Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (22): 5650-5658.doi: 10.12307/2026.161
Previous Articles Next Articles
Cobalt chloride-induced hypoxic environment accelerates knee cartilage degeneration in New Zealand rabbits
Xu Peng1, Jiang Wei1, Yu You1, Lei Zhengliang1, Tian Yang1, Zhang Jie1, Liu Luchang2
- 1Orthopedic Center, 2Stomatology Center, The Second People’s Hospital of Yibin, Yibin Hospital of West China Hospital of Sichuan University, Yibin 644000, Sichuan Province, China
-
Received:2025-05-06Accepted:2025-08-28Online:2026-08-08Published:2025-12-26 -
Contact:Liu Luchang, Attending physician, Stomatology Center, The Second People’s Hospital of Yibin, Yibin Hospital of West China Hospital of Sichuan University, Yibin 644000, Sichuan Province, China -
About author:Xu Peng, PhD, Associate chief physician, Orthopedic Center, The Second People’s Hospital of Yibin, Yibin Hospital of West China Hospital of Sichuan University, Yibin 644000, Sichuan Province, China -
Supported by:Sichuan Provincial Natural Science Foundation (General Project), No. 2023NSFSC0546 (to XP)
CLC Number:
Cite this article
Xu Peng, Jiang Wei, Yu You, Lei Zhengliang, Tian Yang, Zhang Jie, Liu Luchang. Cobalt chloride-induced hypoxic environment accelerates knee cartilage degeneration in New Zealand rabbits[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(22): 5650-5658.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

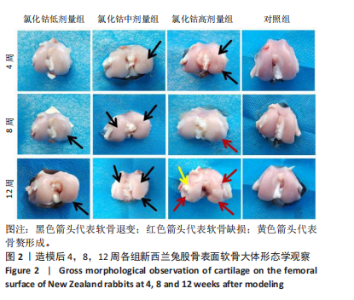
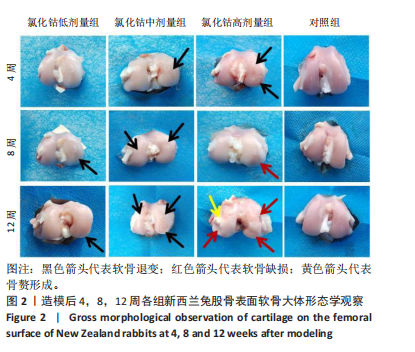
氯化钴低剂量组:术后4周时,股骨软骨完整性、光泽度尚可,软骨表面较为平整,未见骨赘或异常增生物形成;术后8周时,股骨外髁中央软骨骨质厚度变薄,软骨表面尚平整,光滑度有所下降,可见淡红色软骨下骨;术后12周时,软骨表面明显干燥,光泽度及平滑度进一步降低,股骨外髁中央及前下部均出现明显软骨退变,范围及程度较8周时更大,提示骨关节炎病理进程进一步加重。 氯化钴中剂量组:术后4周时,股骨外髁中央软骨表面干燥、色泽暗淡、平整度较差,软骨虽无缺损但可见明显软骨下骨成分;术后8周时,股骨外髁软骨退变进一步加重,可见细小软骨裂隙,平整度欠规则,股骨内髁中央部亦出现较轻微软骨损伤,表面有轻微颗粒感;术后12周时,股骨软骨多处退变,内外髁多范围累及,光泽度、平整度较前进一步降低,尤其在股骨外下髁可见一片状楔样软骨退变,甚至累及软骨下骨。 氯化钴高剂量组:术后4周时,股骨外髁软骨呈多处、多中心退变,表面干燥、光泽度低,可见明显深红色软骨下骨;术后8周时,股骨外髁中下部出现一较表浅软骨缺损,表面凹陷直达软骨下骨;术后12周时,股骨内外髁均出现较深软骨缺损,缺损深度深达软骨下骨,周围骨质凹凸不平,股骨内髁中部可见一明显凸起赘生物形成。 对照组:术后4,8,12周,股骨软骨平整度、光滑度、完整性均较好,未见明显的软骨退变或缺损。 2.3 软骨苏木精-伊红染色切片观察 见图3。"
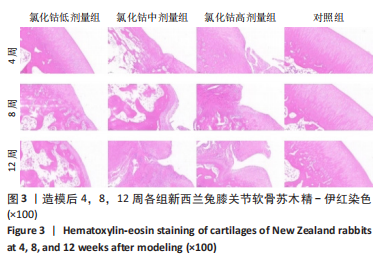

氯化钴低剂量组:术后4周,软骨表面平整光滑,细胞分布均匀一致、排列整齐、极性强,软骨厚度与对照组相似;术后8周,软骨层部分变薄,厚度不均,软骨细胞排列尚可,但整齐性和极性均不及4周时;术后12周,软骨层明显变薄,软骨细胞数量减少,部分细胞胞质出现肿胀,形态欠规则。 氯化钴中剂量组:术后4周,软骨中央出现一小缺损,但未达软骨下层,有部分软骨细胞肿胀明显,细胞数量减少;术后8周,软骨层破坏明显加重,部分细胞极性完全消失,排列紊乱,软骨裂隙进一步增大,部分累及软骨下层;术后12周,软骨层中部呈一“楔样”裂隙,深达软骨深层,靠近骨质部分的细胞数量明显减少。 氯化钴高剂量组:术后4周,软骨下表面出现明显裂隙伴部分塌陷,塌陷周围细胞排列紊乱,极性部分丧失;术后8周,靠近骨质部分的软骨细胞数量进一步减少,细胞裂隙进一步加深;术后12周,软骨破坏已深达软骨下层,形成一巨大裂隙,大量细胞染色缺失,细胞肿胀、极性消失、排列紊乱。 对照组:术后4,8,12周,软骨表面平整,细胞排列整齐、形态良好、极性一致,软骨下骨生长良好,未见软骨破坏及软骨裂隙。 2.4 软骨番红-固绿染色切片观察 见图4。 氯化钴低剂量组:术后4周,软骨表面光滑平整、软骨基质染色均匀一致,软骨细胞形态良好、排列"
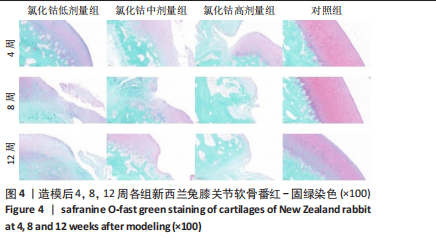
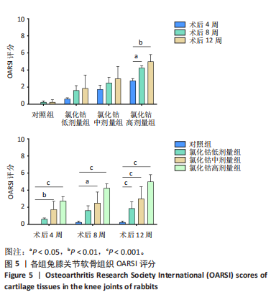
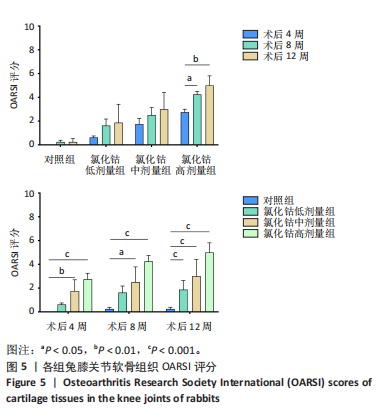
呈现明显极性,未见“潮线”紊乱及番红染色缺失;术后8周,软骨层变薄,软骨基质染色变浅,番红染色出现轻微不均,未出现失染现象;术后12周,软骨下部出现一较为明显的骨质破坏,周围软骨细胞排列紊乱,上部软骨面变薄,染色不均,软骨下方“潮线”轻微破坏,似不连续,软骨下骨未受明显侵袭。 氯化钴中剂量组:术后4周,软骨表面即出现一横向缺损,软骨表层明显破坏,平整度欠佳,软骨下层未受侵袭;术后8周,软骨中部出现一“楔样”裂隙且伴有番红染色缺失,软骨细胞排列较为紊乱,极性差,软骨浅、中、深层均受累及,软骨下骨有轻微破坏;术后12周,软骨表层出现一“圆弧状”缺损,细胞“潮线”不完整,软骨下骨破坏进一步加重。 氯化钴高剂量组:术后4周,软骨缺损严重,出现明显糜烂及裂缝,软骨细胞排列紊乱,极性较差;术后8周,软骨层缺损进一步加重,出现番红染色大量缺失,“潮线”破坏范围较大,软骨细胞缺失较多;术后12周,骨质表层几乎不见番红染色,“潮线”破坏殆尽,极不连续,软骨浅、中、深层及钙化软骨均受破坏,软骨下骨破坏严重。 对照组:术后4,8,12周软骨表面平整光滑、未见番红染色缺失,软骨各层及软骨下骨形态良好,软骨细胞排列极性强,未见软骨破坏及软骨裂隙。 2.5 OARSI 软骨评分 组间比较:在同一时间点,随着造模试剂氯化钴溶液浓度的升高,低、中、高剂量氯化钴组OARSI评分逐渐升高,且显著高于对照组,差异有显著性意义(P < 0.05)。 组内比较:在造模浓度相同的情况下,随着造模时间的延长,低、中、高剂量氯化钴组OARSI评分逐渐升高,且显著高于对照组,差异有显著性意义 (P < 0.05),但对照组评分随着时间的延长不具有统计学意义(P > 0.05),见图5。"

2.6 白细胞介素1 、肿瘤坏死因子 α免疫组化染色结果 见图6,7。 氯化钴低剂量组:术后4周,软骨表面结构较为完整光滑,软骨浅中、深层细胞排列较为整齐,有少许细胞极性不一,白细胞介素1及肿瘤坏死因子α阳性细胞的表达量稍增加;术后8周,软骨表面较为毛躁,软骨细胞出现聚集现象,呈团簇样集中,细胞极性较不明显,深部可见部分褐色颗粒聚集,白细胞介素1及肿瘤坏死因子α阳性细胞的表达量进一步增加;术后12周,可见软骨表面有一浅线样裂隙,软骨表面较粗糙,软骨细胞聚集成团,褐色颗粒进一步增多,白细胞介素1及肿瘤坏死因子α阳性细胞的表达量显著增加。 氯化钴中剂量组:术后4周,软骨表面毛躁程度较低剂量组重,细胞极性不明显,褐色颗粒广泛分布,基质染色分布较不均匀;术后8周,软骨退变进一步加重,软骨细胞排列不整齐,极性进一步丧失,基质染色及分布极不均匀;术后12周,软骨表面出现一“楔样”深裂隙,深达软骨下骨,裂隙周围有大量白细胞介素1及肿瘤坏死因子α阳性细胞聚集,提示炎症状态较明显。 氯化钴高剂量组:术后4周,软骨表面出现一较明显缺损及片状脱落,软骨表面粗糙不平整,软"

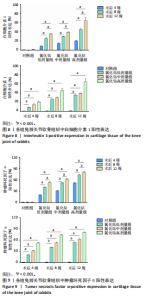
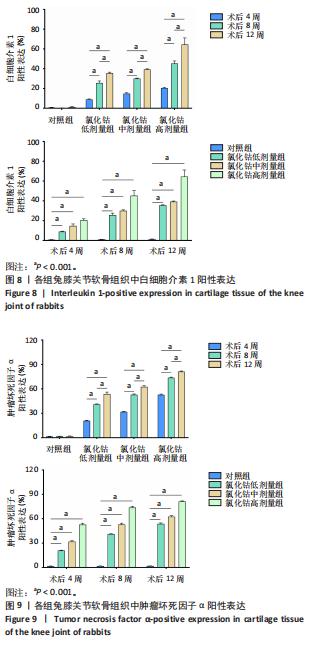
骨细胞大量聚集成团簇样,深部褐色颗粒染色明显;术后8周,软骨缺损进一步加重,软骨表层及中层出现大量白细胞介素1及肿瘤坏死因子α阳性细胞聚集;术后12周,软骨缺损严重,难以分辨正常的软骨表层,细胞内出现大量褐色颗粒,基质部分染色极不均匀,阳性细胞表达较多。 对照组:术后4,8,12周,软骨表面较为光滑平整,白细胞介素1及肿瘤坏死因子α阳性细胞表达较少。 2.7 白细胞介素1、肿瘤坏死因子α染色强度分析 组间比较:在术后同一时间,随着造模试剂氯化钴溶液浓度的升高,低、中、高剂量氯化钴组白细胞介素1及肿瘤坏死因子α阳性表达均逐渐升高,且显著高于对照组,差异有显著性意义(P < 0.01)。组内比较:在同一造模浓度下,随着造模时间的延长,从第4周到第12周,白细胞介素1及肿瘤坏死因子α阳性表达逐渐升高,显著高于对照组,差异有显著性意义(P < 0.01),见图8,9。"
| [1] 张莹莹,李旭东,杨佳娟,等.中国 40 岁及以上人群骨关节炎患病率的 Meta 分 析[J].中国循证医学杂志,2021,21(4):407-414. [2] PERRUCCIO AV, YOUNG JJ, WILFONG JM, et al. Osteoarthritis year in review 2023: Epidemiology & therapy. Osteoarthritis Cartilage. 2024;32(2):159-165. [3] ROELOFS AJ, DE BARI C. Osteoarthritis year in review 2023: Biology. Osteoarthritis Cartilage. 2024;32(2):148-158. [4] 关尚琪,滕菲,张志毅,等.骨关节炎流行病学研究进展 [J].中华内科杂志,2017,56(6):450-452. [5] 吴玥,薛婧,魏强,等.国家动物模型资源共享信息平台的建立[J].中国实验动物学报,2022,30(8):1080-1086. [6] LIAO Q, XIA W, CHEN J, et al. Circular RNA DNAH14 molecular mechanism in an experimental model of hepatocellular carcinoma treated with Cobalt chloride to mimic the hypoxia-like response of transcatheter arterial chemoembolization. Sci Rep. 2024;14(1):1992. [7] AMARA R, ZEINEH N, MONGA S, et al. The Effect of the Classical TSPO Ligand PK 11195 on In Vitro Cobalt Chloride Model of Hypoxia-like Condition in Lung and Brain Cell Lines. Biomolecules. 2022;12(10):1397. [8] SADRI M, DELBANDI AA, RASHIDI N, et al. Cobalt Chloride-induced Hypoxia Can Lead SKBR3 and HEK293T Cell Lines toward Epithelial-mesenchymal Transition. Iran J Allergy Asthma Immunol. 2022;21(4):449-457. [9] LU J, TANG X, ZHANG D, et al. Didang Tang inhibits intracerebral hemorrhage-induced neuronal injury via ASK1/MKK7/JNK signaling pathway, network pharmacology-based analyses combined with experimental validation. Heliyon. 2022;8(11):e11407. [10] 李欣怡,王洪伸,谭傲威,等.氯化钴诱导体外人髓核细胞缺氧模型的建立[J].广东医学,2023,44(6):729-734. [11] 李晓娟,李浩,马永壮,等.缺氧环境通过 HIF-1α/YAP 信号促进大鼠生长板软骨细胞表型维持[J].骨科,2019,10(2):134-139. [12] 李晓峰,罗世兴,赵劲民,等.芒果苷对缺氧损伤骨髓间充质干细胞凋亡的保护[J].中国组织工程研究,2013,17(49):8481-8487. [13] 熊波涵,卢晓君,薛文强,等.内减张技术辅助前交叉韧带重建对滇南小耳猪关 节软骨的保护作用[J].中国组织工程研究,2024, 28(14):2221-2226. [14] GLASSON SS, CHAMBERS MG, VAN DEN BERG WB, et al. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18 Suppl 3:S17-23. [15] KNIGHTS AJ, REDDING SJ, MAERZ T. Inflammation in osteoarthritis: the latest progress and ongoing challenges. Curr Opin Rheumatol. 2023;35(2):128-134. [16] NEDUNCHEZHIYAN U, VARUGHESE I, SUN AR, et al. Obesity, Inflammation, and Immune System in Osteoarthritis. Front Immunol. 2022;13:907750. [17] SAFIRI S, KOLAHI AA, SMITH E, et al. Global,regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis. 2020;79(6): 819-828. [18] HUNTER DJ, SCHOFIELD D, CALLANDER E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014; 10(7):437-441. [19] WALLACE IJ, WORTHINGTON S, FELSON DT, et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci U S A. 2017;114(35):9332-9336. [20] WU J, PAN Y, YU Y, et al. Axial Compressive Loading Attenuates Early Osteoarthritis by Reducing Subchondral Bone Remodeling. Am J Sports Med. 2023;51(7):1752-1764. [21] TAKAHASHI I, TAKEDA K, TOYAMA T, et al. Histological and immunohistochemical analyses of articular cartilage during onset and progression of pre- and early-stage osteoarthritis in a rodent model. Sci Rep. 2024;14(1):10568. [22] YU Y, KIM SM, PARK K, et al. Therapeutic Nanodiamonds Containing Icariin Ameliorate the Progression of Osteoarthritis in Rats. Int J Mol Sci. 2023;24(21):15977. [23] 刘晓辰, 付维力.骨关节炎动物模型的选择[J].中国组织工程研究, 2020,24(11):1769-1776. [24] 张立,王培民.膝骨关节炎动物模型的选择[J].世界中西医结合杂志,2014,9(7):782-786. [25] 彭诗,刘娟,朱兆荣,等.电针对木瓜蛋白酶致骨关节炎模型犬血清中 IL-1、TNF-α、SP 的影响[J].中国兽医杂志,2014,50(6):52-54. [26] KELLY S, DUNHAM JP, MURRAY F, et al. Spontaneous firing in C-fibers and increased mechanical sensitivity in A-fibers of knee joint-associated mechanoreceptive primary afferent neurones during MIA-induced osteoarthritis in the rat. Osteoarthritis Cartilage. 2012;20(4):305-313. [27] 曹斌,李彦林,李晓林,等.骨关节炎的转基因动物模型[J].中国组织工程研究与临床康复,2011,15(7):1269-1272. [28] MCCOY AM. Animal Models of Osteoarthritis: Comparisons and Key Considerations. Vet Pathol. 2015;52(5):803-818. [29] DA SILVA LA, THIRUPATHI A, COLARES MC, et al. The effectiveness of treadmill and swimming exercise in an animal model of osteoarthritis. Front Physiol. 2023;14:1101159. [30] CHRISTIANSEN BA, CHAN DD, VAN DER MEULEN MCH, et al. Small-Animal Compression Models of Osteoarthritis. Methods Mol Biol. 2023; 2598:345-356. [31 ] 吴伟,李慧,邹军,等.骨关节炎小动物模型的制备及量表评价[J].中国组织工程研究,2017,21(28):4529-4535. [32] 刘宇涵,樊渝江,王启光.早期创伤性膝骨关节炎动物模型构建方案的比较 [J].中国组织工程研究,2024,28(4):542-549. [33] YOUNG C, KOBAYASHI T. Limited roles of Piezo mechanosensing channels in articular cartilage development and osteoarthritis progression. Osteoarthritis Cartilage. 2023;31(6):775-779. [34] THAMPI P, SEABAUGH KA, PEZZANITE LM, et al. A pilot study to determine the optimal dose of scAAVIL-1ra in a large animal model of post-traumatic osteoarthritis. Gene Ther. 2023;30(12):792-800. [35] 孙雪莲,刘渊,周红海.牛膝总皂苷对兔膝骨关节炎软骨组织形态变化及关节液中IL-1β、TGF-β1含量的影响[J].中药新药与临床药理,2016,27(3):321-326. [36] 李情,薛平聚,张小琴,等.不同施灸时间对膝骨关节炎大鼠膝关节软骨组织形态及TNF-α和IL-10表达的影响[J]. 针灸推拿医学(英文版),2023,21(3):187-196. [37] LI X, CHEN W, LIU D, et al. Pathological progression of osteoarthritis: a perspective on subchondral bone. Front Med. 2024;18(2):237-257. [38] OLÁH T, CUCCHIARINI M, MADRY H. Temporal progression of subchondral bone alterations in OA models involving induction of compromised meniscus integrity in mice and rats: A scoping review. Osteoarthritis Cartilage. 2024;32(10):1220-1234. [39] WOJDASIEWICZ P, PONIATOWSKI ŁA, SZUKIEWICZ D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. [40] LIANG S, LV ZT, ZHANG JM, et al. Necrostatin-1 Attenuates Trauma-Induced Mouse Osteoarthritis and IL-1β Induced Apoptosis via HMGB1/TLR4/SDF-1 in Primary Mouse Chondrocytes. Front Pharmacol. 2018;9:1378. |
| [1] | Lyu Guoqing, Aizimaitijiang·Rouzi, Xiong Daohai. Irisin inhibits ferroptosis in human articular chondrocytes: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1359-1367. |
| [2] | Bu Yangyang, Ning Xinli, Zhao Chen. Intra-articular injections for the treatment of osteoarthritis of the temporomandibular joint: different drugs with multiple combined treatment options [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1215-1224. |
| [3] | Zhang Qian, Huang Dongfeng. Weighted gene co-expression network analysis combined with machine learning to screen and validate biomarkers for osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1096-1105. |
| [4] | Chen Yixian, Chen Chen, Lu Liheng, Tang Jinpeng, Yu Xiaowei. Triptolide in the treatment of osteoarthritis: network pharmacology analysis and animal model validation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 805-815. |
| [5] | Yang Jing, Wang Houmei, Wang Yi, Song Min, Ren Jie, Dai Lujun, Xiao Ziwen. Constructing a rat animal model of pelvic organ prolapse: a comparison of three modeling methods [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 864-872. |
| [6] | Xie Ziying, Li Songbo, Li Jianwen, Yin Yuchao, Zheng Baichuan, Hu Chengshang. Animal experimental study on the treatment of lumbar intervertebral disc degeneration with Chinese herbal compound: species selection, modeling method and drug administration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(23): 5934-5942. |
| [7] | Wan Ziyi, Jiang Mengyu, Zhou Yuehui, Xue Yuxuan, Wei Yangwenxiang, Zhou Chi. Articular cartilage lesions at different stages of steroid-induced osteonecrosis of the femoral head: characteristics and mechanisms of crescent sign formation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(22): 5659-5670. |
| [8] | Wang Xinyue, Li Hongli, Guo Chunhui, Chen Jibing, Yu Hua. Changes in the expression of six microRNAs in ovarian tissue from animal models of premature ovarian failure and in peripheral blood of patients with premature ovarian failure [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(18): 4675-4684. |
| [9] | Li Feifan, Zhang Yibo, Wang Jing, Zhu Jinqiang, Zheng Wenke. Comparison and evaluation of three methods for preparing insomnia mouse models [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(18): 4685-4693. |
| [10] | Liao Xingzhuan, Li Guangdi, Wu Yabin, Liu Xingyu, Wan Jiajia. Molecular mechanisms underlying non-coding RNA regulation of ferroptosis in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(18): 4713-4725. |
| [11] | Fu Jingyue, Zhou Qinfeng, Li Muzhe, Ma Yong, Pan Yalan, Sun Jie, Huang Xiangyang, Guo Yang. Preparation and evaluation of an animal model of osteoporosis and osteoarthritis comorbidity in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(17): 4299-4308. |
| [12] | Peng Hao, Jiang Yang, Song Yanping, Wu Quan, Yao Na, Chen Qigang, Shen Zhen. H-type angiogenesis and its role in various skeletal disease animal models [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(16): 4154-4165. |
| [13] | Chen Xinlong, Meng Tao, Wang Yaomin, Zhang Kefan, Li Jian, Shi Hui, Zhang Chenchen. Ferroptosis inhibitors in the treatment of osteoarthritis: diversity and multitarget characteristics [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(16): 4166-4179. |
| [14] | Wang Zhengye, Liu Wanlin, Zhao Zhenqun. Multidimensional target regulation of vascular endothelial growth factor A in articular cartilage development [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(16): 4193-4203. |
| [15] | Li Yijin, Li Jiahao, Zhang Haitao, Huang Yiwei, Chen Jinlun, Zeng Yirong, Feng Wenjun. GJK Tablets intervene in cartilage homeostasis to protect articular cartilage of mice with knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(12): 2994-3004. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||