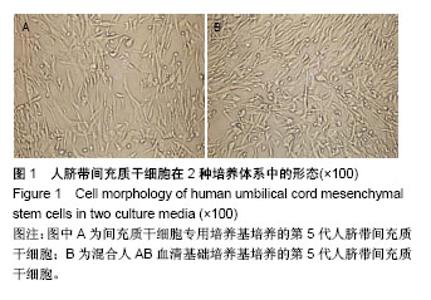
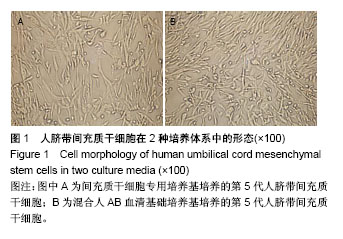
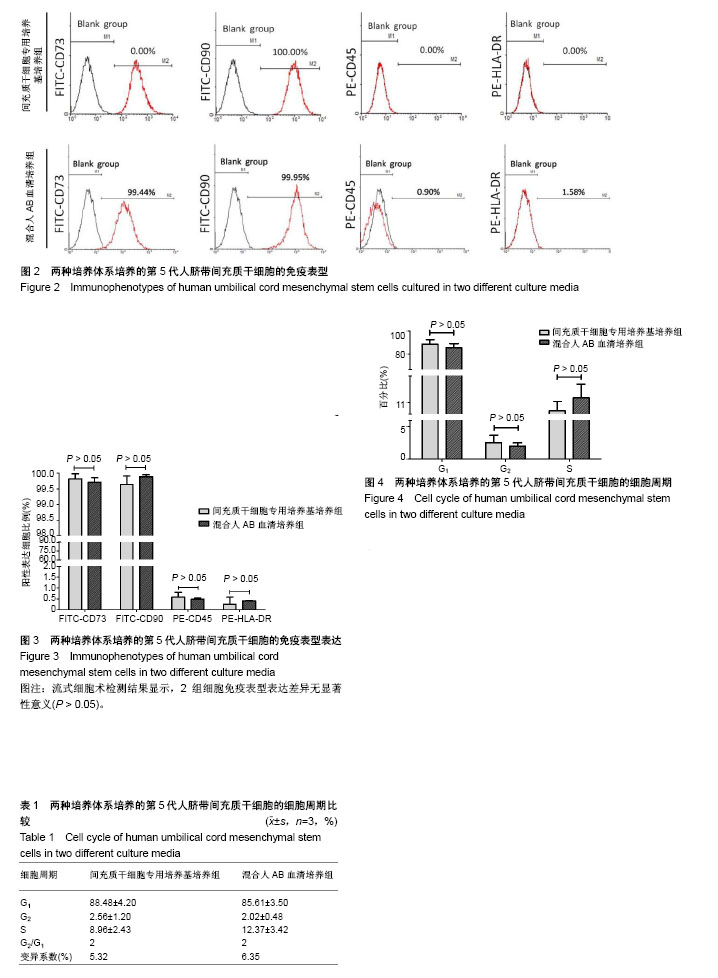
| [1] 刘慧文.人骨髓和脐带来源间充质干细胞体外分离培养及生物学特性比较[J].浙江临床医学,2015,17(11):1872-1873.[2] 韩华,李洁,薛改,等.人脐带华通胶间充质干细胞的分离培养及标记方法研究[J].医疗卫生装备,2016,37(12):28-31.[3] 赵琳,刘广芝,张荷,等.人脐带间充质干细胞分离和初步鉴定[J].中华实用诊断与治疗杂志,2016,30(6):549-551.[4] 任春红,张昕.无动物源成分培养基用于人脐带间充质干细胞培养的研究[J].延安大学学报(医学科学版),2015,13(1):1-4.[5] Banas A. Purification of adipose tissue mesenchymal stem cells and differentiation toward hepatic-like cells. Methods Mol Biol. 2012;826: 61-72.[6] Li CS, Zheng Z, Su XX, et al. Activation of the Extracellular Signal- Regulated Kinase Signaling Is Critical for Human Umbilical Cord Mesenchymal Stem Cell Osteogenic Differentiation. Biomed Res Int. 2016;2016:3764372.[7] Ruan Z, Zhu L, Yin Y, et al. Overexpressing NKx2.5 increases the differentiation of human umbilical cord drived mesenchymal stem cells into cardiomyocyte-like cells. Biomed Pharmacother. 2016;78:110-115.[8] 何洁,赵晶,王金祥,等.多次贴壁法高效制备人脐带间充质干细胞[J].西南国防医药,2015,25(8):828-831.[9] 陈津,郭子宽,王立生,等.大规模间充质干细胞培养技术评估报告[J].中国医药生物技术,2013,8(4):274-283. [10] Hsiao ST, Asgari A, Lokmic Z, et al. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev. 2012; 21(12):2189-2203.[11] Rojewski MT, Fekete N, Baila S, et al. GMP-compliant isolation and expansion of bone marrow-derived MSCs in the closed, automated device quantum cell expansion system. Cell Transplant. 2013;22(11): 1981-2000.[12] Xu Y, Hong Y, Xu M, et al. Role of Keratinocyte Growth Factor in the Differentiation of Sweat Gland-Like Cells From Human Umbilical Cord-Derived Mesenchymal Stem Cells. Stem Cells Transl Med. 2016;5(1):106-116.[13] Yan Y, Zhu Y, Sun F, et al. Extracellular regulated protein kinases 1/2 phosphorylation is required for hepatic differentiation of human umbilical cord-derived mesenchymal stem cells. Exp Biol Med (Maywood). 2015;240(4):534-545.[14] 胡建立,李静,陈斌,等.成人AB型血清取代胎牛血清在体外有效扩增骨髓间充质干细胞[J].基础医学与临床, 2010,30(6):576-581.[15] Jung S, Panchalingam KM, Rosenberg L, et al. Ex vivo expansion of human mesenchymal stem cells in defined serum-free media. Stem Cells Int. 2012;2012:123030.[16] Hsiao ST, Asgari A, Lokmic Z, et al. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev. 2012; 21(12):2189-2203.[17] 李嘉.人脐带间充质干细胞在临床治疗中的研究进展[J].医学综述, 2016, 22(10):1931-1934.[18] 赵晶,何洁,王金祥,等.人脐带间充质干细胞体外规模化、标准化制备技术研究[J].西南国防医药,2016,26(12):1395-1397.[19] 施沁,杨晓清,张玉泉.人脐带华通氏胶间充质干细胞定向分化:现状与前景[J].中国组织工程研究,2018,22(5):793-800.[20] Hematti P. Human embryonic stem cell-derived mesenchymal progenitors: an overview. Methods Mol Biol. 2011;690:163-174.[21] 戴如飞,叶英,曾因明,等.不同来源血清体外培养骨髓间充质干细胞的研究[J].国际麻醉学与复苏杂志,2009,30(5):390-398.[22] Insausti CL, Blanquer MB, Olmo LM, et al. Isolation and characterization of mesenchymal stem cells from the fat layer on the density gradient separated bone marrow. Stem Cells Dev. 2012;21(2): 260-272.[23] Bagher Z, Azami M, Ebrahimi-Barough S, et al. Differentiation of Wharton's Jelly-Derived Mesenchymal Stem Cells into Motor Neuron-Like Cells on Three-Dimensional Collagen-Grafted Nanofibers. Mol Neurobiol. 2016;53(4):2397-2408.[24] 屈玟,宋磊,赵瑶,等.人脐带间充质干细胞不同培养方案的比较与优化[J].海南医学院学报,2017,23(8):1009-1013.[25] Xue L, Li Y, Chen J. Duration of simulated microgravity affects the differentiation of mesenchymal stem cells. Mol Med Rep. 2017;15(5): 3011-3018.[26] Yan Y, Jiang W, Tan Y, et al. hucMSC Exosome-Derived GPX1 Is Required for the Recovery of Hepatic Oxidant Injury. Mol Ther. 2017; 25(2):465-479.[27] Fayyad-Kazan M, Najar M, Fayyad-Kazan H, et al. Identification and Evaluation of New Immunoregulatory Genes in Mesenchymal Stromal Cells of Different Origins: Comparison of Normal and Inflammatory Conditions. Med Sci Monit Basic Res. 2017;23:87-96.[28] Kim N, Cho SG. Overcoming immunoregulatory plasticity of mesenchymal stem cells for accelerated clinical applications. Int J Hematol. 2016;103(2):129-137.[29] Ma XW, Cui DP, Zhao DW. Vascular endothelial growth factor/bone morphogenetic protein-2 bone marrow combined modification of the mesenchymal stem cells to repair the avascular necrosis of the femoral head. Int J Clin Exp Med. 2015;8(9):15528-15534.[30] 于丽华,赵书平,程薇莉,等.AB血清对人胚脑神经干细胞体外培养的作用[J].实用医药杂志,2005,22(2):137-138. |