Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (22): 5671-5681.doi: 10.12307/2026.169
Previous Articles Next Articles
Potential mechanism by which iroquois homeobox 3 regulates the browning of perivascular adipose tissue in vascular injury
Hu Xiaoyong, Song Qianhua, Yang Zhaoying, Tang Rui, Li Hongjian
- Department of Hypertension, The Fifth Affiliated Hospital of Xinjiang Medical University, Urumqi 830000, Xinjiang Uyghur Autonomous Region, China
-
Received:2025-06-06Accepted:2025-09-16Online:2026-08-08Published:2025-12-26 -
Contact:Li Hongjian, MD, Professor, Department of Hypertension, The Fifth Affiliated Hospital of Xinjiang Medical University, Urumqi 830000, Xinjiang Uyghur Autonomous Region, China -
About author:Hu Xiaoyong, MD candidate, Department of Hypertension, The Fifth Affiliated Hospital of Xinjiang Medical University, Urumqi 830000, Xinjiang Uyghur Autonomous Region, China -
Supported by:Health and Wellness Research Project of Xinjiang Uygur Autonomous Region, No. 2025001CXKYXM650025463 (to LHJ); Xinjiang 'Tianshan Talents' High-level Talent Training Program in Medicine and Health - Leading Talent Project, No. TSYC202301A057 (to LHJ); the National Natural Science Foundation of China, No. 8226020195 (to LHJ)
CLC Number:
Cite this article
Hu Xiaoyong, Song Qianhua, Yang Zhaoying, Tang Rui, Li Hongjian. Potential mechanism by which iroquois homeobox 3 regulates the browning of perivascular adipose tissue in vascular injury[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(22): 5671-5681.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
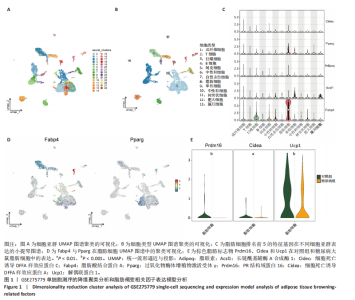
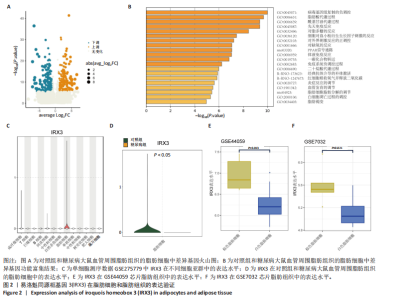
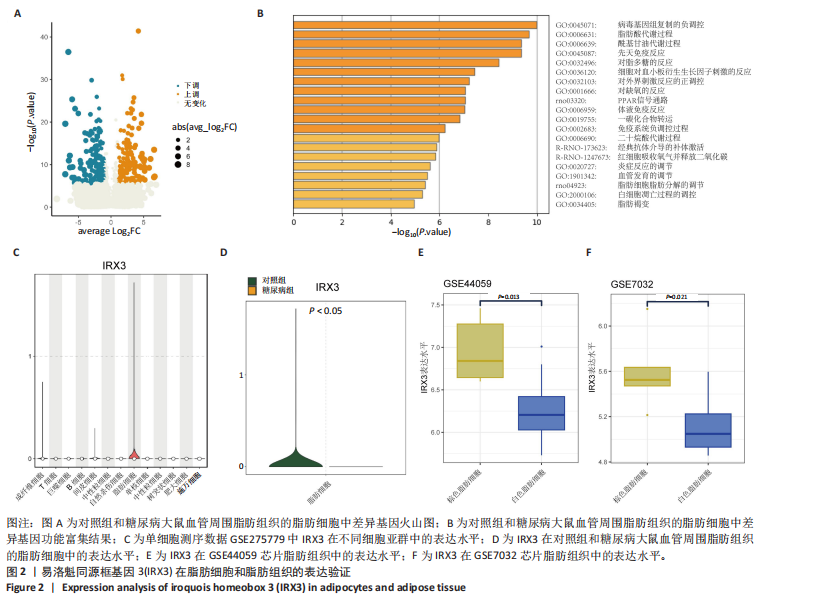
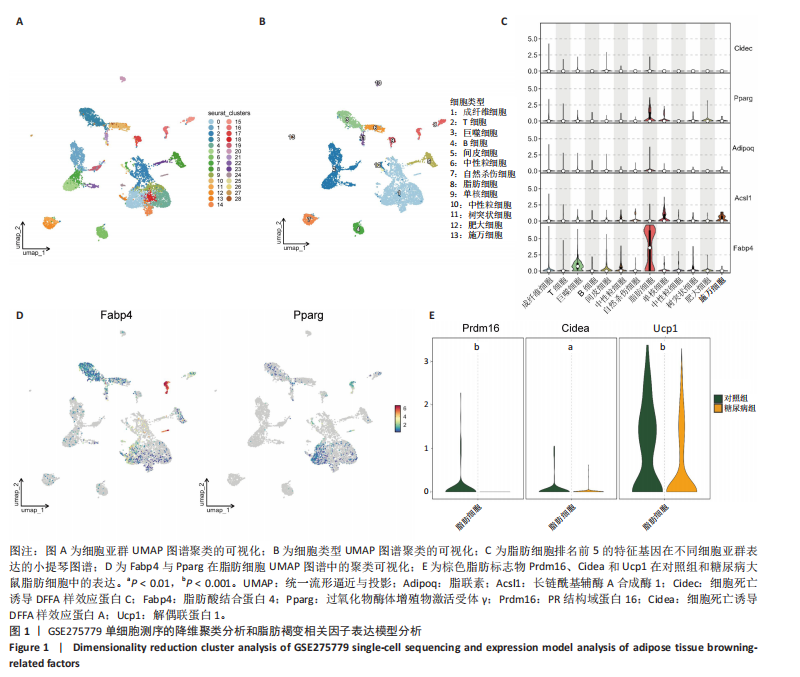
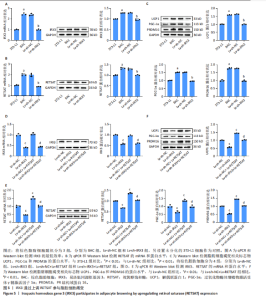
2.1 单细胞测序分析发现损伤血管周围脂肪组织褐变减少 血管损伤是糖尿病的常见并发症[20]。GSE275779数据集展示了正常和糖尿病大鼠血管周围脂肪组织中单细胞景观。经Seurat降维得到28个细胞亚群(图1A),然后根据细胞标志物的表达特异性进一步降维,得到13个细胞亚群,分别为成纤维细胞、脂肪细胞、T细胞、巨噬细胞、B细胞、间皮细胞、内皮细胞、NKT细胞、神经施万细胞、肥大细胞、中性粒细胞、树突状细胞、单核细胞(图1B)。对脂肪细胞进一步分析,表达排名前5的特征因子包括脂肪酸结合蛋白4、过氧化物酶体增殖物激活受体γ、脂联素、长链酰基辅酶A合成酶1和细胞死亡诱导DFFA样效应蛋白C(图1C),其中脂肪酸结合蛋白4、过氧化物酶体增殖物激活受体γ的统一流形逼近与投影聚类图谱如图1D所示。研究发现与对照组相比,PR结构域蛋白16、细胞死亡诱导DFFA样效应蛋白A和解偶联蛋白1等在糖尿病大鼠的血管周围脂肪组织中显著下调,且这些基因均参与脂肪褐变(图1E)[23]。结果表明,糖尿病血管损伤伴随着血管周围脂肪组织褐变的抑制。 2.2 IRX3在脂肪细胞中特异性表达并且在棕色脂肪中表达上调 对单细胞测序数据GSE275779中对照和糖尿病大鼠脂肪细胞中差异基因(∣logFC∣> 1,adjusted P.value < 0.05)进行筛选(图2A)及功能富集发现差异基因富集到脂肪褐变这一过程(图2B),表明具有血管损伤特性的糖尿病大鼠血管周围脂肪组织确实存在脂肪褐变的改变。进一步分析发现IRX3只在脂肪细胞中特征表达,并且在对照组血管周围脂肪组织的脂肪细胞中高表达,在糖尿病大鼠的血管周围脂肪组织的脂肪细胞中下调(图2C,D),提示血管损伤时血管周围脂肪组织褐变减少可能与IRX3表达减少有关联。同样,在棕色脂肪相关芯片表达数据(GSE44059和GSE7032)中,也发现相较于白色脂肪组织,IRX3在棕色脂肪组织中显著高表达(图2E,F,P < 0.05)。 2.3 IRX3在脂肪褐变过程中表达上调 使用GSE185518数据,包括诱导多潜能干细胞、分化过程中的棕色脂肪以及胚胎棕色脂肪组织验证,发现随着棕色脂肪细胞的成熟,IRX3的mRNA表达显著提高,并且在棕色脂肪组织中表达最高(图3A)。使用GSE168387展示了未分化的胚胎干细胞(Undiff-Stem cells)、中胚层期(Mesoderm stage)、棕色脂肪前体细胞(Brown adipocytes precursor)以及成熟的棕色脂肪细胞(Brown adipocytes)这一棕色脂肪诱导分化过程中基因表达数据,同样也观察到类似"
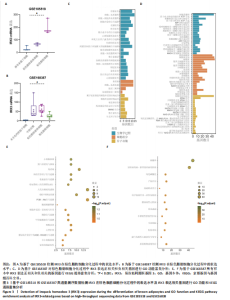
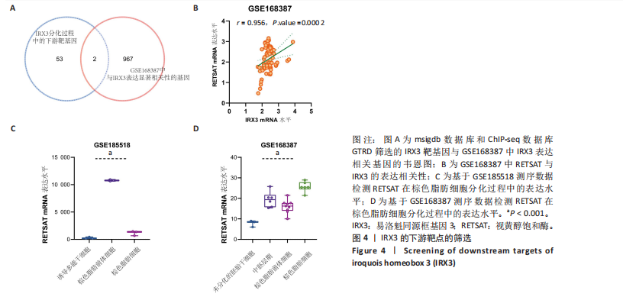
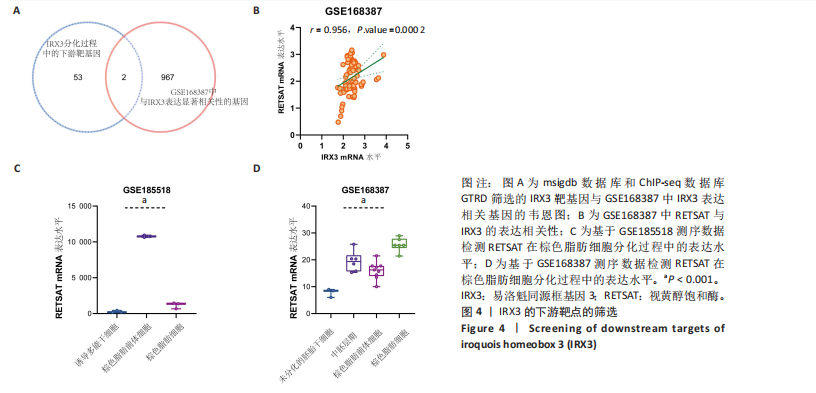
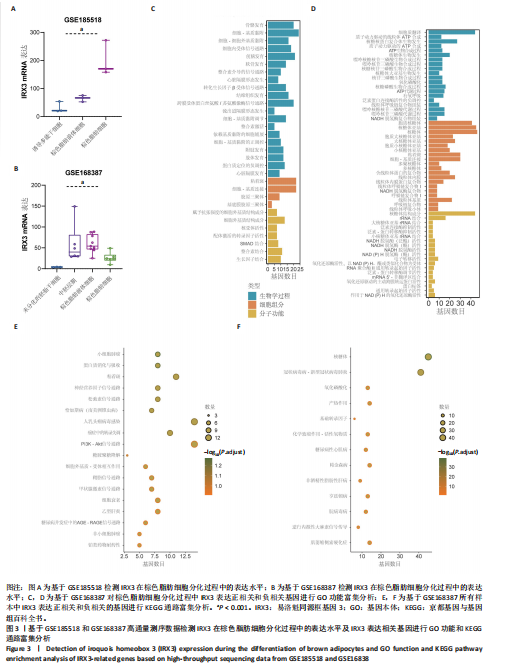
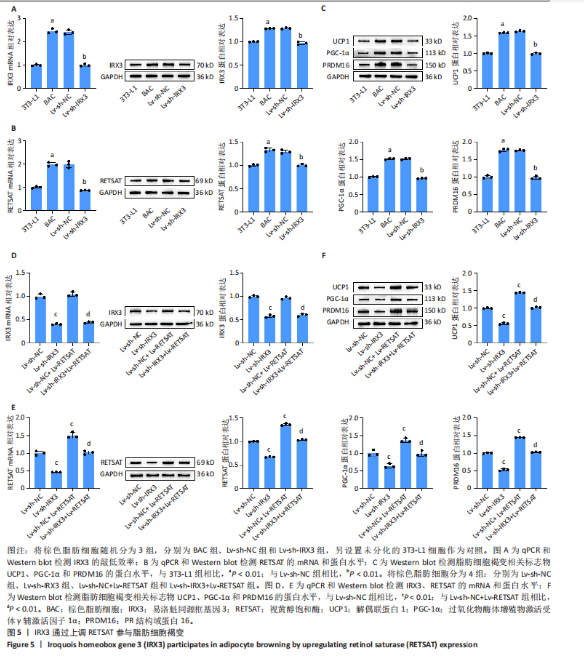
结果,从未分化的干细胞到成熟褐色脂肪细胞分化过程中IRX3的mRNA表达显著提高,其中以棕色脂肪前体细胞的表达量最高(图3B)。总之,棕色脂肪细胞分化过程中IRX3的mRNA表达显著提高。进一步基于GSE168387所有样本数据得到与IRX3表达具有相关性的基因(∣r∣> 0.95,P < 0.05),并对其进行功能富集分析(图3C,D)和KEGG通路富集分析(图3E,F),结果表明,正相关基因大多参与多潜能分化调控以及心肌细胞发育调控,而负相关基因参与能量平衡代谢。以上结果表明,IRX3可能参与棕色脂肪细胞分化过程。 2.4 IRX3可能介导RETSAT参与血管周围脂肪组织褐变 IRX3作为IRX家族的转录因子调控基因表达,为了进一步探讨IRX3参与棕色脂肪细胞分化的可能机制。此研究筛选IRX3分化过程中的下游靶基因。从msigdb (https://www.gsea-msigdb.org/)得到了IRX3的靶基因(IRX3_TARGET_GENES),其中55个基因经ChIP-seq数据库GTRD鉴定,在其启动子区(TSS-1000,+100 bp) 含有1个或多个IRX3蛋白(UniProt:P78415) 结合位点。将IRX3的靶基因与GSE168387数据中和IRX3表达具有显著相关性的基因取交集(r > 0.95,OR < -0.95,P < 0.05),发现核糖体蛋白S13、RETSAT可能是IRX3的靶基因(图4A)。RETSAT和IRX3表达也具有显著的正相关性(图4B)。进一步研究结果显示,在棕色脂肪细胞分化测序数据GSE185518和GSE168387中,观察到RETSAT的mRNA水平随着棕色脂肪细胞分化程度增加而有不同程度的升高(图4C,D,P < 0.05),说明IRX3极有可能介导RETSAT参与棕色脂肪细胞的分化过程。 2.5 IRX3可上调RETSAT参与脂肪细胞褐变 为了进一步验证IRX3/RETSAT是否调控脂肪褐变,检测3T3-L1脂肪前体细胞诱导成棕色脂肪细胞后IRX3表达变化,发现IRX3 mRNA和蛋白表达水平都显著升高(图5A)。在3T3-L1细胞中敲低IRX3并进行棕色脂"
肪细胞诱导分化,qPCR和Western blot检测细胞中IRX3的mRNA和蛋白表达水平以评估转染效率,结果表明,Lv-sh-IRX3敲低模型构建成功。进一步在体外对IRX3和RETSAT的关系进行验证,采用qPCR和Western blot检测RETSAT的蛋白表达水平,结果显示,成棕色脂肪诱导分化后RETSAT表达也上调。与Lv-NC组相比,IRX3敲低会明显下调RETSAT的蛋白水平(图5B)。最后采用Western blot检测脂肪细胞褐变相关标志物解偶联蛋白1、过氧化物酶体增殖物激活受体γ辅激活因子1α、PR结构域蛋白16的表达水平,结果显示,成棕色脂肪诱导分化后,上述标志物表达显著上调。与Lv-NC组相比,IRX3敲低组解偶联蛋白1、过氧化物酶体增殖物激活受体γ辅激活因子1α和PR结构域蛋白16的表达水平显著降低,表明脂肪细胞褐变受到抑制(图5C)。 为进一步验证IRX3通过RETSAT参与脂肪细胞褐变,在IRX3敲低细胞中进行RETSAT过表达的回复实验,3T3-L1脂肪前体细胞转染RETSAT过表达载体后进行诱导分化。qPCR和Western blot结果显示,与Lv-sh-NC组或Lv-sh-IRX3组相比,Lv-RETSAT成功增加了RETSAT的mRNA和蛋白水平,但对IRX3的表达无影响(图5D,E)。Western blot检测脂肪细胞褐变相关标志物,结果显示,Lv-RETSAT显著增加了解偶联蛋白1、过氧化物酶体增殖物激活受体γ辅激活因子1α和PR结构域蛋白16的表达水平,脂肪细胞褐变水平增强(图5F)。综上所述,IRX3可能通过RETSAT参与脂肪细胞褐变。IRX3/RETSAT调控脂肪细胞褐变的功能也提示其可能通过促进脂肪细胞褐变发挥保护血管的功能。"
| [1] KABEIL M, KAUVAR DS, BENNETT L, et al. Recent advances and the future of abdominopelvic and lower extremity vascular injury management. Semin Vasc Surg. 2023;36(2):268-282. [2] SHOKOPLES BG, BERILLO O, COMEAU K, et al. P2RX7 gene knockout or antagonism reduces angiotensin II-induced hypertension, vascular injury and immune cell activation. J Hypertens. 2023;41(11):1701-1712. [3] WANG X, ZHANG M, MAO C, et al. Icariin alleviates ferroptosis-related atherosclerosis by promoting autophagy in xo-LDL-induced vascular endothelial cell injury and atherosclerotic mice. Phytother Res. 2023;37(9): 3951-3963. [4] TAI GJ, MA YJ, FENG JL, et al. NLRP3 inflammasome-mediated premature immunosenescence drives diabetic vascular aging dependent on the induction of perivascular adipose tissue dysfunction. Cardiovasc Res. 2025;121(1):77-96. [5] PENG H, LV Y, LI C, et al. Cathepsin S inhibition in dendritic cells prevents Th17 cell differentiation in perivascular adipose tissues following vascular injury in diabetic rats. J Biochem Mol Toxicol. 2023;37(9):e23419. [6] JIANG T, WEI Y, XU R, et al. Renal denervation alleviates vascular remodeling in spontaneously hypertensive rats by regulating perivascular adipose tissue. Hypertens Res. 2024;47(10):2760-2772. [7] CHENG CK, DING H, JIANG M, et al. Perivascular adipose tissue: Fine-tuner of vascular redox status and inflammation. Redox Biol. 2023;62:102683. [8] HILLOCK-WATLING C, GOTLIEB AI. The pathobiology of perivascular adipose tissue (PVAT), the fourth layer of the blood vessel wall. Cardiovasc Pathol. 2022;61:107459. [9] PERSSON P, MARCHETTI M, FRIEDERICH-PERSSON M. Browning of perivascular adipose tissue prevents vascular dysfunction and reduces hypertension in angiotensin II-infused mice. Am J Physiol Regul Integr Comp Physiol. 2023;325(3):R290-R298. [10] KATSIKI N, MIKHAILIDIS DP. Perivascular Adipose Tissue: Pathophysiological Links With Inflammation, Atherosclerosis, and Thrombosis. Angiology. 2022;73(3):195-196. [11] KANWISCHER L, XU X, SAIFUDDIN AB, et al. Low levels of circulating methylated IRX3 are related to worse outcome after transcatheter aortic valve implantation in patients with severe aortic stenosis. Clin Epigenetics. 2023;15(1):149. [12] LIU W, OH Y, YIN W, et al. The Combinatorial Role of Iroquois Homebinatorial Role of Iroquois Homeobox Genes 3 and 4 in the Compaction of the Ventricular Myocardium. Can J Cardiol. 2021;37(10, Supplement):S5. [13] NAGEL S, MEYER C, POMMERENKE C. IRX-related homeobox gene MKX is a novel oncogene in acute myeloid leukemia. PLoS One. 2024;19(12): e0315196. [14] MØLLER AL, VASAN RS, LEVY D, et al. Integrated omics analysis of coronary artery calcifications and myocardial infarction: the Framingham Heart Study. Sci Rep. 2023;13(1):21581. [15] SMEMO S, TENA JJ, KIM KH, et al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature. 2014; 507(7492):371-375. [16] TU P, BHASIN S, HRUZ PW, et al. Genetic disruption of myostatin reduces the development of proatherogenic dyslipidemia and atherogenic lesions in Ldlr null mice. Diabetes. 2009;58(8):1739-1748. [17] ZHOU X, CHEN Y, CUI L, et al. Advances in the pathogenesis of psoriasis: from keratinocyte perspective.Cell Death Dis. 2022;13(1):81. [18] LI J, TIAN Z, ZHANG T, et al. Single-cell view and a novel protective macrophage subset in perivascular adipose tissue in T2DM. Cell Mol Biol Lett. 2024;29(1):148. [19] BONDAREVA O, RODRÍGUEZ-AGUILERA JR, OLIVEIRA F, et al. Single-cell profiling of vascular endothelial cells reveals progressive organ-specific vulnerabilities during obesity. Nat Metab. 2022;4(11):1591-1610. [20] TIMMONS JA, WENNMALM K, LARSSON O, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A. 2007; 104(11):4401-4406. [21] RAO J, DJEFFAL Y, CHAL J, et al. Reconstructing human brown fat developmental trajectory in vitro. Dev Cell. 2023;58(21):2359-2375. [22] CASTELLÁ M, BLASCO-ROSET A, PEYROU M, et al. Adipose tissue plasticity in pheochromocytoma patients suggests a role of the splicing machinery in human adipose browning. iScience. 2023;26(6):106847. [23] LIU H, WANG X, GAO H, et al. Physiological and pathological characteristics of vascular endothelial injury in diabetes and the regulatory mechanism of autophagy. Front Endocrinol (Lausanne). 2023;14:1191426. [24] TRAN KV, FITZGIBBONS T, MIN SY, et al. Distinct adipocyte progenitor cells are associated with regional phenotypes of perivascular aortic fat in mice. Mol Metab. 2018;9:199-206. [25] HOU N, DU G, HAN F, et al. Irisin Regulates Heme Oxygenase-1/Adiponectin Axis in Perivascular Adipose Tissue and Improves Endothelial Dysfunction in Diet-Induced Obese Mice. Cell Physiol Biochem. 2017;42(2):603-614. [26] GBD 2015 OBESITY COLLABORATORS, AFSHIN A, FOROUZANFAR MH, et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377(1):13-27. [27] JIANG M, REN X, HAN L, et al. Associations between sarcopenic obesity and risk of cardiovascular disease: A population-based cohort study among middle-aged and older adults using the CHARLS. Clin Nutr. 2024;43(3):796-802. [28] KOENEN M, HILL MA, COHEN P, et al. Obesity, Adipose Tissue and Vascular Dysfunction. Circ Res. 2021;128(7):951-968. [29] TING P, WANG T, FU M, et al. Prevalence and inequalities of obesity and associated complications in China: A multicentre nationwide survey. Public Health. 2024;237:97-106. [30] LI HF, LIU HT, CHEN PY, et al. Role of PVAT in obesity-related cardiovascular disease through the buffering activity of ATF3. iScience. 2022;25(12): 105631. [31] HUANG CL, HUANG YN, YAO L, et al. Thoracic perivascular adipose tissue inhibits VSMC apoptosis and aortic aneurysm formation in mice via the secretome of browning adipocytes. Acta Pharmacol Sin. 2023;44(2):345-355. [32] HAGBERG CE, SPALDING KL. White adipocyte dysfunction and obesity-associated pathologies in humans. Nat Rev Mol Cell Biol. 2024;25(4):333. [33] PEIU SN, ZUGUN-ELOAE F, STOICA B, et al. Obesity-Induced PVAT Dysfunction and Atherosclerosis Development: The Role of GHSR-1a in Increased Macrophage Infiltration and Adipocytokine Secretion. J Cardiovasc Dev Dis. 2025;12(3):87. [34] VAN DAM AD, BOON MR, BERBÉE JFP, et al. Targeting white, brown and perivascular adipose tissue in atherosclerosis development. Eur J Pharmacol. 2017;816:82-92. [35] SHI H, GOO B, KIM D, et al. Perivascular adipose tissue promotes vascular dysfunction in murine lupus. Front Immunol. 2023;14:1095034. [36] MU W, QIAN S, SONG Y, et al. BMP4-mediated browning of perivascular adipose tissue governs an anti-inflammatory program and prevents atherosclerosis. Redox Biol. 2021;43:101979. [37] BROWN RM, WANG L, FU A, et al. Irx3 promotes gap junction communication between uterine stromal cells to regulate vascularization during embryo implantation. Biol Reprod. 2022;106(5):1000-1010. [38] YAN XY, LUO YY, CHEN HJ, et al. IRX3 promotes adipose tissue browning and inhibits fibrosis in obesity-resistant mice. Int J Biochem Cell Biol. 2024; 175:106638. [39] YAO J, WU D, ZHANG C, et al. Macrophage IRX3 promotes diet-induced obesity and metabolic inflammation. Nat Immunol. 2021;22(10):1268-1279. [40] ZOU Y, LU P, SHI J, et al. IRX3 Promotes the Browning of White Adipocytes and Its Rare Variants are Associated with Human Obesity Risk. EBioMedicine. 2017;24:64-75. [41] TU Q, LIU X, YAO X, et al. RETSAT associates with DDX39B to promote fork restarting and resistance to gemcitabine based chemotherapy in pancreatic ductal adenocarcinoma. J Exp Clin Cancer Res. 2022;41(1):274. [42] JIANG X, HE Y, SHEN Q, et al. RETSAT Mutation Selected for Hypoxia Adaptation Inhibits Tumor Growth. Front Cell Dev Biol. 2021;9:744992. [43] KIEFER MF, MENG Y, YANG N, et al. Intestinal retinol saturase is implicated in the development of obesity and epithelial homeostasis upon injury. Am J Physiol Endocrinol Metab. 2024;327(2):E203-E216. [44] PANG XY, WANG S, JURCZAK MJ, et al. Retinol saturase modulates lipid metabolism and the production of reactive oxygen species. Arch Biochem Biophys. 2017;633:93-102. |
| [1] | Lyu Guoqing, Aizimaitijiang·Rouzi, Xiong Daohai. Irisin inhibits ferroptosis in human articular chondrocytes: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1359-1367. |
| [2] | Zhu Kuicheng, Du Chunyan, Zhang Jintao. Mechanism by which hairless gene mutation promotes white adipose tissue browning in hairless mice [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1424-1430. |
| [3] | Liu Kexin, , Hao Kaimin, Zhuang Wenyue, , Li Zhengyi. Autophagy-related gene expression in pulmonary fibrosis models: bioinformatic analysis and experimental validation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1129-1138. |
| [4] | Zhou Wen, Yang Hongwei. Molecular mechanism and natural drug screening for ferroptosis-targeted therapy in rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(23): 6051-6061. |
| [5] | Liao Guibin, Wu Yixuan, Tang Jing, Huang Jinke, Wang Jun, Yan Ziqi, Liu Shujun, Zhang Haiyan. Shared genetic basis and causal relationship between nutrition, nutritional status and inflammatory bowel disease [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(22): 5876-5885. |
| [6] | Tang Cen, Hu Wanqin. Establishing a diagnostic model for recurrent spontaneous abortion based on the levels of autophagy-related genes in the endometrium [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(22): 5728-5738. |
| [7] | Li Wenhui, Fan Weijing, Liu Guobin. Impact of Zi-Zhu ointment on the miRNA expression profile in mouse models of diabetic ulcers: a high-throughput sequencing analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(17): 4337-4346. |
| [8] | Lu Liwei, Huang Keqi, Chen Yueping, Zhuo Yinghong, Zhu Naihui, Wei Peng. Bioinformatics-based analysis of shared genes and associations in immune mechanisms between rheumatoid arthritis and Crohn’s disease [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(16): 4253-4264. |
| [9] | Zhou Man, Long Meiting, Xin Guoyan, Huang Mengjun, Yao Zhenglian, Zhao Huajuan, Shen Linqiang, Wu Xijun, Yang Xiaoyan. Bioinformatics screening and experimental verification of core genes in chronic myeloid leukemia and imatinib resistance [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(13): 3331-3342. |
| [10] | Tian Minghao, Liao Yehui, Zhou Wenyang, He Baoqiang, Leng Yebo, Xu Shicai, Zhou Jiajun, Li Yang, Tang Chao, Tang Qiang, Zhong Dejun . Neuroprotective regulation of the IRF9 gene after spinal cord injury: bioinformatics analysis combined with experimental validation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(11): 2712-2726. |
| [11] | Qi Xiang, Cao Shan, Chen Jian, Zhang Yijia, Liu Keke, Xu Zifu, Liu Wang, Fu Xiaoxiao, Yin Xiaolei. Screening of genes related to mitochondrial dysfunction and ferroptosis in atherosclerosis and target prediction of regulatory traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(10): 2641-2652. |
| [12] | Deng Keqi, Li Guangdi, Goswami Ashutosh, Liu Xingyu, He Xiaoyong. Screening and validation of Hub genes for iron overload in osteoarthritis based on bioinformatics [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1972-1980. |
| [13] | Liu Lin, Liu Shixuan, Lu Xinyue, Wang Kan. Metabolomic analysis of urine in a rat model of chronic myofascial trigger points [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1585-1592. |
| [14] | Zhao Jiacheng, Ren Shiqi, Zhu Qin, Liu Jiajia, Zhu Xiang, Yang Yang. Bioinformatics analysis of potential biomarkers for primary osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1741-1750. |
| [15] | Zhang Zhenyu, Liang Qiujian, Yang Jun, Wei Xiangyu, Jiang Jie, Huang Linke, Tan Zhen. Target of neohesperidin in treatment of osteoporosis and its effect on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1437-1447. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||