Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (31): 6727-6732.doi: 10.12307/2025.622
Previous Articles Next Articles
Optimal parameters for physical interventions in bone marrow mesenchymal stem cell differentiation
Liu Xun, Ouyang Hougan, Pan Rongbin, Wang Zi, Yang Fen, Tian Jiaxuan
- Jiangxi University of Chinese Medicine, Nanchang 330004, Jiangxi Province, China
-
Received:2024-04-30Accepted:2024-07-10Online:2025-11-08Published:2025-02-25 -
Contact:Ouyang Hougan, MD, Professor, Doctoral supervisor, Jiangxi University of Chinese Medicine, Nanchang 330004, Jiangxi Province, China -
About author:Liu Xun, Master candidate, Jiangxi University of Chinese Medicine, Nanchang 330004, Jiangxi Province, China -
Supported by:National Natural Science Foundation of China, No. 82260862 (to OYHG)
CLC Number:
Cite this article
Liu Xun, Ouyang Hougan, Pan Rongbin, Wang Zi, Yang Fen, Tian Jiaxuan . Optimal parameters for physical interventions in bone marrow mesenchymal stem cell differentiation[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6727-6732.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
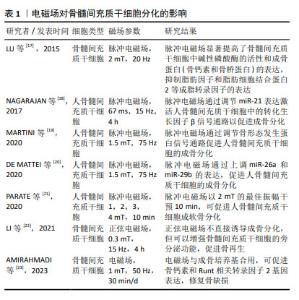
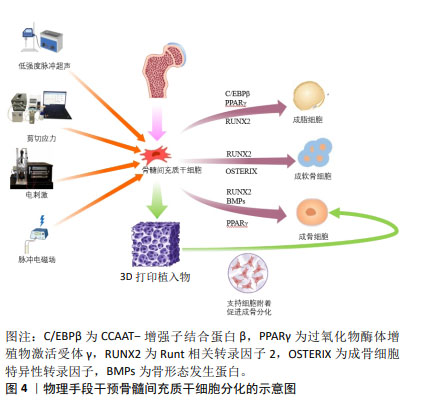
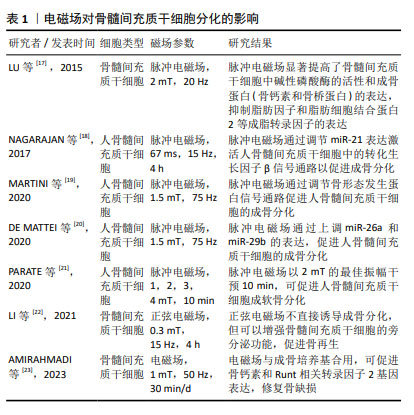
2.2 影响骨髓间充质干细胞成骨、成软骨分化的相关物理因素及作用机制 2.2.1 电磁场 电磁场作为电磁作用的一种媒介,对骨组织和成骨细胞均有着重要的影响,常用于脊髓损伤和骨折的辅助治疗。电磁场对骨髓间充质干细胞的增殖和分化具有积极作用,TU等[15]研究表明电磁场预处理后骨髓间充质干细胞的成骨和成软骨分化能力增强,成脂分化能力减弱,电磁场暴露联合骨髓间充质干细胞对大鼠胫骨骨不连具有显著的治疗作用。脉冲电磁场是脉冲电流通过线圈时在线圈中所产生的一种瞬态电磁场,利用脉冲发生装置的可调性产生特定的脉冲电流,从而产生特定频率、特定强度、特定上升时间及特定脉宽的脉冲电磁场。研究表明,脉冲电磁场可改善骨微结构[16],上调骨髓间充质干细胞中Runt相关转录因子2和碱性磷酸酶的表达,同时,脉冲电磁场干预可通过降低Beclin1、LC3、P62的表达,抑制骨髓间充质干细胞的自噬,为干细胞提供适度自噬水平从而促进成骨分化。研究表明,电磁场对干细胞分化具有积极作用,然而电磁场的场强度、场频率、持续时间和应用方法仍无统一的标准,需要在未来的工作中进行更多的探究。 文章总结了电磁场对骨髓间充质干细胞成骨、成软骨分化的研究进展[17-23],见表1。 "
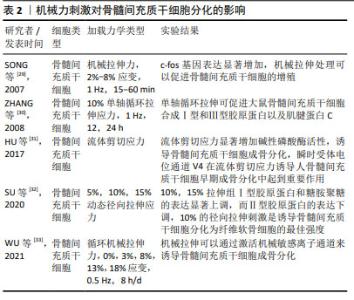
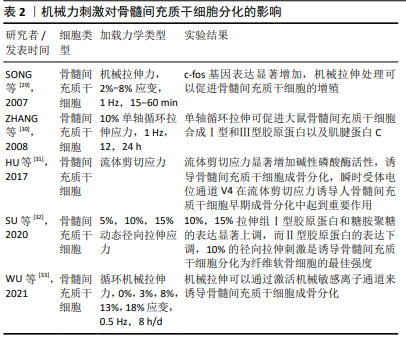
2.2.2 机械力刺激 在人体中,骨组织与细胞代谢受到多重力学刺激的影响,包括剪切应力、静水压力、流体动力压力、拉伸应力等,骨组织感知机械应力,将其转化为生化信号,通过分子机制将其传导到细胞内,并最终诱导细胞反应,如启动关键基因表达的信号通路、蛋白质合成、细胞表型修饰等。研究表明,对骨髓间充质干细胞施加牵张应力刺激可调控Notch1信号通路,使Notch1和Jagged1蛋白表达显著升高,从而促进骨髓间充质干细胞的增殖和成骨分化,抑制成脂分化[24-25]。ZAYZAFOON等[26]发现微重力环境可抑制人骨髓间充质干细胞成骨分化,并促进成脂分化。BECQUART等[27]对骨髓间充质干细胞给予剪切应力刺激可激活细胞外信号调节激酶1/2信号通路,促进骨髓间充质干细胞成骨分化。REIPRICH等[28]将人骨髓间充质干细胞暴露于10 Pa的流体剪切应力下,发现流体剪切应力可上调人骨髓间充质干细胞中骨矿化相关基因的表达。 文章总结了机械力刺激对骨髓间充质干细胞成骨、成软骨分化的研究进展[29-33],见表2。 "
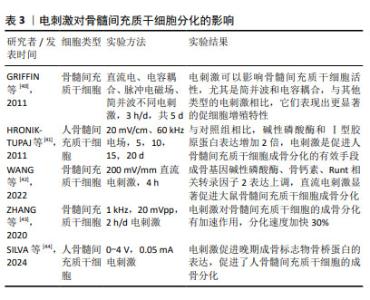
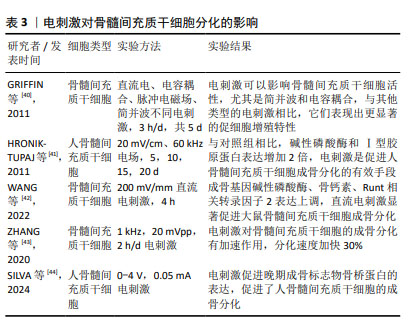
2.2.3 电刺激 近年来,电刺激对干细胞诱导分化具有一定的促进作用,施加一定电刺激可适当促进间充质干细胞的成骨分化。陈艳梅等[34]研究发现,通过给予物理电刺激,可激活磷脂酰肌醇-3-羟激酶(phosphatidylinositol 3- hydroxykinase,PI3K)、丝氨酸/苏氨酸蛋白激酶B(serine/ threonine protein kinase,AkT)通路和细胞外调节蛋白激酶(extracellular regulated protein kinases,ERK)通路,从而调控骨髓间充质干细胞向平滑肌细胞转化。HU等[35]发现直流电场治疗可上调成骨分化相关基因Runt相关转录因子2的表达,促进骨髓间充质干细胞成骨分化。LEPPIK等[36]研究表明,间充质干细胞与支架暴露在体外电刺激3周可显著促进其成骨分化,在体内实验中也可以一定程度上促进骨形成及成骨相关基因表达。UZIELIENE等[37]发现在不存在生长因子的情况下,仅使用电刺激也能有效诱导骨髓间充质干细胞成软骨分化。 电刺激方法已被广泛应用于骨组织工程研究中,能促进细胞增殖和分化。YU等[38]开发的新型耦合生物纳米发电机(HCBG),不仅克服了一般植入式自供电发电机结构复杂、能源不稳定的缺点,而且通过上调胞质钙离子来激活成骨分化,实现了电刺激增强骨髓间充质干细胞的体外成骨分化和体内骨再生。SHEN等[39]将新型压电聚偏二氟乙烯-三氟乙烯层涂覆在氧化铟锡平面微电极上,发现电刺激能够通过钙介导的蛋白激酶C信号通路调节骨髓间充质干细胞的成骨分化。 文章总结了电刺激对骨髓间充质干细胞成骨、成软骨分化的研究进展[40-44],见表3。 "
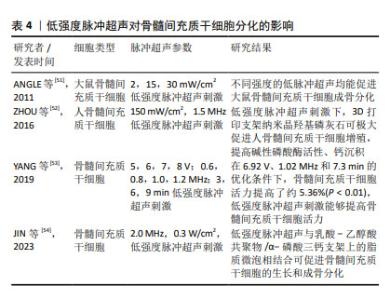
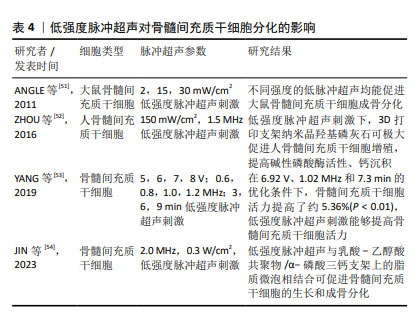
2.2.4 低强度脉冲超声 低强度脉冲超声是一种安全有效的加速骨愈合的疗法[45],具有机械效应、空化效应、热效应等生物物理学特性,已广泛应用于治疗骨折、关节炎、肌腱和韧带损伤以及其他疾病[46],研究发现低强度脉冲超声可以改善干细胞的治疗效果[47]。 细胞迁移是细胞的基本生命活动之一,受到细胞内外信号源的刺激和调节,研究表明,低强度脉冲超声可以通过调节自噬来促进骨髓间充质干细胞迁移和治疗骨关节炎[48],CHEN等[49]研究发现低强度脉冲超声可通过激活黏着斑激酶和细胞外调控激酶1/2信号通路促进骨髓间充质干细胞在体内和体外的迁移。此外,WANG等[42]发现使用强度为30 mW/cm2的低强度脉冲超声干预3-7 d, 可显著促进骨髓间充质干细胞向牙槽骨缺损区的迁移和归巢,并不同程度地促进Ⅰ型胶原蛋白和骨桥蛋白的表达,提示低强度脉冲超声可促进骨髓间充质干细胞的成骨分化。CHEN等[50]结合压电BaTiO3涂层Ti6Al4V支架和低强度脉冲超声,使得支架具备良好的力电响应性,压电与超声共刺激来触发电流上调成骨相关基因表达。 文章总结了低强度脉冲超声对骨髓间充质干细胞成骨、成软骨分化的研究进展[51-54],见表4。 "
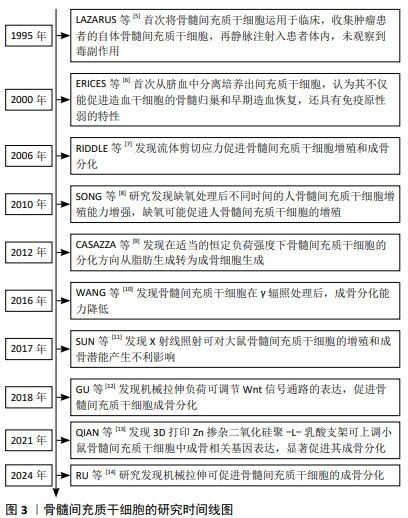
| [1] 王帅,张霄雁,李哲海.骨髓间充质干细胞成骨细胞分化研究进展[J].医学综述,2015,21(12):2137-2139. [2] MARUYAMA M, RHEE C, UTSUNOMIYA T, et al. Modulation of the Inflammatory Response and Bone Healing. Front Endocrinol (Lausanne). 2020;11:386. [3] WU D, CHANG X, TIAN J, et al. Bone mesenchymal stem cells stimulation by magnetic nanoparticles and a static magnetic field: release of exosomal miR-1260a improves osteogenesis and angiogenesis. J Nanobiotechnology. 2021;19(1):209. [4] DING P, GAO C, GAO Y, et al. Osteocytes regulate senescence of bone and bone marrow. Elife. 2022;11:e81480. [5] LAZARUS HM, HAYNESWORTH SE, GERSON SL, et al. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16(4):557-564. [6] ERICES A, CONGET P, MINGUELL JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109(1):235-242. [7] RIDDLE RC, TAYLOR AF, GENETOS DC, et al. MAP kinase and calcium signaling mediate fluid flow-induced human mesenchymal stem cell proliferation. Am J Physiol Cell Physiol. 2006;290(3):C776-784. [8] SONG WW, BAI H, WANG CB, et al. Effects of hypoxia on the proliferation of human bone marrow mesenchymal stem cells. Zhonghua Yi Xue Za Zhi. 2010;90(30):2149-2152. [9] CASAZZA K, HANKS LJ, HIDALGO B, et al. Short-term physical activity intervention decreases femoral bone marrow adipose tissue in young children: a pilot study. Bone. 2012;50(1):23-27. [10] WANG Y, ZHU G, WANG J, et al. Irradiation alters the differentiation potential of bone marrow mesenchymal stem cells. Mol Med Rep. 2016;13(1):213-223. [11] SUN R, ZHU G, WANG J, et al. Indirect effects of X-irradiation on proliferation and osteogenic potential of bone marrow mesenchymal stem cells in a local irradiated rat model. Mol Med Rep. 2017;15(6): 3706-3714. [12] GU Q, TIAN H, ZHANG K, et al. Wnt5a/FZD4 Mediates the Mechanical Stretch-Induced Osteogenic Differentiation of Bone Mesenchymal Stem Cells. Cell Physiol Biochem. 2018;48(1):215-226. [13] QIAN G, ZHANG L, WANG G, et al. 3D Printed Zn-doped Mesoporous Silica-incorporated Poly-L-lactic Acid Scaffolds for Bone Repair. Int J Bioprint. 2021;7(2):346. [14] RU Y, GU H, SUN L, et al. Mechanical Stretch-Induced ATP Release from Osteocytes Promotes Osteogenesis of Bone Marrow Mesenchymal Stem Cells. Discov Med. 2024;36(182):494-508. [15] TU C, XIAO Y, MA Y, et al. The legacy effects of electromagnetic fields on bone marrow mesenchymal stem cell self-renewal and multiple differentiation potential. Stem Cell Res Ther. 2018;9(1):215. [16] 肖豪,黄福锦,黄夏荣,等.脉冲电磁场抑制骨质疏松大鼠骨髓间充质干细胞自噬的机制研究[J].中国康复医学杂志,2023,38(10): 1351-1357. [17] LU T, HUANG YX, ZHANG C, et al. Effect of pulsed electromagnetic field therapy on the osteogenic and adipogenic differentiation of bone marrow mesenchymal stem cells. Genet Mol Res. 2015;14(3): 11535-11542. [18] SELVAMURUGAN N, HE Z, RIFKIN D, et al. Pulsed Electromagnetic Field Regulates MicroRNA 21 Expression to Activate TGF-β Signaling in Human Bone Marrow Stromal Cells to Enhance Osteoblast Differentiation. Stem Cells Int. 2017;2017:2450327. [19] MARTINI F, PELLATI A, MAZZONI E, et al. Bone Morphogenetic Protein-2 Signaling in the Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells Induced by Pulsed Electromagnetic Fields. Int J Mol Sci. 2020;21(6):2104. [20] DE MATTEI M, GRASSILLI S, PELLATI A, et al. Pulsed Electromagnetic Fields Modulate miRNAs During Osteogenic Differentiation of Bone Mesenchymal Stem Cells: a Possible Role in the Osteogenic-angiogenic Coupling. Stem Cell Rev Rep. 2020;16(5):1005-1012. [21] PARATE D, KADIR ND, CELIK C, et al. Pulsed electromagnetic fields potentiate the paracrine function of mesenchymal stem cells for cartilage regeneration. Stem Cell Res Ther. 2020;11(1):46. [22] LI W, LIU W, WANG W, et al. Sinusoidal electromagnetic fields accelerate bone regeneration by boosting the multifunctionality of bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2021;12(1):234. [23] AMIRAHMADI F, HAJI GHASEM KASHANI M, NASIRI M, et al. Osteogenic effect of electromagnetic fields on stem cells derived from rat bone marrow cultured in osteogenic medium versus conditioned medium in vitro. Cell Tissue Bank. 2023;24(2):317-328. [24] 邓海艳,孙江伟,古丽再努·依不拉音,等.牵张应力调控Notch1信号通路促进大鼠BMSCs增殖和成骨分化[J].中国骨质疏松杂志, 2023,29(6):802-806+824. [25] 钱岳鹏.miR-140-5P对应力作用下的BMSCs成骨与成脂分化调控作用的研究[D].广州:南方医科大学,2019. [26] ZAYZAFOON M, GATHINGS WE, MCDONALD JM. Modeled microgravity inhibits osteogenic differentiation of human mesenchymal stem cells and increases adipogenesis. Endocrinology. 2004;145(5):2421-2432. [27] BECQUART P, CRUEL M, HOC T, et al. Human mesenchymal stem cell responses to hydrostatic pressure and shear stress. Eur Cell Mater. 2016;31:160-173. [28] REIPRICH S, AKOVA E, ASZÓDI A, et al. Hyaluronan Synthases’ Expression and Activity Are Induced by Fluid Shear Stress in Bone Marrow-Derived Mesenchymal Stem Cells. Int J Mol Sci. 2021;22(6):3123. [29] SONG G, JU Y, SHEN X, et al. Mechanical stretch promotes proliferation of rat bone marrow mesenchymal stem cells. Colloids Surf B Biointerfaces. 2007;58(2):271-277. [30] ZHANG L, KAHN CJ, CHEN HQ, et al. Effect of uniaxial stretching on rat bone mesenchymal stem cell: orientation and expressions of collagen types I and III and tenascin-C. Cell Biol Int. 2008;32(3):344-352. [31] HU K, SUN H, GUI B, et al. TRPV4 functions in flow shear stress induced early osteogenic differentiation of human bone marrow mesenchymal stem cells. Biomed Pharmacother. 2017;91:841-848. [32] SU X, WANG J, KANG H, et al. Effects of dynamic radial tensile stress on fibrocartilage differentiation of bone marrow mesenchymal stem cells. Biomed Eng Online. 2020;19(1):8. [33] WU T, YIN F, WANG N, et al. Involvement of mechanosensitive ion channels in the effects of mechanical stretch induces osteogenic differentiation in mouse bone marrow mesenchymal stem cells. J Cell Physiol. 2021;236(1):284-293. [34] 陈艳梅,刘琦石,王莉辉,等.电刺激调控骨髓间充质干细胞向平滑肌细胞分化作用及机制研究[J].生物医学工程与临床,2022, 26(2):137-142. [35] HU WW, CHEN TC, TSAO CW, et al. The effects of substrate-mediated electrical stimulation on the promotion of osteogenic differentiation and its optimization. J Biomed Mater Res B Appl Biomater. 2019; 107(5):1607-1619. [36] LEPPIK L, ZHIHUA H, MOBINI S, et al. Combining electrical stimulation and tissue engineering to treat large bone defects in a rat model. Sci Rep. 2018;8(1):6307. [37] UZIELIENE I, BERNOTAS P, MOBASHERI A, et al. The Role of Physical Stimuli on Calcium Channels in Chondrogenic Differentiation of Mesenchymal Stem Cells. Int J Mol Sci. 2018;19(10):2998. [38] YU B, QIAO Z, CUI J, et al. A host-coupling bio-nanogenerator for electrically stimulated osteogenesis. Biomaterials. 2021;276:120997. [39] SHEN S, HE X, CHEN X, et al. Enhanced osteogenic differentiation of mesenchymal stem cells on P(VDF-TrFE) layer coated microelectrodes. J Biomed Mater Res B Appl Biomater. 2021;109(12):2227-2236. [40] GRIFFIN M, IQBAL SA, SEBASTIAN A, et al. Degenerate wave and capacitive coupling increase human MSC invasion and proliferation while reducing cytotoxicity in an in vitro wound healing model. PLoS One. 2011;6(8):e23404. [41] HRONIK-TUPAJ M, RICE WL, CRONIN-GOLOMB M, et al. Osteoblastic differentiation and stress response of human mesenchymal stem cells exposed to alternating current electric fields. Biomed Eng Online. 2011;10:9. [42] WANG Y, LI J, ZHOU J, et al. Low-intensity pulsed ultrasound enhances bone marrow-derived stem cells-based periodontal regenerative therapies. Ultrasonics. 2022;121:106678. [43] ZHANG Z, ZHENG T, ZHU R. Microchip with Single-Cell Impedance Measurements for Monitoring Osteogenic Differentiation of Mesenchymal Stem Cells under Electrical Stimulation. Anal Chem. 2020;92(18):12579-12587. [44] SILVA JC, MENESES J, GARRUDO FFF, et al. Direct coupled electrical stimulation towards improved osteogenic differentiation of human mesenchymal stem/stromal cells: a comparative study of different protocols. Sci Rep. 2024;14(1):5458. [45] PALANISAMY P, ALAM M, LI S, et al. Low-Intensity Pulsed Ultrasound Stimulation for Bone Fractures Healing: A Review. J Ultrasound Med. 2022;41(3):547-563. [46] LAI WC, IGLESIAS BC, MARK BJ, et al. Low-Intensity Pulsed Ultrasound Augments Tendon, Ligament, and Bone-Soft Tissue Healing in Preclinical Animal Models: A Systematic Review. Arthroscopy. 2021;37(7):2318-2333.e3. [47] TAN Y, GUO Y, REED-MALDONADO AB, et al. Low-intensity pulsed ultrasound stimulates proliferation of stem/progenitor cells: what we need to know to translate basic science research into clinical applications. Asian J Androl. 2021;23(6):602-610. [48] XIA P, WANG X, WANG Q, et al. Low-Intensity Pulsed Ultrasound Promotes Autophagy-Mediated Migration of Mesenchymal Stem Cells and Cartilage Repair. Cell Transplant. 2021;30:963689720986142. [49] CHEN J, JIANG J, WANG W, et al. Low intensity pulsed ultrasound promotes the migration of bone marrow- derived mesenchymal stem cells via activating FAK-ERK1/2 signalling pathway. Artif Cells Nanomed Biotechnol. 2019;47(1):3603-3613. [50] CHEN J, LI S, JIAO Y, et al. In Vitro Study on the Piezodynamic Therapy with a BaTiO3-Coating Titanium Scaffold under Low-Intensity Pulsed Ultrasound Stimulation. ACS Appl Mater Interfaces. 2021;13(41): 49542-49555. [51] ANGLE SR, SENA K, SUMNER DR, et al. Osteogenic differentiation of rat bone marrow stromal cells by various intensities of low-intensity pulsed ultrasound. Ultrasonics. 2011;51(3):281-288. [52] ZHOU X, CASTRO NJ, ZHU W, et al. Improved Human Bone Marrow Mesenchymal Stem Cell Osteogenesis in 3D Bioprinted Tissue Scaffolds with Low Intensity Pulsed Ultrasound Stimulation. Sci Rep. 2016;6:32876. [53] YANG X, WU Y, LI J, et al. A Pilot Study of Parameter-Optimized Low-Intensity Pulsed Ultrasound Stimulation for the Bone Marrow Mesenchymal Stem Cells Viability Improvement. Comput Math Methods Med. 2019;2019:8386024. [54] JIN L, SHAN J, HAO Y, et al. Enhanced bone regeneration by low-intensity pulsed ultrasound and lipid microbubbles on PLGA/TCP 3D-printed scaffolds. BMC Biotechnol. 2023;23(1):13. [55] WANG B, WEN H, SMITH W, et al. Regulation effects of melatonin on bone marrow mesenchymal stem cell differentiation. J Cell Physiol. 2019;234(2):1008-1015. [56] LI C, WANG Q, GU X, et al. Porous Se@SiO2 nanocomposite promotes migration and osteogenic differentiation of rat bone marrow mesenchymal stem cell to accelerate bone fracture healing in a rat model. Int J Nanomedicine. 2019;14:3845-3860. [57] LI L, WANG B, LI Y, et al. Celastrol regulates bone marrow mesenchymal stem cell fate and bone-fat balance in osteoporosis and skeletal aging by inducing PGC-1α signaling. Aging (Albany NY). 2020;12(17):16887-16898. [58] MAHBOUDI H, KAZEMI B, SOLEIMANI M, et al. Enhanced chondrogenesis of human bone marrow mesenchymal Stem Cell (BMSC) on nanofiber-based polyethersulfone (PES) scaffold. Gene. 2018;643:98-106. |
| [1] | Lai Pengyu, Liang Ran, Shen Shan. Tissue engineering technology for repairing temporomandibular joint: problems and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [2] | Yuan Weibo, Liu Chan, Yu Limei. Potential application of liver organoids in liver disease models and transplantation therapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1684-1692. |
| [3] | Hu Taotao, Liu Bing, Chen Cheng, Yin Zongyin, Kan Daohong, Ni Jie, Ye Lingxiao, Zheng Xiangbing, Yan Min, Zou Yong. Human amniotic mesenchymal stem cells overexpressing neuregulin-1 promote skin wound healing in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1343-1349. |
| [4] | Jin Kai, Tang Ting, Li Meile, Xie Yuan. Effects of conditioned medium and exosomes of human umbilical cord mesenchymal stem cells on proliferation, migration, invasion, and apoptosis of hepatocellular carcinoma cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1350-1355. |
| [5] | Li Dijun, Jiu Jingwei, Liu Haifeng, Yan Lei, Li Songyan, Wang Bin. Three-dimensional gelatin microspheres loaded human umbilical cord mesenchymal stem cells for chronic tendinopathy repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1356-1362. |
| [6] | Lou Guo, Zhang Min, Fu Changxi. Exercise preconditioning for eight weeks enhances therapeutic effect of adipose-derived stem cells in rats with myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1363-1370. |
| [7] | Liu Qi, Li Linzhen, Li Yusheng, Jiao Hongzhuo, Yang Cheng, Zhang Juntao. Icariin-containing serum promotes chondrocyte proliferation and chondrogenic differentiation of stem cells in the co-culture system of three kinds of cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1371-1379. |
| [8] | Huang Ting, Zheng Xiaohan, Zhong Yuanji, Wei Yanzhao, Wei Xufang, Cao Xudong, Feng Xiaoli, Zhao Zhenqiang. Effects of macrophage migration inhibitory factor on survival, proliferation, and differentiation of human embryonic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1380-1387. |
| [9] | Aikepaer · Aierken, Chen Xiaotao, Wufanbieke · Baheti. Osteogenesis-induced exosomes derived from human periodontal ligament stem cells promote osteogenic differentiation of human periodontal ligament stem cells in an inflammatory microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1388-1394. |
| [10] | Zhang Zhenyu, Liang Qiujian, Yang Jun, Wei Xiangyu, Jiang Jie, Huang Linke, Tan Zhen. Target of neohesperidin in treatment of osteoporosis and its effect on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1437-1447. |
| [11] | Xie Liugang, Cui Shuke, Guo Nannan, Li Aoyu, Zhang Jingrui. Research hotspots and frontiers of stem cells for Alzheimer’s disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1475-1485. |
| [12] | Li Jialin, Zhang Yaodong, Lou Yanru, Yu Yang, Yang Rui. Molecular mechanisms underlying role of mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1512-1522. |
| [13] | Cao Yue, Ye Xinjian, Li Biyao, Zhang Yining, Feng Jianying. Effect of extracellular vesicles for diagnosis and therapy of oral squamous cell carcinoma [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1523-1530. |
| [14] | Sun Yuting, Wu Jiayuan, Zhang Jian. Physical factors and action mechanisms affecting osteogenic/odontogenic differentiation of dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1531-1540. |
| [15] | Yu Ting, Lyu Dongmei, Deng Hao, Sun Tao, Cheng Qian. Icariin pretreatment enhances effect of human periodontal stem cells on M1-type macrophages [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1328-1335. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||