Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (23): 6122-6133.doi: 10.12307/2026.355
Previous Articles Next Articles
Potential targets of glucagon-like peptide 1 receptor agonist ticagrelor in the treatment of Alzheimer’s disease
Zhang Xiaomin, Du Pengyang, Zhang Xiuping, Xue Guofang
- Department of Neurology, Second Hospital of Shanxi Medical University, Taiyuan 030001, Shanxi Province, China
Zhang Xiaomin, MS, Attending physician, Department of Neurology, Second Hospital of Shanxi Medical University, Taiyuan 030001, Shanxi Province, China
-
Received:2025-07-26Accepted:2025-08-27Online:2026-08-18Published:2026-01-05 -
Contact:Zhang Xiaomin, Department of Neurology, Second Hospital of Shanxi Medical University, Taiyuan 030001, Shanxi Province, China -
About author:Du Pengyang, MS candidate, Department of Neurology, Second Hospital of Shanxi Medical University, Taiyuan 030001, Shanxi Province, China Zhang Xiaomin and Du Pengyang contributed equally to this work. -
Supported by:Shanxi Province Higher Education "Billion Project" Science and Technology Guidance Project, MOE Key Laboratory of Coal Environmental Pathogenicity and Prevention, No. BY-ZB-2024012 (to XGF); Natural Science Research Project of Shanxi Province Basic Research, No. 202303021211213 (to XGF)
CLC Number:
Cite this article
Zhang Xiaomin, Du Pengyang, Zhang Xiuping, Xue Guofang. Potential targets of glucagon-like peptide 1 receptor agonist ticagrelor in the treatment of Alzheimer’s disease[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(23): 6122-6133.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
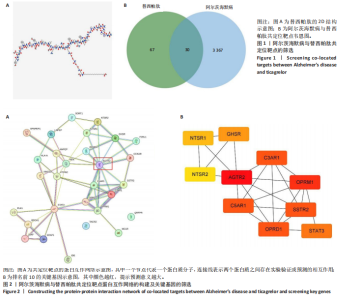
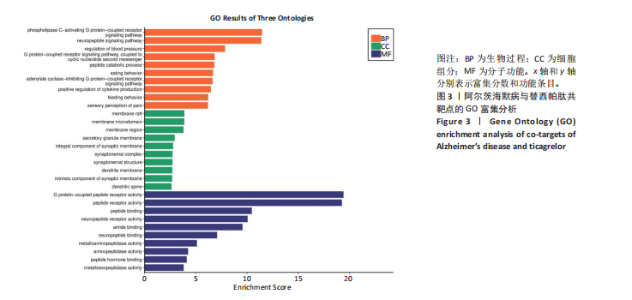
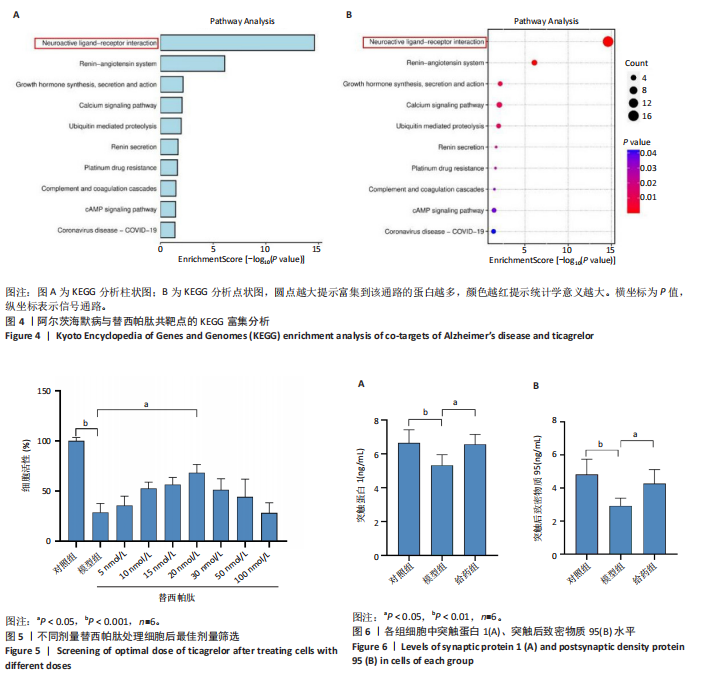
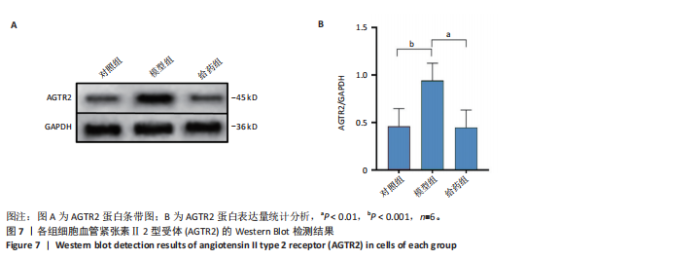
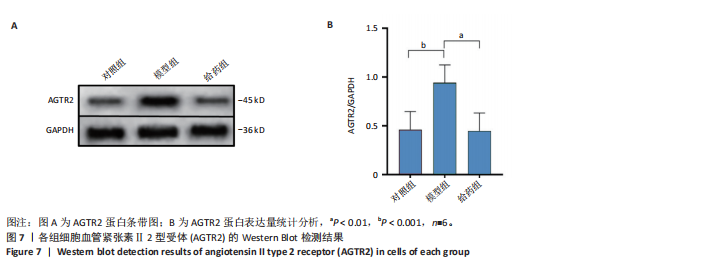
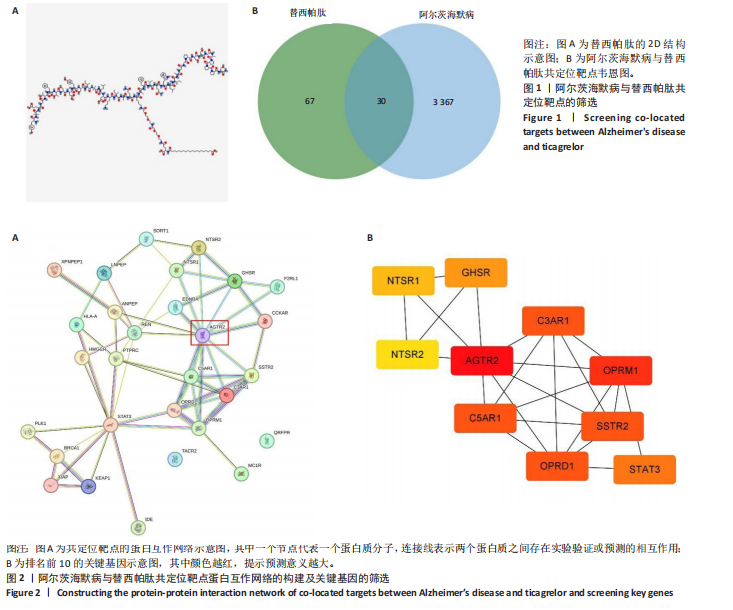
2.1 阿尔茨海默病与替西帕肽共定位靶点的筛选 从DisGeNET数据库筛选出阿尔茨海默病相关联的基因,共得到3 397个关联基因。从PubChem上获取替西帕肽的2D结构(图1A)和SMILES编码结构式,随后将SMILES编码结构式输入至SEA数据库中,得到97个替西帕肽的潜在作用靶点。随后使用R语言筛选出阿尔茨海默病与替西帕肽共定位靶点,共30个,并使用R语言进行可视化(图1B)。 2.2 蛋白互作网络的构建及关键基因的筛选 将先前得到的30个共定位靶点蛋白导入至SRTING在线数据库进行蛋白互作网络构建,见图2A。使用Cytoscape软件中的cytoHubba插件,根据蛋白关联度筛选出了10个连接度最高的关键基因(Hub gene):AGTR2、NTSR1、NTSR2、GHSR、C5AR1、C3AR1、OPRM1、SSTR2、OPRD1、STAT3,可视化结果见图2B。通过对关键基因功能的初步查询,此次研究选用与阿尔茨海默病和替西帕肽排名前10的关键基因进行后续的GO/KEGG分析。 2.3 关键基因的GO富集分析和KEGG通路分析 使用DAVID在线数据库进行GO富集分析及KEGG通路分析。GO结果显示排名前10的关键基因主要富集在:①生物过程(Biological Process,BP):磷脂酰肌醇特异性磷脂酶C激活的G蛋白偶联受体;②细胞组分(Cell Component,CC):膜筏;③分子功能(Molecular Function,MF):G蛋白偶联肽受体活性;GO结果符合预期,见图3。KEGG主要富集在“神经活性配体-受体相互作用”信号通路上,血管紧张素Ⅱ2型受体属于G蛋白偶联受体1家族成员,见图4。结果提示替西帕肽可能通过改善神经受体-配体功能来改善阿尔茨海默病,此次研究将以此通路为切入点进行讨论并佐以实验验证。 2.4 细胞水平验证结果 2.4.1 替西帕肽给药剂量筛选 使用CCK-8技术筛选替西帕肽治疗阿尔茨海默病的最佳给药剂量。对照组、模型组、给药组(分别给予5,10,15,20,30,50,100 nmol/L替西帕肽处理)的存活细胞百分比分别为(100.00±3.46)%,(28.83±8.86)%,(35.50±9.39)%,(52.67±6.28)%,(56.50±7.09)%,(68.17±8.35)%,(51.33±11.04)%,(44.17±17.79)%,(28.17±10.19)%。与对照组相比,模型组细胞活性明显降低(P < 0.001),提示β-淀粉样蛋白1-42对HT22细胞具有细胞毒性作用;不同浓度替西帕肽给药组中,20 nmol/L给药组的细胞活性最高(P=0.018 3),提示拮抗β-淀粉样蛋白1-42毒性作用效果最好,见图5。因此后续研究选用20 nmol/L为给药干预剂量。 2.4.2 基于ELISA技术探讨替西帕肽治疗阿尔茨海默病的机制 突触蛋白1属于突触蛋白家族,主要表达在成熟神经元突触前膜中。它通过结合于突触小泡的表面,介导位于突触前膜的突触小泡的释放,以发挥促进神经元发育和维持突触正常功能的作用[19];突触后致密物质95是突触后致密区(PSD)的核心支架蛋白,起到支撑骨架的作用,对突触结构的稳定性、信号传导整合及可塑性调控具有重要意义。KEGG结果提示关键蛋白富集在调控突触功能信号通路上,因此此次研究通过对突触蛋白1及突触后致密物质95的表达情况分析,讨论替西帕肽治疗阿尔茨海默病的潜在机制。 ELISA检测结果如图6A所示,突触蛋白1在对照组、模型组、给药组中的质量浓度分别为(6.651±0.77),(5.330±0.62),(6.561±0.58) ng/mL;如图6B所示,突触后致密物质95在对照组、模型组、给药组中的质量浓度分别为(4.822±0.91),(3.258±0.71),(4.438±0.67) ng/mL。突触蛋白1(P= 0.009 7)、突触后致密物质95(P=0.008 8)在阿尔茨海默病模型中显著下调,使用替西帕肽干预后突触蛋白1(P=0.015 4)、突触后致密物质95(P=0.047 0)的表达显著上调,提示替西帕肽可能通过改善阿尔茨海默病的突触功能来发挥治疗作用,与此项研究前期的KEGG富集分析结果相符,符合预期。 2.4.3 关键基因血管紧张素Ⅱ2型受体的Western Blot检测结果 通过Cytoscape对所有关键基因的筛选,发现血管紧张素Ⅱ2型受体是关联度排名最高、最显著的关键基因,因此此次研究使用Western Blot技术对3组HT22 细胞中血管紧张素Ⅱ2型受体的蛋白表达情况进行检测,见图7。血管紧张素Ⅱ2型受体蛋白在对照组、模型组和给药组的相对表达量分别为0.461 3±0.185 2,0.941 8±0.182 7,0.448 5±0.183 8。与正常组相比,模型组血管紧张素Ⅱ2型受体蛋白表达量显著上调(P=0.001 1);与模型组相比,实验组血管紧张素Ⅱ2型受体蛋白表达量下调(P=0.000 9)。提示血管紧张素Ⅱ2型受体可能是替西帕肽治疗阿尔茨海默病的潜在靶点。"
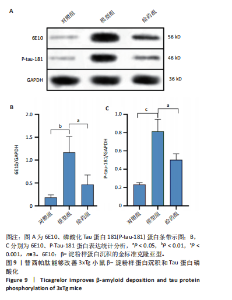
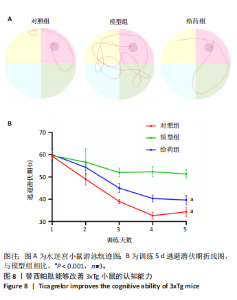
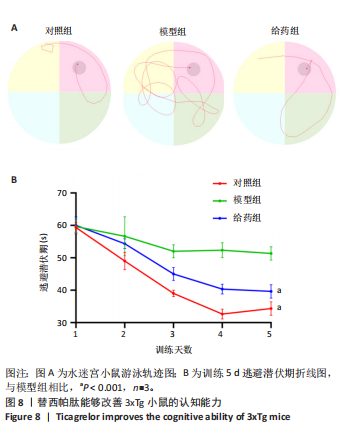
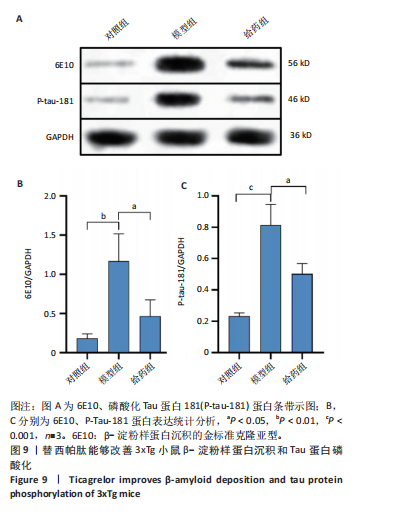
2.5.2 替西帕肽能够改善3xTg小鼠β-淀粉样蛋白沉积和Tau蛋白磷酸化 6E10是研究β-淀粉样蛋白沉积的金标准克隆亚型,常被学术界用来评估β-淀粉样蛋白的表达情况[17];Tau蛋白是神经元细胞中众多微管相关蛋白之一,是一种低分子量含磷糖蛋白。在阿尔茨海默病早期阶段,血浆磷酸化的Tau蛋白水平随时间逐渐增加。在大规模临床试验研究中,血浆磷酸化的Tau蛋白已被证明可以准确区分阿尔茨海默病型痴呆与非阿尔茨海默病型神经退行性疾病相关痴呆,并且具有较高的准确性;此外,在轻度认知障碍患者中,血浆P-tau-181已被证明可以准确预测未来2-6年内出现认知能力下降和转化为阿尔茨海默病痴呆的患者[18]。 Western Blot结果显示,6E10在对照组、模型组、给药组中的相对表达量分别为0.28±0.05,1.16±0.35,0.46±0.20,模型组与对照组相比,6E10表达量明显上调,提示β-淀粉样蛋白在3xTg小鼠脑海马组织中明显高表达(P=0.005 5);给药组与模型组相比,6E10表达量明显下调(P=0.026 3),提示替西帕肽能够改善3xTg小鼠模型脑内的异常β-淀粉样蛋白沉积,见图9。 P-tau-181在对照组、模型组、给药组中的相对表达量分别为0.23±0.02,0.81±0.13,0.50±0.06,型组与对照组相比,P-tau-181表达量明显上调,提示P-tau-181在3xTg小鼠脑海马组织中明显高表达(P=0.000 4);给药组与模型组相比,P-tau-181表达量明显下调(P=0.010 9),提示替西帕肽能够改善3xTg小鼠模型脑内的Tau蛋白磷酸化,见图9。"
| [1] SCHELTENS P, DE STROOPER B, KIVIPELTO M, et al.Alzheimer’s disease. Lancet. 2021;397(10284):1577-1590. [2] GRIFFITHS J, GRANT SGN. Synapse pathology in Alzheimer’s disease.Semin Cell Dev Biol. 2023;139:13-23. [3] KAMATHAM PT, SHUKLA R, KHATRI DK, et al. Pathogenesis, diagnostics, and therapeutics for Alzheimer’s disease: Breaking the memory barrier. Ageing Res Rev. 2024;101:102481. [4] LIU E, ZHANG Y, WANG JZ. Updates in Alzheimer’s disease: from basic research to diagnosis and therapies. Transl Neurodegener. 2024;13(1): 45. [5] RAJENDRAN K, KRISHNAN UM. Mechanistic insights and emerging therapeutic stratagems for Alzheimer’s disease. Ageing Res Rev. 2024; 97:102309. [6] HUANG N, HUANG W, WANG M, et al. Biomaterials in Alzheimer’s Disease: An Anti-Neuroinflammatory Perspective. Adv Healthc Mater. 2025;14(19):e2500498. [7] TZIORAS M, MCGEACHAN RI, DURRANT CS, et al. Synaptic degeneration in Alzheimer disease. Nat Rev Neurol. 2023;19(1):19-38. [8] ZHANG X, LIU F, GU Z. Tissue Engineering in Neuroscience: Applications and Perspectives. BME Front. 2023;4:0007. [9] YAN Y, LI X, GAO Y, et al. 3D bioprinting of human neural tissues with functional connectivity. Cell Stem Cell. 2024;31(2):260-74.e7. [10] YAO X, XUE T, CHEN B, et al. Advances in biomaterial-based tissue engineering for peripheral nerve injury repair. Bioact Mater. 2025;46: 150-172. [11] LIAO W, SHI Y, LI Z, et al. Advances in 3D printing combined with tissue engineering for nerve regeneration and repair. J Nanobiotechnol. 2025; 23(1):5. [12] ZHANG B, HU Y, DU H, et al. Tissue engineering strategies for spiral ganglion neuron protection and regeneration. J Nanobiotechnol. 2024; 22(1):458. [13] ZIVARI-GHADER T, VALIOGLU F, EFTEKHARI A, et al. Recent progresses in natural based therapeutic materials for Alzheimer’s disease. Heliyon. 2024;10(4):e2635. [14] WU P, XU C, ZOU X, et al. Capacitive-Coupling-Responsive Hydrogel Scaffolds Offering Wireless In Situ Electrical Stimulation Promotes Nerve Regeneration. Adv Mater. 2024;36(14):e2310483. [15] NOWELL J, BLUNT E, EDISON P. Incretin and insulin signaling as novel therapeutic targets for Alzheimer’s and Parkinson’s disease . Mol Psychiatry. 2023;28(1):217-229. [16] GUO X, LEI M, ZHAO J, et al. Tirzepatide ameliorates spatial learning and memory impairment through modulation of aberrant insulin resistance and inflammation response in diabetic rats. Front Pharmacol. 2023; 14:1146960. [17] PIRTTILÄ T, KIM KS, MEHTA PD, et al. Soluble amyloid beta-protein in the cerebrospinal fluid from patients with Alzheimer’s disease, vascular dementia and controls. J Neurol Sci. 1994;127(1):90-95. [18] JANELIDZE S, MATTSSON N, PALMQVIST S, et al. Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med. 2020;26(3):379-386. [19] WEI Y, XIA X, WANG X, et al. Enhanced BBB penetration and microglia-targeting nanomodulator for the two-pronged modulation of chronically activated microglia-mediated neuroinflammation in Alzheimer’s disease. Acta Pharm Sin B. 2025;15(2):1098-1111. [20] WANG ZJ, LI XR, CHAI SF, et al. Semaglutide ameliorates cognition and glucose metabolism dysfunction in the 3xTg mouse model of Alzheimer’s disease via the GLP-1R/SIRT1/GLUT4 pathway. Neuropharmacology. 2023;240:109716. [21] DU P, ZHANG X, LIAN X, et al. O-GlcNAcylation and Its Roles in Neurodegenerative Diseases. J Alzheimers Dis. 2024;97(3):1051-1568. [22] HÖLSCHER C. Glucagon-like peptide-1 class drugs show clear protective effects in Parkinson’s and Alzheimer’s disease clinical trials: A revolution in the making? . Neuropharmacology. 2024;253:109952. [23] HONG CT, CHEN JH, HU CJ. Role of glucagon-like peptide-1 receptor agonists in Alzheimer’s disease and Parkinson’s disease. J Biomed Sci. 2024;31(1):102. [24] 柴世凡, 李欣儒, 叶育采, 等. 索马鲁肽治疗阿尔茨海默病的潜在靶点:沉默信息调节因子1 [J]. 中国组织工程研究,2024,28(20):3235-3239. [25] MÜLLER TD, ADRIAENSSENS A, AHRÉN B, et al. Glucose-dependent insulinotropic polypeptide (GIP). Mol Metab. 2025;95:102118. [26] ZHOU ZD, YI L, POPŁAWSKA-DOMASZEWICZ K, et al. Glucagon-like peptide-1 receptor agonists in neurodegenerative diseases: Promises and challenges. Pharmacol Res. 2025;216:107770. [27] DIZ-CHAVES Y, MAASTOR Z, SPUCH C, et al. Glucagon-like peptide 1 receptor activation: anti-inflammatory effects in the brain. Neural Regen Res. 2024;19(8):1671-1677. [28] KARIMI MA, GHOLAMI CHAHKAND MS, DADKHAH PA, et al. Comparative effectiveness of semaglutide versus liraglutide, dulaglutide or tirzepatide: a systematic review and meta-analysis. Front Pharmacol. 2025;16:1438318. [29] KARAGIANNIS T, MALANDRIS K, AVGERINOS I, et al. Subcutaneously administered tirzepatide vs semaglutide for adults with type 2 diabetes: a systematic review and network meta-analysis of randomised controlled trials. Diabetologia. 2024;67(7):1206-1222. [30] HEISE T, MARI A, DEVRIES JH, et al. Effects of subcutaneous tirzepatide versus placebo or semaglutide on pancreatic islet function and insulin sensitivity in adults with type 2 diabetes: a multicentre, randomised, double-blind, parallel-arm, phase 1 clinical trial. Lancet Diabetes. Endocrinol 2022;10(6):418-429. [31] MELSON E, ASHRAF U, PAPAMARGARITIS D, et al. What is the pipeline for future medications for obesity? . Int J Obes. 2025;49(3):433-451. [32] MASKERY M, GOULDING EM, GENGLER S, et al. The Dual GLP-1/GIP Receptor Agonist DA4-JC Shows Superior Protective Properties Compared to the GLP-1 Analogue Liraglutide in the APP/PS1 Mouse Model of Alzheimer’s Disease. Am J Alzheimers Dis Other Demen. 2020;35:1533317520953041. [33] SALLES GN, CALIÓ ML, HÖLSCHER C, et al. Neuroprotective and restorative properties of the GLP-1/GIP dual agonist DA-JC1 compared with a GLP-1 single agonist in Alzheimer’s disease. Neuropharmacology. 2020;162:107813. [34] ZHANG Z, SHI M, LI Z, et al. A Dual GLP-1/GIP Receptor Agonist Is More Effective than Liraglutide in the A53T Mouse Model of Parkinson’s Disease. Parkinsons Dis. 2023;2023:7427136. [35] YANG J, GU Y, CHEN H, et al. Tirzepatide’s innovative applications in the management of type 2 diabetes and its future prospects in cardiovascular health. Front Pharmacol. 2024;15:1453825. [36] CIARDULLO S, MORIERI ML, DANIELE G, et al. GLP1-GIP receptor co-agonists: a promising evolution in the treatment of type 2 diabetes. Acta Diabetol. 2024;61(8):941-950. [37] PARVANOVA A, ABBATE M, RESEGHETTI E, et al. Mechanisms and treatment of obesity-related hypertension-Part 2: Treatments. Clin Kidney J. 2025;18(3):sfaf035. [38] FONTANELLA RA, GHOSH P, PESAPANE A, et al. Tirzepatide prevents neurodegeneration through multiple molecular pathways .J Transl Med. 2024;22(1):114. [39] ALSHEHRI GH, AL-KURAISHY HM, WAHEED HJ, et al. Tirzepatide: a novel therapeutic approach for Alzheimer’s disease. Metab Brain Dis. 2025; 40(5):221. [40] YANG S, ZHAO X, ZHANG Y, et al. Tirzepatide shows neuroprotective effects via regulating brain glucose metabolism in APP/PS1 mice. Peptides. 2024;179:171271. [41] FATIMA N, PATEL SN, HUSSAIN T. Angiotensin II Type 2 Receptor: A Target for Protection Against Hypertension, Metabolic Dysfunction, and Organ Remodeling. Hypertension. 2021;77(6):1845-1856. [42] PORRELLO ER, DELBRIDGE LM, THOMAS WG. The angiotensin II type 2 (AT2) receptor: an enigmatic seven transmembrane receptor. Front Biosci (Landmark Ed). 2009;14(3):958-972. [43] SHEN L, CHEN DY, LOU QQ, et al. Angiotensin Type 2 Receptor Pharmacological Agonist Relieves Neurocognitive Deficits via Reducing Neuroinflammation and Microglial Engulfment of Dendritic Spines. J Neuroimmune Pharmacol. 2023;18(1-2):41-57. [44] SMITH HC, YU Z, IYER L, et al. Sex-Dependent Effects of Angiotensin Type 2 Receptor-Expressing Medial Prefrontal Cortex Interneurons in Fear Extinction Learning. Biol Psychiatry Glob Open Sci. 2024;4(5):100340. [45] TAYLER HM, MACLACHLAN R, GÜZEL Ö, et al. Altered Gene Expression Within the Renin-Angiotensin System in Normal Aging and Dementia. J Gerontol A Biol Sci Med Sci. 2024;79(1):glad241. [46] THATHIAH A, DE STROOPER B. G protein-coupled receptors, cholinergic dysfunction, and Abeta toxicity in Alzheimer’s disease. Sci Signal. 2009;2(93):re8. [47] MONTEIRO AR, BARBOSA DJ, REMIÃO F, et al. Alzheimer’s disease: Insights and new prospects in disease pathophysiology, biomarkers and disease-modifying drugs. Biochem Pharmacol. 2023;211:115522. [48] 李欣儒, 柴世凡, 李蔚然, 等 阿尔茨海默病发病机制相关基因生物信息学分析及实验验证[J]. 中国组织工程研究,2023,27(35):5653-5658. [49] KRISHNAMURTHY HK, JAYARAMAN V, KRISHNA K, et al. An overview of the genes and biomarkers in Alzheimer’s disease. Ageing Res Rev. 2025;104:102599. [50] ZHANG Y, CHEN H, LI R, et al. Amyloid β-based therapy for Alzheimer’s disease: challenges, successes and future. Signal Transduct Target Ther. 2023;8(1):248. [51] ZHENG Q, WANG X. Alzheimer’s disease: insights into pathology, molecular mechanisms, and therapy. Protein Cell. 2025;16(2):83-120. [52] AHN EH, PARK JB. Molecular Mechanisms of Alzheimer’s Disease Induced by Amyloid-β and Tau Phosphorylation Along with RhoA Activity: Perspective of RhoA/Rho-Associated Protein Kinase Inhibitors for Neuronal Therapy. Cells. 2025;14(2):89. [53] JIA B, XU Y, ZHU X. Cognitive resilience in Alzheimer’s disease: Mechanism and potential clinical intervention. Ageing Res Rev. 2025;106:102711. [54] LAZAROV O, GUPTA M, KUMAR P, et al. Memory circuits in dementia: The engram, hippocampal neurogenesis and Alzheimer’s disease. Prog Neurobiol. 2024;236:102601. [55] LEE C, KAANG BK. Clustering of synaptic engram: Functional and structural basis of memory. Neurobiol Learn Mem.2024; 216: 107993. [56] TADDEI RN, KED. Synapse vulnerability and resilience underlying Alzheimer’s disease. EBioMedicine. 2025;112:105557. [57] BÄHLER M, GREENGARD P. Synapsin I bundles F-actin in a phosphorylation-dependent manner. Nature. 1987;326(6114):704-707. [58] GYLYS KH, FEIN JA, YANG F, et al. Synaptic changes in Alzheimer’s disease: increased amyloid-beta and gliosis in surviving terminals is accompanied by decreased PSD-95 fluorescence. Am J Pathol. 2004;165(5):1809-1817. [59] SUN J, XU J, LING Y, et al. Fecal microbiota transplantation alleviated Alzheimer’s disease-like pathogenesis in APP/PS1 transgenic mice. Transl Psychiatry. 2019;9(1):189. [60] AJWAD N, MUSTAPHA M, IDRIS Z, et al. The Recent Applications of Stem Cell-Derived Exosomes and Hydrogels in Neurological Disorders. Tissue Eng Part B Rev. 2025. doi: 10.1089/ten.teb.2024.0353. [61] JIN J, ZHANG H, LU Q, et al. Nanocarrier-mediated siRNA delivery: a new approach for the treatment of traumatic brain injury-related Alzheimer’s disease. Neural Regen Res. 2025;20(9):2538-5555. [62] IYASWAMY A, THAKUR A, GUAN XJ, et al. Fe65-engineered neuronal exosomes encapsulating corynoxine-B ameliorate cognition and pathology of Alzheimer’s disease. Signal Transduct Target Ther. 2023; 8(1):404. |
| [1] | Zhou Sirui, Xu Yukun, Zhao Kewei. Ideas and methods of anti-melanogenesis of Angelica dahurica extracellular vesicles [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1747-1754. |
| [2] | Chen Yulin, He Yingying, Hu Kai, Chen Zhifan, Nie Sha Meng Yanhui, Li Runzhen, Zhang Xiaoduo , Li Yuxi, Tang Yaoping. Effect and mechanism of exosome-like vesicles derived from Trichosanthes kirilowii Maxim. in preventing and treating atherosclerosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1768-1781. |
| [3] | Peng Zhiwei, Chen Lei, Tong Lei. Luteolin promotes wound healing in diabetic mice: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1398-1406. |
| [4] | Liu Huan, Zeng Shaopeng, Chen Jun, He Linqian, Yang Ying, Zhang Jing. Aging-related dysregulation of glucose metabolism: crossroads of cancer and neurodegenerative diseases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1527-1538. |
| [5] | Chen Yixian, Chen Chen, Lu Liheng, Tang Jinpeng, Yu Xiaowei. Triptolide in the treatment of osteoarthritis: network pharmacology analysis and animal model validation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 805-815. |
| [6] | He Qiwang, , , Chen Bo, Liang Fuchao, Kang Zewei, Zhou Yuan, Ji Anxu, Tang Xialin, . Relationship between Alzheimer’s disease and sarcopenia and body mass index: analysis of GWAS datasets for European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1036-1046. |
| [7] | Hu Yalin, Huang Fengqin, Yang Boyin, Luo Xingmei. Transcription factor EB improves Alzheimer’s disease via the autophagy-lysosome pathway [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(22): 5844-5858. |
| [8] | Pei Xiaxia, Li Tian, Zhang Yanli, Gao Yanping, Su Qiang. A novel treadmill-based method for assessing learning and memory in mice [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(18): 4694-4701. |
| [9] | Mi Baolai, Liu Yufei, Yang Qiaoli, Kang Yanlan, Yuan Liang, Cao Jianchun. Limb lymphedema: network pharmacology and molecular docking analysis of core drug mechanisms in traditional Chinese medicine treatment [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(18): 4814-4824. |
| [10] | Wu Xue, Zhang Linao, Luo Shifang, Liu Feifan, Wan Yan, Bai Yuanmei, Cao Julin, Xie Yuhuan, Guo Peixin. Dandeng Tongnao soft capsules against ischemic stroke: fingerprinting and network pharmacological analysis of efficacy and mechanism of action [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(17): 4517-4528. |
| [11] | Wu Yilin, Tian Hongying, Sun Jiale, Jiao Jiajia, Zhao Zihan, Shao Jinhuan, Zhao Kaiyue, Zhou Min, Li Qian, Li Zexin, Yue Changwu. Intervention effect and mechanism of Compound Herba Gueldenstaedtiae in a mouse model of breast hyperplasia [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(17): 4377-4389. |
| [12] | Zhao Yu, Xue Yun, Huang Jiajun, Wu Diyou, Yang Bin, Huang Junqing. Total flavonoids from Semen Cuscutae inhibits osteoblast apoptosis in hormone-induced femoral head avascular necrosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(17): 4289-4298. |
| [13] | Zhou Wu, Zhang Jingxin, Liu Yuancheng, Hu Chenglong, Wang Siqi, Xu Jianxia, Huang Hai, Wei Sixi. Treatment of acute myeloid leukemia with corynoline: network pharmacology analysis of potential mechanisms and experimental validation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(16): 4088-4104. |
| [14] | Liu Annan, Li Jianhui, Gao Wei, Li Xue, Song Jing, Xing Liping, Li Honglin. Bibliometric analysis of ferroptosis and Alzheimer’s disease [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(16): 4278-4288. |
| [15] | Jiang Huanhuan, Mu Sheng, Ma Wenxin, Liu Chang, Liu Ziyu, Pu Jing, Zhu Xiangdong, Hui Hong, Ma Huiming. Mechanism of multi-target intervention of the active ingredient of Allii Tuberosi Semen in rats with oligoasthenozoospermia [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(12): 3044-3057. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||