Mandibular reconstruction: meta-analysis of iliac- versus fibula-free flaps. ANZ J Surg. 2016;86(5):337-342. [29] Van Genechten ML, Batstone MD. The relative survival of composite free flaps in head and neck reconstruction. Int J Oral Maxillofac Surg. 2016;45(2):163-166. [30] Micev AJ, Kalainov DM, Soneru AP. Masquelet technique for treatment of segmental bone loss in the upper extremity. J Hand Surg Am. 2015;40(3):593-598. [31] Morelli I, Drago L, George DA, et al. Masquelet technique: myth or reality? A systematic review and meta-analysis. Injury. 2016;47 Suppl 6:S68-S76. [32] Tarchala M, Harvey EJ, Barralet J. Biomaterial-Stabilized Soft Tissue Healing for Healing of Critical-Sized Bone Defects: the Masquelet Technique. Adv Healthc Mater. 2016;5(6):630-640. [33] Guo ZM, ShangGuan TC, Zhang M, et al. Prevention and treatment of the related complications of tibial fractures bone defect by bone transport. Zhongguo Gu Shang. 2016;29(8): 756-760. [34] Tsitskaris K, Havard H, Bijlsma P, et al. Internal bone transport using a cannulated screw as a mounting device in the treatment of a post-infective ulnar defect. Strategies Trauma Limb Reconstr. 2016;11(1):63-67. [35] Chen L, Li B, Xiao X, et al. Preparation and evaluation of an Arg-Gly-Asp-modified chitosan/hydroxyapatite scaffold for application in bone tissue engineering. Mol Med Rep. 2015; 12(5):7263-7270. [36] Nguyen DT, Burg KJ. Bone tissue engineering and regenerative medicine: targeting pathological fractures. J Biomed Mater Res A. 2015;103(1):420-429. [37] De Francesco F, Ricci G, D'Andrea F, et al. Human Adipose Stem Cells: From Bench to Bedside. Tissue Eng Part B Rev. 2015;21(6):572-584. [38] Romagnoli C, Zonefrati R, Galli G, et al. In Vitro Behavior of Human Adipose Tissue-Derived Stem Cells on Poly(ε-caprolactone) Film for Bone Tissue Engineering Applications. Biomed Res Int. 2015;2015:323571. [39] Zanetti AS, McCandless GT, Chan JY, et al. Characterization of novel akermanite: poly-?-caprolactone scaffolds for human adipose-derived stem cells bone tissue engineering. J Tissue Eng Regen Med. 2015;9(4):389-404. [40] Yin X, Xu H, Jiang Y, et al. The effect of lentivirus-mediated PSPN genetic engineering bone marrow mesenchymal stem cells on Parkinson's disease rat model. PLoS One. 2014;9(8): e105118. [41] Liu X, Joshi S, Ravishankar B, et al. Bone morphogenetic protein signaling in rotator cuff muscle atrophy and fatty infiltration. Muscles Ligaments Tendons J. 2015;5(2):113-119. [42] Yin Y, Wang Y. Association of BMP-14 rs143383 ploymorphism with its susceptibility to osteoarthritis: A meta-analysis and systematic review according to PRISMA guideline. Medicine (Baltimore). 2017;96(42):e7447. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [3] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [4] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| [5] | Wang Shiqi, Zhang Jinsheng. Effects of Chinese medicine on proliferation, differentiation and aging of bone marrow mesenchymal stem cells regulating ischemia-hypoxia microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1129-1134. |
| [6] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [7] | Kong Desheng, He Jingjing, Feng Baofeng, Guo Ruiyun, Asiamah Ernest Amponsah, Lü Fei, Zhang Shuhan, Zhang Xiaolin, Ma Jun, Cui Huixian. Efficacy of mesenchymal stem cells in the spinal cord injury of large animal models: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1142-1148. |
| [8] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [9] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [10] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [11] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [12] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [13] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [14] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [15] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||