Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (18): 4638-4648.doi: 10.12307/2026.753
Previous Articles Next Articles
Moderate-intensity exercise improves renal injury and inflammatory response in mice with hyperuricemia
Yang Ling, Dai Jiahui, Zhou Han, Yang Lin, Bian Bogao, Liu Gang
- School of Sports and Health, Guangzhou University of Chinese Medicine, Guangzhou 510006, Guangdong Province, China
-
Received:2025-06-06Accepted:2025-10-15Online:2026-06-28Published:2025-12-04 -
Contact:Liu Gang, MS, Associate professor, School of Sports and Health, Guangzhou University of Chinese Medicine, Guangzhou 510006, Guangdong Province, China -
About author:Yang Ling, MS, School of Sports and Health, Guangzhou University of Chinese Medicine, Guangzhou 510006, Guangdong Province, China -
Supported by:the 2024-2025 Guangdong Sports Science Research Program for Technological Advancement and Cultural Development in Sports, No. GDSS2024N062 (to LG); the Guangdong Undergraduate Teaching Quality and Reform Project, No. 561 (to LG)
CLC Number:
Cite this article
Yang Ling, Dai Jiahui, Zhou Han, Yang Lin, Bian Bogao, Liu Gang. Moderate-intensity exercise improves renal injury and inflammatory response in mice with hyperuricemia[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(18): 4638-4648.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
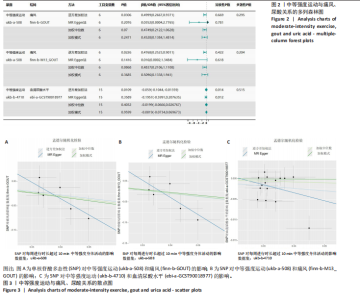
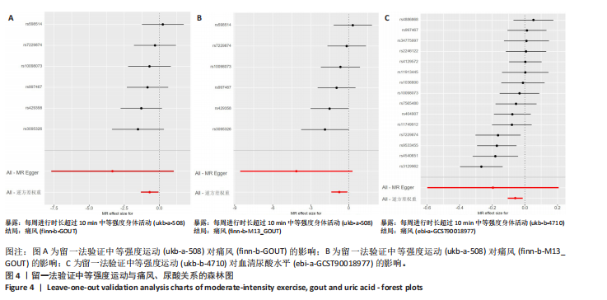
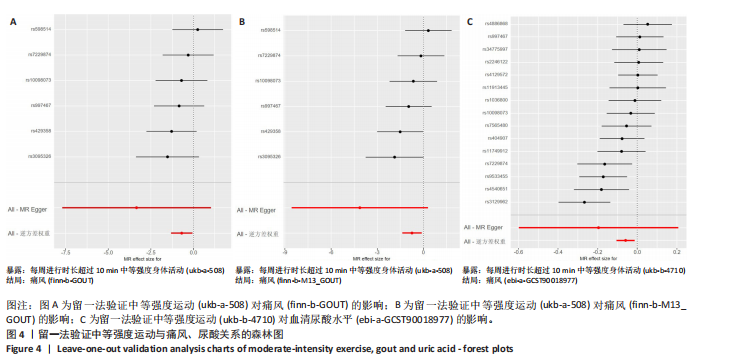
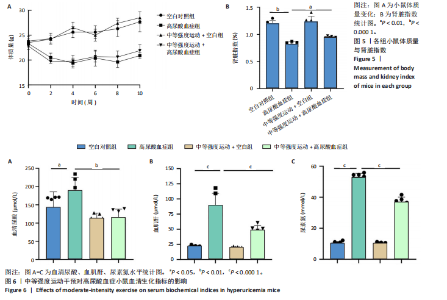
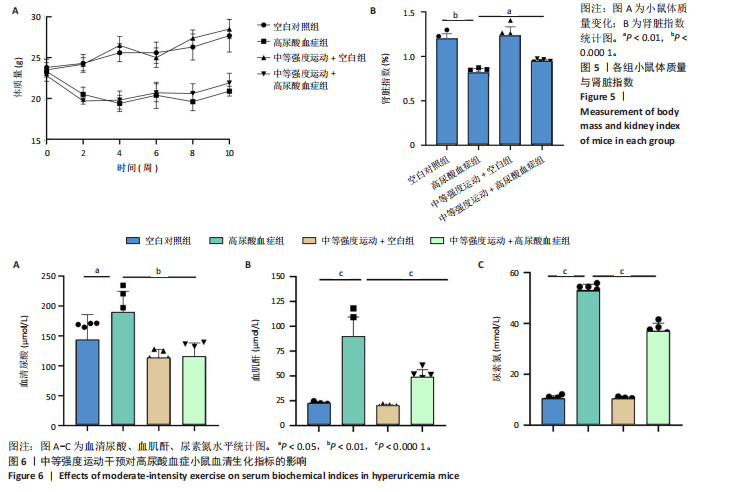
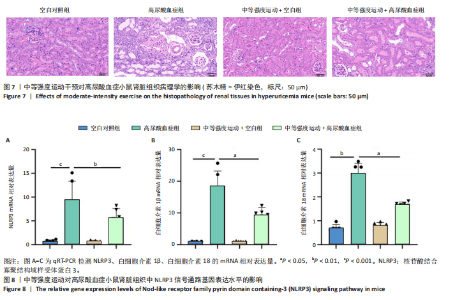
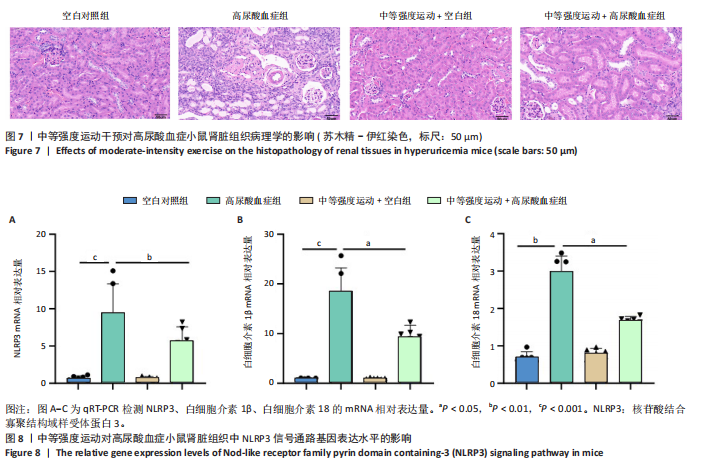
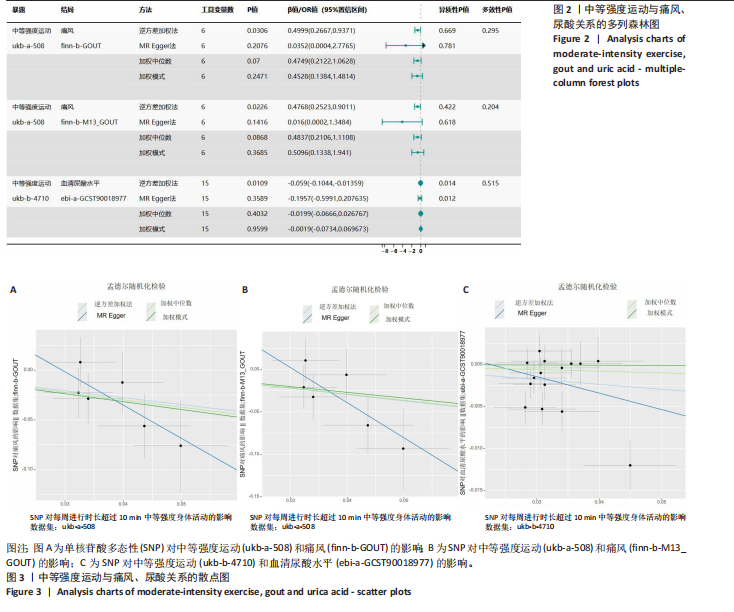
2.1 孟德尔随机化 如图2所示,以逆方差加权法为主要分析方法,对中等强度运动与痛风、血清尿酸水平的关系进行评估。其中,痛风作为分类变量,选用OR值评估;血清尿酸水平作为连续型变量,则采用效应值(β)评估,分别为0.499 9,0.476 8,?0.059,提示中等强度运动可能有助于降低痛风风险和血清尿酸水平。中等强度运动与痛风的异质性P值与多效性P均> 0.05,可以排除其他混杂因素的干扰;而中等强度运动与血清尿酸水平异质性P < 0.05,存在显著异质性,这可能是不同遗传变异通过不同生物学机制影响二者联系,也可能与纳入人群在种族、年龄、性别等方面存在差异有关,但综合整体分析结果仍支持中等强度运动与血清尿酸水平的负相关性。 如图3所示,散点图A和散点图B展示中等强度运动与不同数据集痛风之间的相关性,散点图C展示中等强度运动与血清尿酸水平的关系。各图的横、纵坐标分别表示单核苷酸多态性对中等强度运动的影响,以及单核苷酸多态性对痛风或血清尿酸水平的影响,从整体散点分布和线条趋势来看,多数散点分布相对集中,部分线条呈现向下倾斜趋势,提示中等强度运动可能对痛风和血清尿酸水平有负向影响,即中等强度运动增加,痛风发生风险和血清尿酸水平可能降低。 如图4所示,选用留一法进行分析,结果提示排除任何单个工具变量均未明显改变总体结果,为孟德尔随机化的结果稳健性提供了有力的证据。图中大多数单个单核苷酸多态性的黑色线段位于零线左侧,且各单核苷酸多态性效应值有差异,图4A、B综合分析以及图4C逆方差加权法分析的红色线段也在零线左侧,提示中等强度运动可能对痛风与尿酸水平有负向影响,即中等强度运动增加可能降低痛风风险与尿酸水平。 2.2 实验动物数量分析 参加实验共32只小鼠,因为模型组存在个别小鼠尿酸水平升高不够显著,未达到造模成功标准,故剔除模型组2只,为排除数量差异影响,在其余3组各剔除最大值和最小值即每组剔除2只,最终进入结果分析数量共24只,每组6只。 2.3 小鼠体质量和肾脏指数的测定 连续观察小鼠体质量10周,每周日记录,实验结束后,称小鼠肾脏质量并计算肾脏指数,观察小鼠肾脏外观。如图5A所示,空白对照组和中等强度运动+空白组小鼠体质量逐渐升高,因氧嗪酸钾和腺嘌呤药物灌胃导致中等强度运动+高尿酸血症组和高尿酸血症组小鼠体质量早期下降明显,运动干预2周后中等强度运动+高尿酸血症组体质量有所回升,造模小鼠整体均比正常小鼠体质量低。如图5B所示,与空白对照组相比,高尿酸血症组小鼠肾脏指数显著下降;与高尿酸血症组相比,中等强度运动+高尿酸血症组肾脏指数升高。小鼠体质量和肾脏指数的变化提示实验过程中小鼠存在肾脏损伤,而运动可改善体质量和肾脏指数。 2.4 血清生化指标 高尿酸血症的主要特征为血清尿酸水平升高,当高尿酸血症损害肾脏的排泄功能时,可使血尿素氮和肌酐水平急剧上升[32]。如图6所示,与空白对照组相比,高尿酸血症组血清尿酸(P < 0.05)、血肌酐(P < 0.000 1)、血尿素氮(P < 0.000 1)均显著升高,提示高尿酸血症小鼠模型构建成功,且小鼠肾脏排泄功能显著减退。与高尿酸血症组相比,中等强度运动+高尿酸血症组血清尿酸(P < 0.01)、血肌酐(P < 0.000 1)、血尿素氮(P < 0.000 1)显著降低,提示中等强度运动可以降低血清尿酸水平,减轻高尿酸血症小鼠肾脏损伤。 2.5 各组小鼠肾组织病理形态表现 尿酸水平过高在肾脏中沉积形成单钠尿酸盐晶体会对肾脏组织造成严重损伤[33],肾脏苏木精-伊红染色显示,空白对照组小鼠肾组织结构正常,无明显病变,肾小球与肾小管结构清晰完整。高尿酸血症组小鼠肾小球代偿性肥大,肾小管上皮细胞空泡变性,细胞核脱落,伴有不同程度的管腔水肿扩张,肾间质局部有炎性细胞浸润。与空白对照组相比,中等强度运动+空白组肾小球和肾小管结构形态相近无明显差异变化。与高尿酸血症组相比,中等强度运动+高尿酸血症组肾小球轻度增大,肾小管扩张和上皮细胞水肿程度有所减轻,中等强度运动干预可减轻高尿酸血症小鼠肾脏组织病变,提示运动可改善高尿酸血症小鼠肾脏损伤和炎症反应,见图7。 2.6 各组小鼠肾脏组织NLRP3信号通路基因表达 如图8所示,与空白对照组相比,高尿酸血症组小鼠肾脏组织中NLRP3、白细胞介素1β及白细胞介素18基因表达量显著升高(NLRP3:P < 0.001、白细胞介素1β:P < 0.001、白细胞介素18:P < 0.01);而中等强度运动+高尿酸血症组NLRP3、白细胞介素1β及白细胞介素18基因表达量均显著低于高尿酸血症组(NLRP3:P < 0.01、白细胞介素1β:P < 0.05、白细胞介素18:P < 0.05),以上基因表达量变化提示高尿酸血症能够激活NLRP3炎性小体,进而促进白细胞介素1β、白细胞介素18成熟,而中等强度运动干预后上述基因表达水平显著降低,提示运动干预有可能抑制NLRP3信号通路的激活。 2.7 各组小鼠肾脏组织中白细胞介素6、白细胞介素11、NLRP3、Caspase-1和白细胞介素1β蛋白表达 如图9所示,与空白对照组相比,高尿酸血症组小鼠肾脏组织中NLRP3、Caspase-1、白细胞介素1β、白细胞介素6、白细胞介素11蛋白表达量均显著升高(分别为P < 0.05、P < 0.01、P < 0.05、P < 0.05、P < 0.01),而中等强度运动+高尿酸血症组上述蛋白表达量均显著降低于高尿酸血症组(分别为P < 0.05、P < 0.05、P < 0.01、P < 0.05、P < 0.01),提示高尿酸血症诱导炎症因子蛋白表达上调造成肾脏炎症,运动干预后可以降低炎症因子表达量,发挥肾脏保护作用。"
| [1] HUANG Z, XIE N, ILLES P, et al. From purines to purinergic signalling: molecular functions and human diseases. Signal Transduct Target Ther. 2021;6(1):162. [2] DU L, ZONG Y, LI H, et al. Hyperuricemia and its related diseases: mechanisms and advances in therapy. Signal Transduct Target Ther. 2024;9(1):212. [3] ZHAO H, LV J, CHEN B, et al. RAGE deficiency obstructs high uric acid-induced oxidative stress and inflammatory response. Int Immunopharmacol. 2025;151:114316. [4] SONG J, JIN C, SHAN Z, et al. Prevalence and Risk Factors of Hyperuricemia and Gout: A Cross-sectional Survey from 31 Provinces in Mainland China. J Transl Int Med. 2022;10(2):134-145. [5] WU ZD, YANG XK, HE YS, et al. Environmental factors and risk of gout. Environ Res. 2022;212(Pt C):113377. [6] ZHANG M, ZHU X, WU J, et al. Prevalence of Hyperuricemia Among Chinese Adults: Findings From Two Nationally Representative Cross-Sectional Surveys in 2015-16 and 2018-19. Front Immunol. 2022;12:791983. [7] DANVE A, SEHRA ST, NEOGI T. Role of diet in hyperuricemia and gout. Best Pract Res Clin Rheumatol. 2021;35(4):101723. [8] TERKELTAUB R. Emerging Urate-Lowering Drugs and Pharmacologic Treatment Strategies for Gout: A Narrative Review. Drugs. 2023;83(16): 1501-1521. [9] LI D, YUAN S, DENG Y, et al. The dysregulation of immune cells induced by uric acid: mechanisms of inflammation associated with hyperuricemia and its complications. Front Immunol. 2023;14:1282890. [10] GUPTA S, CASSEL SL, SUTTERWALA FS, et al. Regulation of the NLRP3 inflammasome by autophagy and mitophagy. Immunol Rev. 2025;329(1):e13410. [11] BARNETT KC, LI S, LIANG K, et al. A 360° view of the inflammasome: Mechanisms of activation, cell death, and diseases. Cell. 2023;186(11): 2288-2312. [12] PANG Q, WANG P, PAN Y, et al. Irisin protects against vascular calcification by activating autophagy and inhibiting NLRP3-mediated vascular smooth muscle cell pyroptosis in chronic kidney disease. Cell Death Dis. 2022;13(3):283. [13] WEN L, YANG H, MA L, et al. The roles of NLRP3 inflammasome-mediated signaling pathways in hyperuricemic nephropathy. Mol Cell Biochem. 2021;476(3):1377-1386. [14] SCHEFFER DDL, LATINI A. Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochim Biophys Acta Mol Basis Dis. 2020;1866(10):165823. [15] ZHANG T, LIU W, GAO S. Exercise and hyperuricemia: an opinion article. Ann Med. 2024;56(1):2396075. [16] SPRICK JD, JEONG J, SABINO-CARVALHO JL, et al. Neurocirculatory regulation and adaptations to exercise in chronic kidney disease. Am J Physiol Heart Circ Physiol. 2023;324(6):H843-H855. [17] JIA E, ZHU H, GENG H, et al. The effects of aerobic exercise on body composition in overweight and obese patients with gout: a randomized, open-labeled, controlled trial. Trials. 2022;23(1):745. [18] HOU Y, MA R, GAO S, et al. The Effect of Low and Moderate Exercise on Hyperuricemia: Protocol for a Randomized Controlled Study. Front Endocrinol (Lausanne). 2021;12:716802. [19] COSTANTI-NASCIMENTO AC, BRELAZ-ABREU L, BRAGANÇA-JARDIM E, et al. Physical exercise as a friend not a foe in acute kidney diseases through immune system modulation. Front Immunol. 2023;14: 1212163. [20] MOECKE DMP, MARTINS GHC, GARLET TC, et al. Aerobic Exercise Attenuates Kidney Injury, Improves Physical Performance, and Increases Antioxidant Defenses in Lungs of Adenine-Induced Chronic Kidney Disease Mice. Inflammation. 2022;45(5):1895-1910. [21] CHEN J, JIA S, XUE X, et al. Gut microbiota: a novel target for exercise-mediated regulation of NLRP3 inflammasome activation. Front Microbiol. 2025;15:1476908. [22] LIAN X, GUO Z, LIU J, et al. Aerobic Exercise Affected Lymphocyte Apoptosis by Modulating ROS Release and NLRP3 Inflammasome Activation. Bull Exp Biol Med. 2025;178(5):685-690. [23] ZHANG T, DING S, WANG R. Research Progress of Mitochondrial Mechanism in NLRP3 Inflammasome Activation and Exercise Regulation of NLRP3 Inflammasome. Int J Mol Sci. 2021;22(19):10866. [24] 吴昱苇,朱江,郑兵,等.运动调控尿酸的作用机制[J].中国组织工程研究,2024,28(34):5552-5557. [25] LEE S, SHIN YA, CHO J, et al. Moderate-Intensity Exercise Preserves Bone Mineral Density and Improves Femoral Trabecular Bone Microarchitecture in Middle-Aged Mice. J Bone Metab. 2022;29(2): 103-111. [26] ELSWORTH B, LYON M, ALEXANDER T, et al. The MRC IEU OpenGWAS data infrastructure. bioRxiv, 2020: 2020.08.10.244293. [27] DOHERTY A, JACKSON D, HAMMERLA N, et al. Large Scale Population Assessment of Physical Activity Using Wrist Worn Accelerometers: The UK Biobank Study. PLoS One. 2017;12(2):e0169649. [28] LI Y, XIE Y, LI J, et al. Diastolic and systolic blood pressure and gout: a Mendelian randomization study. Front Endocrinol (Lausanne). 2024; 15:1367621. [29] ZHANG Y, TANG Z, TONG L, et al. Serum uric acid and risk of diabetic neuropathy: a genetic correlation and mendelian randomization study. Front Endocrinol (Lausanne). 2023;14:1277984. [30] BIRNEY E. Mendelian Randomization. Cold Spring Harb Perspect Med. 2022;12(4):a041302. [31] SEKULA P, DEL GRECO MF, PATTARO C, et al. Mendelian Randomization as an Approach to Assess Causality Using Observational Data. J Am Soc Nephrol. 2016;27(11):3253-3265. [32] GUO XL, GAO YY, YANG YX, et al. Amelioration effects of α-viniferin on hyperuricemia and hyperuricemia-induced kidney injury in mice. Phytomedicine. 2023;116:154868. [33] LI D, LI Y, CHEN X, et al. The pathogenic mechanism of monosodium urate crystal-induced kidney injury in a rat model. Front Endocrinol (Lausanne). 2024;15:1416996. [34] DOGRU S, YASAR E, YESILKAYA A. Effects of uric acid on oxidative stress in vascular smooth muscle cells. Biomed Rep. 2024;21(6):171. [35] GE P, GUO Y, CHE B, et al. Modulation of NLRP3 Inflammasome Activation by QYHT Decoction: Implications for the Treatment of Erectile Dysfunction in Hyperuricemia. Am J Mens Health. 2025;19(1): 15579883251318307. [36] YANAI H, ADACHI H, HAKOSHIMA M, et al. Molecular Biological and Clinical Understanding of the Pathophysiology and Treatments of Hyperuricemia and Its Association with Metabolic Syndrome, Cardiovascular Diseases and Chronic Kidney Disease. Int J Mol Sci. 2021;22(17):9221. [37] CHEN LG, TUBBS JD, LIU Z, et al. Mendelian randomization: causal inference leveraging genetic data. Psychol Med. 2024;54(8):1461-1474. [38] WANG Q, DAI H, HOU T, et al. Dissecting Causal Relationships Between Gut Microbiota, Blood Metabolites, and Stroke: A Mendelian Randomization Study. J Stroke. 2023;25(3):350-360. [39] NGUYEN K, MITCHELL BD. A Guide to Understanding Mendelian Randomization Studies. Arthritis Care Res (Hoboken). 2024;76(11): 451-1460. [40] MALOBERTI A, TOGNOLA C, GAROFANI I, et al. Uric acid and metabolic syndrome: Importance of hyperuricemia cut-off. Int J Cardiol. 2024; 417:132527. [41] SONG P, WANG H, XIA W, et al. Prevalence and correlates of hyperuricemia in the middle-aged and older adults in China. Sci Rep. 2018;8(1):4314. [42] GAN Y, ZENG Y, HUANG J, et al. Polysaccharide extracted from Phellinus igniarius attenuated hyperuricemia by modulating bile acid metabolism and inhibiting uric acid synthesis in adenine/potassium oxonate-treated mice. J Ethnopharmacol. 2025;342:119365. [43] WEINER DE, LIU CK, MIAO S, et al. Effect of Long-term Exercise Training on Physical Performance and Cardiorespiratory Function in Adults With CKD: A Randomized Controlled Trial. Am J Kidney Dis. 2023;81(1):59-66. [44] JEONG J, SPRICK JD, DACOSTA DR, et al. Exercise modulates sympathetic and vascular function in chronic kidney disease. JCI Insight. 2023;8(4): e164221. [45] WATSON EL, BAKER LA, WILKINSON TJ, et al. Inflammation and physical dysfunction: responses to moderate intensity exercise in chronic kidney disease. Nephrol Dial Transplant. 2022;37(5):860-868. [46] NAKAYAMA A, KURAJOH M, TOYODA Y, et al. Dysuricemia. Biomedicines. 2023;11(12):3169. [47] JIANG Z, CAO J, SU H, et al. Exercise serum regulates uric acid transporters in normal rat kidney cells. Sci Rep. 2022;12(1):18086. [48] LIU Y, ZHANG P, JIN Y, et al. Is lactic acid a misunderstood trigger of gout attack for a century? Colloids Surf B Biointerfaces. 2024;238:113913. [49] LEVEY AS, BOSCH JP, LEWIS JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461-470. [50] ORANGE ST, LESLIE J, ROSS M, et al. The exercise IL-6 enigma in cancer. Trends Endocrinol Metab. 2023;34(11):749-763. [51] YU J, LI H, WU Y, et al. Inhibition of NLRP3 inflammasome activation by A20 through modulation of NEK7. Proc Natl Acad Sci U S A. 2024; 121(25):e2316551121. [52] MOHAMMED HS, ELARINY HA, SEIF-ELDEIN NA, et al. Investigating the involvement of the NLRP3/ASC/caspase-1 and NF-κb/MAPK pathways in the pathogenesis of gouty arthritis: Insights from irradiated and non-irradiated Trifolium alexandrium L. extracts and some metabolites. J Ethnopharmacol. 2024;334:118566. [53] LIU J, JIA S, YANG Y, et al. Exercise induced meteorin-like protects chondrocytes against inflammation and pyroptosis in osteoarthritis by inhibiting PI3K/Akt/NF-κB and NLRP3/caspase-1/GSDMD signaling. Biomed Pharmacother. 2023;158:114118. |
| [1] | Chen Yulin, He Yingying, Hu Kai, Chen Zhifan, Nie Sha Meng Yanhui, Li Runzhen, Zhang Xiaoduo , Li Yuxi, Tang Yaoping. Effect and mechanism of exosome-like vesicles derived from Trichosanthes kirilowii Maxim. in preventing and treating atherosclerosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1768-1781. |
| [2] | Peng Zhiwei, Chen Lei, Tong Lei. Luteolin promotes wound healing in diabetic mice: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1398-1406. |
| [3] | Wu Yilin, Tian Hongying, Sun Jiale, Jiao Jiajia, Zhao Zihan, Shao Jinhuan, Zhao Kaiyue, Zhou Min, Li Qian, Li Zexin, Yue Changwu. Intervention effect and mechanism of Compound Herba Gueldenstaedtiae in a mouse model of breast hyperplasia [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(17): 4377-4389. |
| [4] | Huang Lei, Wang Xianghong, Zhang Xianxu, Li Shicheng, Luo Zhiqiang. Mechanism and therapeutic potential of nuclear factor E2-related factor 2 in regulating non-infectious spinal diseases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(15): 3971-3982. |
| [5] | Tian Minghao, Liao Yehui, Zhou Wenyang, He Baoqiang, Leng Yebo, Xu Shicai, Zhou Jiajun, Li Yang, Tang Chao, Tang Qiang, Zhong Dejun . Neuroprotective regulation of the IRF9 gene after spinal cord injury: bioinformatics analysis combined with experimental validation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(11): 2712-2726. |
| [6] | Zuo Na, Tang Qi, Yu Meng, Tao Kai. Effect of miR-196b-5p in adipose-derived stem cell exosomes on burn wound healing in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 43-49. |
| [7] | He Bo, Chen Wen, Ma Suilu, He Zhijun, Song Yuan, Li Jinpeng, Liu Tao, Wei Xiaotao, Wang Weiwei, Xie Jing . Pathogenesis and treatment progress of flap ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1230-1238. |
| [8] | Liu Xuan, Ding Yuqing, Xia Ruohan, Wang Xianwang, Hu Shujuan. Exercise prevention and treatment of insulin resistance: role and molecular mechanism of Keap1/nuclear factor erythroid2-related factor 2 signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7578-7588. |
| [9] | Si Juncheng, Peng Lina, Sun Lili, Wang Yu, Shi Lei, Shen Wenhui, Li Mengqi, Zang Wanli. Transcriptome sequencing analysis of the mechanism by which cold water swimming regulates inflammatory response in rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6205-6211. |
| [10] | Liu Hanfei, Cai Zhencun, Zhou Xueting, Wen Hang, Chen Zhenjun. Mechanisms by which traumatic brain injury promotes bone callus formation and fracture healing [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6260-6268. |
| [11] | Lin Meiyu, Zhao Xilong, Gao Jing, Zhao Jing, Ruan Guangping. Action mechanism and progress of stem cells against ovarian granulosa cell senescence [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5414-5421. |
| [12] | Yang Jun, Yin Peng, Zheng Zhonghua. Mechanism of stachydrine-induced autophagy in improving atherosclerosis in high-fat-fed mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(24): 5140-5147. |
| [13] | Huang Xiaomeng, Zhang Zhilan, Shang Wenya, Huang Jing, Wei Huilin, Li Bing, Ren Yafeng. Scientific basis for acupuncture combined with neural stem cells for repairing spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(19): 4111-4121. |
| [14] | He Li, , Ren Lu, , Jiang Xiaoxi, , Liu Xuqian, , Li Chunhui, . Effects of 1,8-cineole on inflammatory response in a rat model of experimental periodontitis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(17): 3605-3613. |
| [15] | Wang Zhikun, Bai Shaoxuan, Zhao Wei, Wang Chenyu. Exercise preconditioning combined with bone marrow mesenchymal stem cell transplantation for myocardial infarction in rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 65-73. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||