Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (19): 4083-4090.doi: 10.12307/2025.054
Previous Articles Next Articles
Stem cell therapy for amyotrophic lateral sclerosis: cell source, number, modification, and administration route
Zhao Wen1, Bi Yulin1, Fu Xuyang1, Duan Hongmei1, Yang Zhaoyang1, Li Xiaoguang1, 2
- 1Department of Neurobiology, School of Basic Medical Sciences, Capital Medical University, Beijing 100069, China; 2Beijing Key Laboratory for Biomaterials and Neural Regeneration, School of Biological Science and Medical Engineering, Beihang University, Beijing 100083, China
-
Received:2024-01-18Accepted:2024-04-11Online:2025-07-08Published:2024-09-13 -
Contact:Li Xiaoguang, MD, Professor, Department of Neurobiology, School of Basic Medical Sciences, Capital Medical University, Beijing 100069, China; Beijing Key Laboratory for Biomaterials and Neural Regeneration, School of Biological Science and Medical Engineering, Beihang University, Beijing 100083, China -
About author:Zhao Wen, Master, Technician-in-charge, Department of Neurobiology, School of Basic Medical Sciences, Capital Medical University, Beijing 100069, China. Bi Yulin, Master candidate, Department of Neurobiology, School of Basic Medical Sciences, Capital Medical University, Beijing 100069, China. Zhao Wen and Bi Yulin contributed equally to this article. -
Supported by:National Natural Science Foundation of China, No. 82271403 (to LXG); National Natural Science Foundation of China, No. 82272171 (to YZY); Beijing Natural Science Foundation, No. 7222004 (to DHM)
CLC Number:
Cite this article
Zhao Wen, Bi Yulin, Fu Xuyang, Duan Hongmei, Yang Zhaoyang, Li Xiaoguang. Stem cell therapy for amyotrophic lateral sclerosis: cell source, number, modification, and administration route[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(19): 4083-4090.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
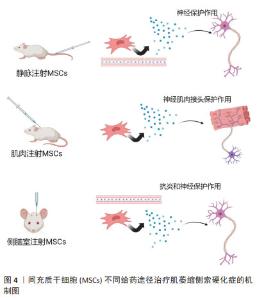
2.1.1 间充质干细胞在ALS领域内的应用 间充质干细胞主要作为细胞治疗手段应用于ALS研究中[18]。间充质干细胞治疗ALS的机制仍不完全明确,目前发现的治疗机制主要为:①抗炎作用。通过间充质干细胞衍生的外泌体作用于小胶质细胞和星形胶质细胞[19],减少其异常激活从而发挥保护作用[20]。②神经元保护。通过间充质干细胞自主分泌碱性成纤维细胞生长因子以及促进星形胶质细胞表达胶质细胞源性神经营养因子发挥神经保护作用[21]。 既往已有大量研究应用间充质干细胞治疗ALS,但治疗效果不一。一些研究认为间充质干细胞具有明显的时效性,治疗效果与给药频次相关,虽有研究发现单次间充质干细胞移植具有一定的收益[22-24],但多次细胞移植的治疗效果远优于单次移植[25]。还有一些研究认为给药途径亦为影响治疗效果的重要因素之一,常见的给药途径包括静脉注射、肌肉注射、侧脑室注射(鞘内注射)。间充质干细胞的治疗机制见图4。"
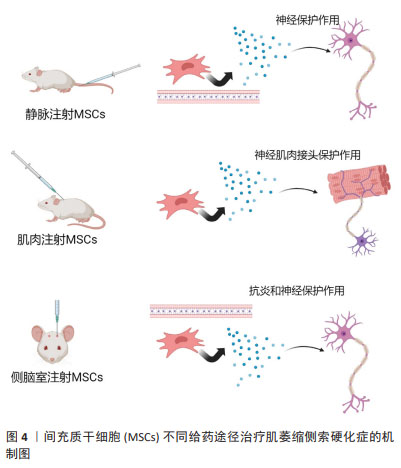

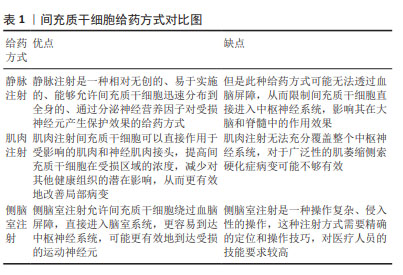
(1)静脉注射:该方法的优点是无创、感染风险小、临床应用时患者更易接受。研究发现,单次静脉注射间充质干细胞可有效抑制血脑屏障的破坏,并且移植的间充质干细胞通过释放神经营养因子发挥了神经保护作用[25],延长了小鼠的生存期[23]。南方医科大学的研究人员通过多次静脉注射源自人羊膜的间充质干细胞,显著延缓了SOD1G93A小鼠的疾病进展。 (2)肌肉注射:由于ALS早期的临床改变主要发生在外周,即神经肌肉接头和神经纤维,而间充质干细胞释放的神经营养因子为靶源性吸收,因此肌肉内注射间充质干细胞被认为可能对延缓ALS疾病进展更为有效[26]。KOOK等[24]通过多次肌肉注射人脐血来源间充质干细胞,抑制了iNOS/NO信号通路,改善了小鼠的运动功能。 (3)侧脑室注射(鞘内注射):相较于以上两种方式,该方式更为直接。侧脑室注射(鞘内注射)间充质干细胞能够有效减少因药物无法透过血脑屏障而导致的药物浓度降低等问题[27]。SIRONI等[28]研究发现,脑室内多次注射人脐血来源间充质干细胞可通过抗炎和神经保护等作用延缓SOD1G93A小鼠的运动神经元死亡。 综上,不同给药途径因治疗的靶标不同,治疗效果不同,见表1。静脉注射靶向为血脑屏障及神经纤维等区域,由于ALS中存在血脑屏障破坏,因此可能部分间充质干细胞通过破坏区域进入到了脊髓中[22],但数量十分有限。肌肉注射间充质干细胞能够明显改善神经肌肉接头的破坏[24],同时提供神经营养因子逆行运输至脊髓运动神经元中,是通过神经营养因子治疗ALS的理想给药途径。侧脑室注射(鞘内注射)是间充质干细胞移植最常见的给药途径,可使细胞大量富集于运动神经元附近,减少细胞损失[29-30]。"
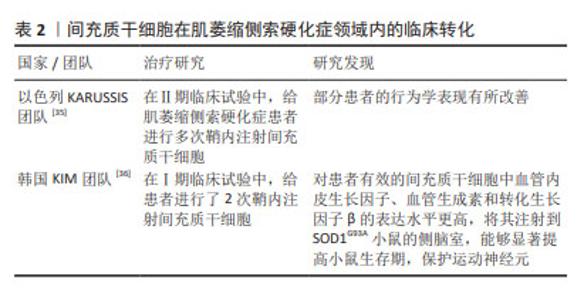
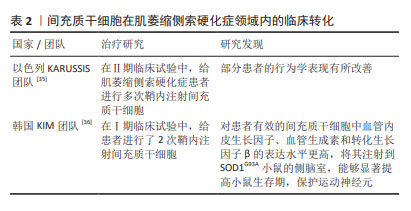
此外,部分学者结合以上不同给药途径的优势,采用了联合给药的方式:如重复鞘内和肌肉内组合注射间充质干细胞可以通过减少细胞凋亡和自噬来保护运动神经元和神经肌肉接头,抑制SOD1G93A大鼠脊髓坏死性凋亡的激活[31]。相比于单一给药方式,联合给药的治疗方式可能具有更好的转化意义。 2.1.2 间充质干细胞在ALS领域内的临床转化 大量的临床前研究证明了间充质干细胞治疗ALS的潜力及可行性,并促成了一些临床研究。有研究发现,ALS患者自体间充质干细胞和健康来源间充质干细胞有相似的免疫调节作用[32];但也有研究发现,ALS患者来源间充质干细胞在释放神经营养因子方面具有功能限制,并表现出衰老表 型[33];还有类似的研究发现ALS患者来源间充质干细胞表达更多的甲基化转移酶,与功能下降有关[34]。虽然ALS患者自体间充质干细胞的有效性仍存在争议,但自体间充质干细胞给药仍具备许多优势,因此大部分研究仍采用自体间充质干细胞进行体外扩增和移植。以色列KARUSSIS团队[35]采用鞘内注射自体间充质干细胞的方法开展了Ⅱ期临床试验,患者共接受1-4次鞘内注射间充质干细胞,间隔3-6个月,未发现明显不良反应,且患者的部分行为学得到改善。韩国KIM团队[36]进行了Ⅰ期临床试验,评估了2次重复鞘内注射自体骨髓来源间充质基质细胞的安全性,随访6个月未观察到严重不良事件,且ALS功能评级量表(ALSFRS-R)评分的下降没有加速,证明该临床试验是安全的。此外,该团队还对间充质干细胞的功能进行了研究:根据患者的治疗效果,将提取的间充质干细胞分为无效间充质干细胞和有效间充质干细胞,首先检测了两组细胞的神经营养因子表达量,发现有效间充质干细胞中血管内皮生长因子、血管生成素和转化生长因子β的表达水平更高;将两组细胞分别注射到SOD1G93A小鼠的侧脑室,发现相较于无效间充质干细胞,有效间充质干细胞能够显著提高小鼠生存期,保护运动神经元,见表2。以上结果提示,自体间充质干细胞注射治疗效果可能更多依赖于这些因子的表达量,同时临床效果和动物模型实验结果的一致性也很好地证明了SOD1G93A小鼠作为ALS临床前药效评估模型的可行性[37-38]。"
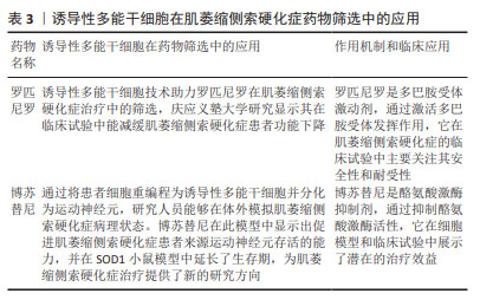
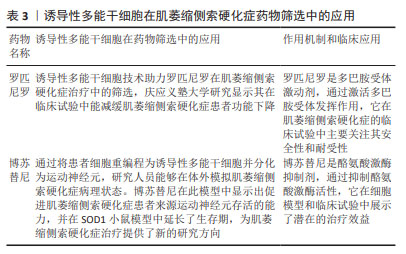
2.2 神经干/祖细胞 神经干/祖细胞存在于胚胎期脑组织中,能够分化为神经元。由于神经干/祖细胞具有神经发生的特性,使得很多研究人员期待将其作为细胞疗法,移植到患者体内发挥代替治疗的作用。 2.2.1 神经干/祖细胞在ALS领域内的应用 ALS是运动神经元退行性疾病,随着疾病进展运动神经元发生不可逆的退行性变[39],因此很多研究人员希望通过移植细胞替代死亡的运动神经元从而实现替代治疗。但由于运动神经元及其祖细胞的形态特殊性,以及无特异表面蛋白等因素,导致该类细胞不易获得。因此很多研究人员期待将神经干/祖细胞诱导分化为运动神经元,并移植到ALS患者体内进行替代治疗,如 LEE 等[40]尝试将神经干细胞在体外高表达Oligo2基因,并经sonic hedgehog(Shh)基因处理后转变成了运动神经元并移植到SOD1G93A小鼠体内,实现了ALS小鼠临床症状延迟7 d,生存期显著延长20 d。但不足的是,该研究并没有提供任何病毒示踪或载体电生理记录等证据证明移植的运动神经元是否与小鼠宿主体内的运动通路建立起连接,以及是否发挥了替代治疗的作用。 此外,移植的神经干/祖细胞还能够通过分化为星形胶质细胞发挥治疗意义。在ALS的发病过程中,星形胶质细胞的异常激活对运动神经元的退行性病变有促进作用[41],如前期研究发现将健康的星形胶质细胞移植到ALS小鼠脊髓中,可明显改善小鼠运动功能障碍,延缓疾病进展,减少运动神经元丢失[42]。一些研究人员将人脊髓源性神经祖细胞移植到SOD1G93A小鼠腰髓后,可产生胶质细胞并释放神经营养因子[43],从而改善ALS小鼠在第18周和第19周的运动功能,并明显延缓ALS小鼠疾病晚期的症状进展,平均生存期延长5 d。这些结果表明,人脊髓源性神经祖细胞移植增加了几种生长因子的内源性分泌,抑制了运动神经元的死亡,但对总生存率影响很小。有研究通过血管内皮生长因子过表达实现了小鼠少数行为学改善,发病延迟,并延长了生存期[40],成功增强了神经干细胞治疗效果。 2.2.2 神经干/祖细胞在ALS领域内的临床转化 在神经干/祖细胞的临床转化方面,Svendsen 团队做了一系列优秀的工作。该团队将皮质分离的人神经祖细胞(human nucleus pulposus cells,hNPCs)在体外进行扩增并使用慢病毒进行修饰,使其分泌胶质细胞源性神经营养因子(glial cell line-derived neurotrophic factor,GDNF)。首先,该团队将hNPCs(GDNF)移植到SOD1G93A大鼠腰髓,这些细胞在动物体内可长期存活达11周[44]。该团队进一步将hNPCs (GDNF)在移植至SOD1G93A大鼠脊髓中,这些细胞转化为了GFAP+的星形胶质细胞,并迁移至病变部位释放GDNF,起到了强大的神经元保护作用;但该研究并未提供神经纤维的保护作用,且干预组与未干预组的神经肌肉接头的丢失情况无差异[45]。为探讨移植的hNPCs(GDNF)是否会导致肿瘤形成,该团队将细胞移植入健康的免疫缺陷大鼠体内,发现 hNPCs(GDNF)可长期存活达7.5个月,并持续释放GDNF,且未观察到肿瘤形成[46]。同时,该团队将 hNPCs(GDNF)移植至SOD1G93A大鼠及食蟹猴皮质,发现这些细胞同样能够分化为星形胶质细胞并表达 GDNF,延缓了疾病进展,且未出现不良反应,证明了 hNPCs(GDNF) 皮质移植的安全性。在临床方面,该团队进行了一项Ⅰ/Ⅱa期临床试验,第一剂量队列(n=9)接受了10次单侧注射,每个注射点200 000个细胞,总共2 000 000个细胞;第二个剂量队列(n=9)每个注射点500 000个细胞,总计5 000 000个细胞。结果发现,与动物实验一致,hNPCs(GDNF)可在体内存活长达42个月,且分化为星形胶质细胞分泌GDNF;所有受试者均达到安全性的主要终点,没有出现与产品相关的严重不良事件;除1例受试者外均未出现排斥或炎症反应。该临床试验为后期ALS的临床治疗奠定了重要基础。后期可尝试多个脊髓阶段注射,以期为患者提供更为全面的运动神经元保护及更佳的临床治疗效果[44]。 2.3 诱导性多能干细胞 诱导性多能干细胞是指通过导入特定的转录因子将终末分化的体细胞重编程为多能性干细胞,具有类似于胚胎干细胞的全能性。诱导性多能干细胞无道德伦理争议,来源广泛,且避免了免疫排斥反应,为整个干细胞领域带来了革命性的改变,对ALS的机制探究及药物开发具有深远的意义。 2.3.1 诱导性多能干细胞在ALS机制研究中的应用 诱导性多能干细胞作为细胞模型在ALS机制研究中广泛应用。既往的ALS研究中使用的模型均依赖于目前已发现的与ALS相关的突变基因,但此类模型理论上仅可部分模拟家族性ALS的表现(家族性患者可能同时存在多种突变基因)。由于基于此类模型研发的药物不能转化到散发性患者中,ALS的研究进展缓慢,且很多临床转化均以失败告终。因此,研发更可靠的ALS模型对于机制研究、药物开发都十分必要。诱导性多能干细胞的发现对于退行性疾病的研究十分重要,研究人员可将患者来源的诱导性多能干细胞进行体外诱导分化为运动神经元,开展机制研究和药物筛选。如目前ALS患者最常见的病理改变是运动神经元中存在TDP43沉淀[47],而这种广泛的病理改变与ALS患者临床表现存在何种关系一直存在疑问。研究人员将存在TDP43突变的患者来源诱导性多能干细胞进行体外诱导分化成运动神经元[48],发现神经元中存在TDP43沉淀;而且这些神经元的TDP43功能丧失导致了轴突生长相关因子STMN2的丢失[49]。该研究为TDP43沉淀与ALS之间提供了机制性联系,有助于帮助人们理解TDP43与ALS临床表现之间的关系。 随着诱导性多能干细胞的研究越来越多,研究人员发现不同的突变基因来源诱导性多能干细胞表现完全不同,这也部分解释了临床前药物研发仅在某种突变基因小鼠中进行,而在转化中失败的原因。不同ALS患者由于基因突变类型不同而出现不同的临床表现,因此更多的研究认为ALS是一种综合征,应更好地区别、鉴定ALS的命名规范。 2.3.2 诱导性多能干细胞在ALS药物筛选中的应用 由于诱导性多能干细胞能够更好地模拟ALS的发病机制,使得目前采用患者来源诱导性多能干细胞诱导的运动神经元模型进行药物筛选,可能获得更好的转化效果。目前正在进行临床试验的药物罗匹尼罗和博舒替尼就是基于此方法研发的药物。 罗匹尼罗作为治疗帕金森病的药物,其作用机制为促进神经营养因子的表达[50-51],增加谷胱甘肽、超氧化物歧化酶和过氧化氢酶活性[52],以及促进脑室下区神经干细胞增殖[53]。研究人员采用携带FUS和TDP43两种突变的诱导性多能干细胞模型进行筛选,发现该药物不仅在FUS和TDP43突变的家族性ALS模型中,在大多数散发性ALS模型中也能起到保护作用[54]。日本庆应义塾大学医学院进行了一项随机可行性、双盲、安慰剂对照试验,结果发现:在所有不良事件中,罗匹尼罗组受试者的胃肠道疾病(主要为暂时性轻度恶心和腹泻)的发生率高达76.9%(安慰剂组为14.3%);在为期6个月的双盲期内,两组受试者的ALSFRS-R评分和功能、生存评分的联合评估并没有显著差异;结果表明,罗匹尼罗的治疗效果仍需更多的研究加以证明。 博苏替尼是一种酪氨酸激酶抑制剂。日本京都大学的 Inoue小组将患者来源诱导性多能干细胞诱导分化为运动神经元,并用于药物筛选,结果提示筛选得到的药物均以Src/c-Abl通路为靶标[55]。作者发现无论是通过药理学抑制还是基因抑制诱导性多能干细胞衍生运动神经元中的Src/c-Abl 通路,均可发挥良好的治疗效果,最终选择了Src/c-Abl激酶抑制剂博舒替尼。研究发现,该药物在诱导性多能干细胞衍生运动神经元中增强了自噬,减少了错误折叠的突变SOD1蛋白的数量,并减弱了线粒体基因的表达改变[56]。作者同时采用TDP43突变或C9orf72重复扩张引起的散发ALS或其他形式的家族性ALS患者诱导性多能干细胞衍生运动神经元进行体外验证[57-58],发现细胞存活率提高;采用SOD1G93A小鼠进行体内验证,同样提高了小鼠的生存期。随后在日本进行了相关的临床试验,日本4家医院使用3+3设计进行开放标签、多中心、剂量升级第Ⅰ阶段研究,以评估博苏替尼在ALS 患者中的安全性和耐受性。在该研究中,研究者使用修订后的ALSFRS-R评估了包括血浆神经丝轻链在内的预测生物标志物的情况,结果发现100-400 mg的剂量范围内, 300 mg患者耐受良好,对治疗反应的患者可以通过较低的血浆神经丝轻链水平来区分。见表3。"
| [1] CAMPISI L, CHIZARI S, HO JSY, et al. Clonally expanded CD8 T cells characterize amyotrophic lateral sclerosis-4. Nature. 2022;606(7916): 945-952. [2] ZONDLER L, MÜLLER K, KHALAJI S, et al. Peripheral monocytes are functionally altered and invade the CNS in ALS patients. Acta Neuropathol. 2016;132(3):391-411. [3] LIU X, HE J, GAO FB, et al. The epidemiology and genetics of Amyotrophic lateral sclerosis in China. Brain Res. 2018;1693(Pt A): 121-126. [4] XU L, CHEN L, WANG S, et al. Incidence and prevalence of amyotrophic lateral sclerosis in urban China: a national population-based study. J Neurol Neurosurg Psychiatry. 2020;91(5):520-525. [5] TENDULKAR S, HEGDE S, GARG L, et al. Caspar, an adapter for VAPB and TER94, modulates the progression of ALS8 by regulating IMD/NFκB-mediated glial inflammation in a Drosophila model of human disease. Hum Mol Genet. 2022;31(17):2857-2875. [6] YAMAWAKI M, AKIBA M, MATSUMOTO N, et al. Defective neuronal and oligodendroglial differentiation by FTD3- and ALS17-associated Ile29-to-Val mutation of CHMP2B. Mol Genet Metab Rep. 2019;19: 100458. [7] LIANG D, LIN WJ, REN M, et al. m6A reader YTHDC1 modulates autophagy by targeting SQSTM1 in diabetic skin. Autophagy. 2022; 18(6):1318-1337. [8] ASSONI AF, GUERRERO EN, WARDENAAR R, et al. IFNγ protects motor neurons from oxidative stress via enhanced global protein synthesis in FUS-associated amyotrophic lateral sclerosis. Brain Pathol. 2024;34(1):e13206. [9] JIN S, SUN Z, FANG X, et al. A patient carrying a heterozygous p.Asn267Ser TARDBP missense mutation diagnosed as ALS and only involving lower motor neurons. Neurol Sci. 2023;44(2):777-782. [10] MCCAULEY ME, O’ROURKE JG, YÁÑEZ A, et al. C9orf72 in myeloid cells suppresses STING-induced inflammation. Nature. 2020;585(7823): 96-101. [11] SHAMMAS MK, HUANG X, WU BP, et al. OMA1 mediates local and global stress responses against protein misfolding in CHCHD10 mitochondrial myopathy. J Clin Invest. 2022;132(14):e157504. [12] YUAN T, WANG T, ZHANG J, et al. Robust and Multifunctional Nanoparticles Assembled from Natural Polyphenols and Metformin for Efficient Spinal Cord Regeneration. ACS Nano. 2023;17(18): 18562-18575. [13] MURPHY S, SCHMITT-JOHN T, DOWLING P, et al. Proteomic profiling of the brain from the wobbler mouse model of amyotrophic lateral sclerosis reveals elevated levels of the astrogliosis marker glial fibrillary acidic protein. Eur J Transl Myol. 2023;33(3):11555. [14] ABE K, ITOYAMA Y, SOBUE G, et al. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(7-8):610-617. [15] YU CH, DAVIDSON S, HARAPAS CR, et al. TDP-43 Triggers Mitochondrial DNA Release via mPTP to Activate cGAS/STING in ALS. Cell. 2020; 183(3):636-649.e18. [16] OKANO H, MORIMOTO S. iPSC-based disease modeling and drug discovery in cardinal neurodegenerative disorders. Cell Stem Cell. 2022;29(2):189-208. [17] WALD-ALTMAN S, PICHINUK E, KAKHLON O, et al. A differential autophagy-dependent response to DNA double-strand breaks in bone marrow mesenchymal stem cells from sporadic ALS patients. Dis Model Mech. 2017;10(5):645-654. [18] SUNOHARA T, MORIZANE A, MATSUURA S, et al. MicroRNA-Based Separation of Cortico-Fugal Projection Neuron-Like Cells Derived From Embryonic Stem Cells. Front Neurosci. 2019;13:1141. [19] BOILLÉE S, YAMANAKA K, LOBSIGER CS, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312(5778):1389-1392. [20] GIUNTI D, MARINI C, PARODI B, et al. Role of miRNAs shuttled by mesenchymal stem cell-derived small extracellular vesicles in modulating neuroinflammation. Sci Rep. 2021;11(1):1740. [21] MARCONI S, BONACONSA M, SCAMBI I, et al. Systemic treatment with adipose-derived mesenchymal stem cells ameliorates clinical and pathological features in the amyotrophic lateral sclerosis murine model. Neuroscience. 2013;248:333-343. [22] MAGOTA H, SASAKI M, KATAOKA-SASAKI Y, et al. Intravenous infusion of mesenchymal stem cells delays disease progression in the SOD1G93A transgenic amyotrophic lateral sclerosis rat model. Brain Res. 2021; 1757:147296. [23] CHIAROTTO GB, CARTAROZZI LP, PEREZ M, et al. Delayed onset, immunomodulation, and lifespan improvement of SOD1G93A mice after intravenous injection of human mesenchymal stem cells derived from adipose tissue. Brain Res Bull. 2022;186:153-164. [24] KOOK MG, LEE S, SHIN N, et al. Repeated intramuscular transplantations of hUCB-MSCs improves motor function and survival in the SOD1 G93A mice through activation of AMPK. Sci Rep. 2020;10(1):1572. [25] MAGOTA H, SASAKI M, KATAOKA-SASAKI Y, et al. Repeated infusion of mesenchymal stem cells maintain the condition to inhibit deteriorated motor function, leading to an extended lifespan in the SOD1G93A rat model of amyotrophic lateral sclerosis. Mol Brain. 2021;14(1):76. [26] SUZUKI M, SVENDSEN CN. Ex Vivo Gene Therapy Using Human Mesenchymal Stem Cells to Deliver Growth Factors in the Skeletal Muscle of a Familial ALS Rat Model. Methods Mol Biol. 2016;1382: 325-336. [27] GOTKINE M, CARACO Y, LERNER Y, et al. Safety and efficacy of first-in-man intrathecal injection of human astrocytes (AstroRx®) in ALS patients: phase I/IIa clinical trial results. J Transl Med. 2023;21(1):122. [28] SIRONI F, VALLAROLA A, VIOLATTO MB, et al. Multiple intracerebroventricular injections of human umbilical cord mesenchymal stem cells delay motor neurons loss but not disease progression of SOD1G93A mice. Stem Cell Res. 2017;25:166-178. [29] IZRAEL M, SLUTSKY SG, ADMONI T, et al. Safety and efficacy of human embryonic stem cell-derived astrocytes following intrathecal transplantation in SOD1G93A and NSG animal models. Stem Cell Res Ther. 2018;9(1):152. [30] TERASHIMA T, KOBASHI S, WATANABE Y, et al. Enhancing the Therapeutic Efficacy of Bone Marrow-Derived Mononuclear Cells with Growth Factor-Expressing Mesenchymal Stem Cells for ALS in Mice. iScience. 2020;23(11):101764. [31] BOHACIAKOVA D, HRUSKA-PLOCHAN M, TSUNEMOTO R, et al. A scalable solution for isolating human multipotent clinical-grade neural stem cells from ES precursors. Stem Cell Res Ther. 2019;10(1):83. [32] JAVORKOVA E, MATEJCKOVA N, ZAJICOVA A, et al. Immunomodulatory Properties of Bone Marrow Mesenchymal Stem Cells from Patients with Amyotrophic Lateral Sclerosis and Healthy Donors. J Neuroimmune Pharmacol. 2019;14(2):215-225. [33] YUN YC, JEONG SG, KIM SH, et al. Reduced sirtuin 1/adenosine monophosphate-activated protein kinase in amyotrophic lateral sclerosis patient-derived mesenchymal stem cells can be restored by resveratrol. J Tissue Eng Regen Med. 2019;13(1):110-115. [34] OH YS, KIM SH, CHO GW. Functional Restoration of Amyotrophic Lateral Sclerosis Patient-Derived Mesenchymal Stromal Cells Through Inhibition of DNA Methyltransferase. Cell Mol Neurobiol. 2016;36(4):613-620. [35] PETROU P, KASSIS I, YAGHMOUR NE, et al. A phase II clinical trial with repeated intrathecal injections of autologous mesenchymal stem cells in patients with amyotrophic lateral sclerosis. Front Biosci (Landmark Ed). 2021;26(10):693-706. [36] OH KW, MOON C, KIM HY, et al. Phase I trial of repeated intrathecal autologous bone marrow-derived mesenchymal stromal cells in amyotrophic lateral sclerosis. Stem Cells Transl Med. 2015;4(6): 590-597. [37] RUEGSEGGER C, MAHARJAN N, GOSWAMI A, et al. Aberrant association of misfolded SOD1 with Na(+)/K(+)ATPase-α3 impairs its activity and contributes to motor neuron vulnerability in ALS. Acta Neuropathol. 2016;131(3):427-451. [38] KIM HY, KIM H, OH KW, et al. Biological markers of mesenchymal stromal cells as predictors of response to autologous stem cell transplantation in patients with amyotrophic lateral sclerosis: an investigator-initiated trial and in vivo study. Stem Cells. 2014;32(10):2724-2731. [39] AN D, FUJIKI R, IANNITELLI DE, et al. Stem cell-derived cranial and spinal motor neurons reveal proteostatic differences between ALS resistant and sensitive motor neurons. Elife. 2019;8:e44423. [40] LEE HJ, KIM KS, AHN J, et al. Human motor neurons generated from neural stem cells delay clinical onset and prolong life in ALS mouse model. PLoS One. 2014;9(5):e97518. [41] GUTTENPLAN KA, WEIGEL MK, ADLER DI, et al. Knockout of reactive astrocyte activating factors slows disease progression in an ALS mouse model. Nat Commun. 2020;11(1):3753. [42] LEPORE AC, RAUCK B, DEJEA C, et al. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci. 2008;11(11):1294-1301. [43] KNIPPENBERG S, RATH KJ, BÖSELT S, et al. Intraspinal administration of human spinal cord-derived neural progenitor cells in the G93A-SOD1 mouse model of ALS delays symptom progression, prolongs survival and increases expression of endogenous neurotrophic factors. J Tissue Eng Regen Med. 2017;11(3):751-764. [44] KLEIN SM, BEHRSTOCK S, MCHUGH J, et al. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum Gene Ther. 2005;16(4):509-521. [45] SUZUKI M, MCHUGH J, TORK C, et al. GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS One. 2007;2(8):e689. [46] GOWING G, SHELLEY B, STAGGENBORG K, et al. Glial cell line-derived neurotrophic factor-secreting human neural progenitors show long-term survival, maturation into astrocytes, and no tumor formation following transplantation into the spinal cord of immunocompromised rats. Neuroreport. 2014;25(6):367-372. [47] CHENNAMPALLY P, SAYED-ZAHID A, SOUNDARARAJAN P, et al. Author Correction: A microfluidic approach to rescue ALS motor neuron degeneration using rapamycin. Sci Rep. 2021;11(1):19743. [48] MELAMED Z, LÓPEZ-ERAUSKIN J, BAUGHN MW, et al. Premature polyadenylation-mediated loss of stathmin-2 is a hallmark of TDP-43-dependent neurodegeneration. Nat Neurosci. 2019;22(2): 180-190. [49] GUERRA SAN JUAN I, NASH LA, SMITH KS, et al. Loss of mouse Stmn2 function causes motor neuropathy. Neuron. 2022;110(10):1671-1688.e6. [50] IIDA M, MIYAZAKI I, TANAKA K, et al. Dopamine D2 receptor-mediated antioxidant and neuroprotective effects of ropinirole, a dopamine agonist. Brain Res. 1999;838(1-2):51-59. [51] TANAKA K, MIYAZAKI I, FUJITA N, et al. Molecular mechanism in activation of glutathione system by ropinirole, a selective dopamine D2 agonist. Neurochem Res. 2001;26(1):31-36. [52] DU F, LI R, HUANG Y, et al. Dopamine D3 receptor-preferring agonists induce neurotrophic effects on mesencephalic dopamine neurons. Eur J Neurosci. 2005;22(10):2422-2430. [53] HÖGLINGER GU, RIZK P, MURIEL MP, et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7(7):726-735. [54] FUJIMORI K, ISHIKAWA M, OTOMO A, et al. Modeling sporadic ALS in iPSC-derived motor neurons identifies a potential therapeutic agent. Nat Med. 2018;24(10):1579-1589. [55] IMAMURA K, IZUMI Y, WATANABE A, et al. The Src/c-Abl pathway is a potential therapeutic target in amyotrophic lateral sclerosis. Sci Transl Med. 2017;9(391):eaaf3962. [56] GOTO K, IMAMURA K, KOMATSU K, et al. Simple Derivation of Spinal Motor Neurons from ESCs/iPSCs Using Sendai Virus Vectors. Mol Ther Methods Clin Dev. 2017;4:115-125. [57] COOK CN, WU Y, ODEH HM, et al. C9orf72 poly(GR) aggregation induces TDP-43 proteinopathy. Sci Transl Med. 2020;12(559): eabb3774. [58] ESHIMA J, O’CONNOR SA, MARSCHALL E, et al. Molecular subtypes of ALS are associated with differences in patient prognosis. Nat Commun. 2023;14(1):95. |
| [1] | Yang Zhihang, Sun Zuyan, Huang Wenliang, Wan Yu, Chen Shida, Deng Jiang. Nerve growth factor promotes chondrogenic differentiation and inhibits hypertrophic differentiation of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1336-1342. |
| [2] | Hu Taotao, Liu Bing, Chen Cheng, Yin Zongyin, Kan Daohong, Ni Jie, Ye Lingxiao, Zheng Xiangbing, Yan Min, Zou Yong. Human amniotic mesenchymal stem cells overexpressing neuregulin-1 promote skin wound healing in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1343-1349. |
| [3] | Jin Kai, Tang Ting, Li Meile, Xie Yuan. Effects of conditioned medium and exosomes of human umbilical cord mesenchymal stem cells on proliferation, migration, invasion, and apoptosis of hepatocellular carcinoma cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1350-1355. |
| [4] | Li Dijun, Jiu Jingwei, Liu Haifeng, Yan Lei, Li Songyan, Wang Bin. Three-dimensional gelatin microspheres loaded human umbilical cord mesenchymal stem cells for chronic tendinopathy repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1356-1362. |
| [5] | Liu Qi, Li Linzhen, Li Yusheng, Jiao Hongzhuo, Yang Cheng, Zhang Juntao. Icariin-containing serum promotes chondrocyte proliferation and chondrogenic differentiation of stem cells in the co-culture system of three kinds of cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1371-1379. |
| [6] | Zhang Zhenyu, Liang Qiujian, Yang Jun, Wei Xiangyu, Jiang Jie, Huang Linke, Tan Zhen. Target of neohesperidin in treatment of osteoporosis and its effect on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1437-1447. |
| [7] | Li Jialin, Zhang Yaodong, Lou Yanru, Yu Yang, Yang Rui. Molecular mechanisms underlying role of mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1512-1522. |
| [8] | He Bo, Chen Wen, Ma Suilu, He Zhijun, Song Yuan, Li Jinpeng, Liu Tao, Wei Xiaotao, Wang Weiwei, Xie Jing . Pathogenesis and treatment progress of flap ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1230-1238. |
| [9] | Chen Yilin, Jiang Xiaobo, Qu Honglin, Liu Ruilian. General pattern of GSK3/Nrf2-regulated biological rhythms in organismal aging [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1257-1264. |
| [10] | Sun Xianjuan, Wang Qiuhua, Zhang Jinyi, Yang Yangyang, Wang Wenshuang, Zhang Xiaoqing. Adhesion, proliferation, and vascular smooth muscle differentiation of bone marrow mesenchymal stem cells on different electrospinning membranes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 661-669. |
| [11] | Deng Li, Liu Yang, Wang Hui, Yang Qiu, Dong Mingqing. Transcription factor NKX2.1 promotes differentiation of induced pluripotent stem cells into lung stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7790-7796. |
| [12] | Zhang Min, Zhang Nini, Huang Guilin, Li Zhuangzhuang, Wang Xue, Wang Huike. Human amniotic mesenchymal stem cell exosomes repair radiation-induced submandibular gland damage in rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7804-7815. |
| [13] | Wang Qingfang, Zhang Fen, Chang Guangping, Li Zihan, Xing Lan, Peng Hao, Zeng Xiuping, Zhong Guiqiang, Chen Hui, Liu Bo, Liu Zhenyu, Liang Xiao. Effect of a novel cryoprotectant in tissues and cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7816-7826. |
| [14] | Guo Jia, Ren Yafeng, Li Bing, Huang Jing, Shang Wenya, Yang Yike, Liu Huiyao. Action mechanism of mesenchymal stem cell-derived exosomes carrying miRNAs in improving spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7827-7838. |
| [15] | Huang Haina, Yu Yanrong, Bi Jian, Huang Miao, Peng Weijie. Epigenetic characteristics of hepatogenic differentiation of mesenchymal stem cells in three-dimensional culture [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7848-7855. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||