Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (25): 4054-4059.doi: 10.12307/2024.175
Previous Articles Next Articles
Role of synaptic input remodeling of corticospinal motor neurons after spinal cord injury
Dai Jiafeng1, Wang Lizhao2, Han Qi2, Shen Hongxing1
- 1Department of Spine Surgery, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200127, China; 2Department of Anatomy and Physiology, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China
-
Received:2023-05-13Accepted:2023-06-25Online:2024-09-08Published:2023-11-24 -
Contact:Shen Hongxing, MD, Chief physician, Department of Spine Surgery, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200127, China -
About author:Dai Jiafeng, Master candidate, Department of Spine Surgery, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200127, China -
Supported by:National Natural Science Foundation of China, No. 82072418 (to SHX)
CLC Number:
Cite this article
Dai Jiafeng, Wang Lizhao, Han Qi, Shen Hongxing. Role of synaptic input remodeling of corticospinal motor neurons after spinal cord injury[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(25): 4054-4059.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
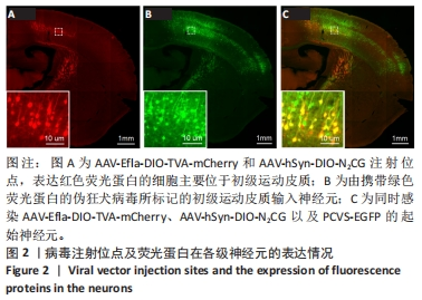
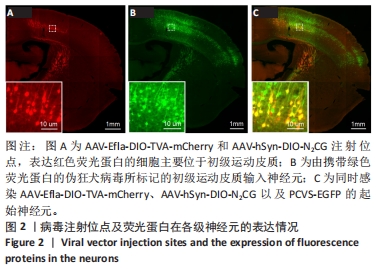
2.1 初级运动皮质中的起始皮质脊髓运动神经元的标记与鉴定 在初级运动皮质,经脊髓注射感染AAV-hSyn-SV40-NLS-Cre的皮质脊髓运动神经元合成Cre重组酶。在胞体内,对Cre重组酶依赖的包含TVA蛋白和RG蛋白序列的腺相关病毒DNA被Cre重组酶剪切和重组,从而诱导TVA和RG蛋白的表达。表达TVA蛋白的神经元会发出红色荧光,见图2A。腺相关病毒注射14 d后,TVA和RG蛋白在初级运动皮质脊髓运动神经元内已经充分表达。此时在初级运动皮质注射表达绿色荧光蛋白的假性狂犬病毒,假性狂犬病毒与皮质脊髓运动神经元内TVA结合,从而进入皮质脊髓运动神经元。当假性狂犬病毒进入初级运动皮质脊髓运动神经元胞体后,病毒基因组进行复制,并加入RG蛋白组装成为有跨突触能力的狂犬病毒颗粒。这些狂犬病毒颗粒逆向跨单突触感染直接支配初级运动皮质脊髓运动神经元的输入神经元,可大量表达绿色荧光蛋白,使得这些输入神经元在荧光显微镜成像中清晰可见,见图2B。由假性狂犬病毒介导的逆向跨单突触感染实验中的起始神经元,是既被表达假性狂犬病毒辅助蛋白的腺相关病毒(红色荧光)感染,又被假性狂犬病毒(绿色荧光)感染的神经元。在小鼠初级运动皮质中,观察到黄色(红色和绿色荧光叠加的结果)起始神经元,见图2C。"
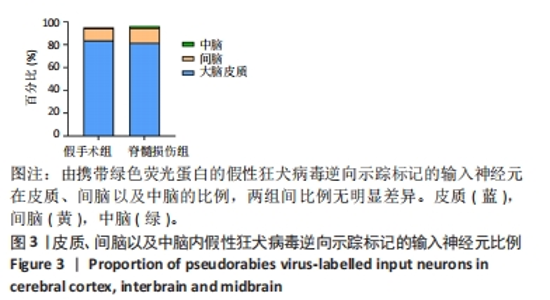
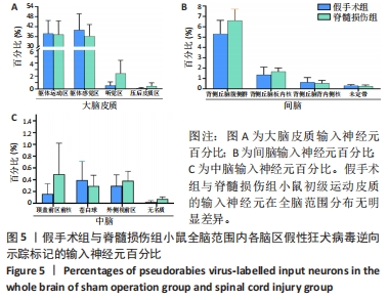
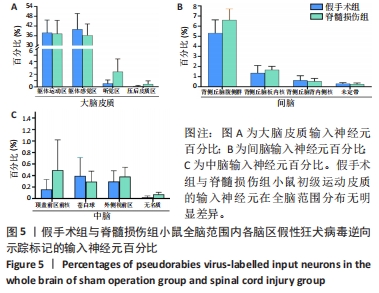
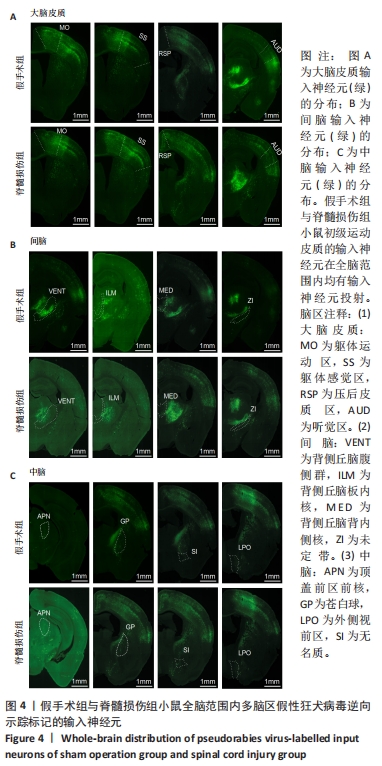
2.2 初级运动皮质中皮质脊髓运动神经元突触输入神经元数量的全脑定量分析 在脊髓损伤组和假手术组小鼠多个脑区观察到有假性狂犬病毒携带的绿色荧光标记的输入神经元,包括皮质(cortical areas)、间脑(interbrain)和中脑(midbrain)。假手术组小鼠皮质的输入神经元占全脑输入神经元总数的(84.0±3.6)%,间脑占(10.6±2.3)%,中脑占(0.7±0.4)%。脊髓损伤组小鼠初级运动皮质的直接输入神经元在皮质、间脑和中脑中的占比分别是(81.7±1.0)%,(13.1±0.5)%和(1.6±0.8)%。脊髓损伤组神经元在3个区域细胞数量的比例与假手术组无明显差异,见图3。在皮质区域,两组输入神经元最多的区域均是躯体运动区(somatomotor area,MO) ,见图4A,图5A。此外,初级运动皮质还与听觉区(auditory areas,AUD)、压后皮质(retrosplenial area,RSP)等皮质结构有解剖学连接,见图4A,5A。"
在间脑,输入神经元主要分布于背侧丘脑,背侧丘脑为大脑重要的感觉传导接替站。来自全身的上行感觉传导通路(除听觉以外)均在背侧丘脑内进行换元,背侧丘脑发出大量神经元投射到大脑皮质。在背侧丘脑内,两组输入神经元最多的区域为背侧丘脑腹侧组,见图4B,5B。背侧丘脑其余区域也与初级运动皮质有解剖学连接,如背侧丘脑板内核(intralaminar nuclei of the dorsal thalamus,ILM)、背侧丘脑背内侧核(medial group of the dorsal thalamus)。此外,初级运动皮质也接受下丘脑于未定带(zona incerta,ZI)的神经元输入,见图4B,图5B。 在中脑,两组输入神经元主要分布于纹状体与中脑运动核团,如顶盖前区前核 (anterior pretectal nucleus,APN)、无名质(substantia innominata,SI)、苍白球(globus pallidus,GP)和外侧视前区(lateral preoptic area,LPO),见图4C,5C。 脊髓损伤后,全脑范围各脑区内有假性狂犬病毒携带的绿色荧光标记的输入神经元数量无明显差异,见图5。"
| [1] BENEDETTI B, WEIDENHAMMER A, REISINGER M, et al. Spinal Cord Injury and Loss of Cortical Inhibition. Int J Mol Sci. 2022;23(10):5622. [2] WELNIARZ Q, DUSART I, ROZE E. The corticospinal tract: Evolution, development, and human disorders. Dev Neurobiol. 2017;77(7):810-829. [3] NISHIMURA Y, ISA T. Cortical and subcortical compensatory mechanisms after spinal cord injury in monkeys. Exp Neurol. 2012;235(1):152-161. [4] COURTINE G, GERASIMENKO Y, VAN DEN BRAND R, et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009;12(10):1333-1342. [5] WICKERSHAM IR, LYON DC, BARNARD RJ, et al. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007; 53(5):639-647. [6] ZHANG S, XU M, CHANG WC, et al. Organization of long-range inputs and outputs of frontal cortex for top-down control. Nat Neurosci. 2016;19(12):1733-1742. [7] DONOGHUE JP, PARHAM C. Afferent connections of the lateral agranular field of the rat motor cortex. J Comp Neurol. 1983;217(4):390-404. [8] BROWN CE, AMINOLTEJARI K, ERB H, et al. In vivo voltage-sensitive dye imaging in adult mice reveals that somatosensory maps lost to stroke are replaced over weeks by new structural and functional circuits with prolonged modes of activation within both the peri-infarct zone and distant sites. J Neurosci. 2009;29(6):1719-1734. [9] FOUAD K, PEDERSEN V, SCHWAB ME, et al. Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr Biol. 2001;11(22):1766-1770. [10] NISHIMURA Y, ISA T. Compensatory changes at the cerebral cortical level after spinal cord injury. Neuroscientist. 2009;15(5):436-444. [11] NISHIMURA Y, ONOE H, MORICHIKA Y, et al. Time-dependent central compensatory mechanisms of finger dexterity after spinal cord injury. Science. 2007;318(5853):1150-1155. [12] HAWASLI AH, RUTLIN J, ROLAND JL, et al. Spinal Cord Injury Disrupts Resting-State Networks in the Human Brain. J Neurotrauma. 2018;35(6):864-873. [13] MARTINEZ M, DELCOUR M, RUSSIER M, et al. Differential tactile and motor recovery and cortical map alteration after C4-C5 spinal hemisection. Exp Neurol. 2010;221(1):186-197. [14] QIAN J, WU W, XIONG W, et al. Longitudinal Optogenetic Motor Mapping Revealed Structural and Functional Impairments and Enhanced Corticorubral Projection after Contusive Spinal Cord Injury in Mice. J Neurotrauma. 2019;36(3):485-499. [15] HILTON BJ, ANENBERG E, HARRISON TC, et al. Re-Establishment of Cortical Motor Output Maps and Spontaneous Functional Recovery via Spared Dorsolaterally Projecting Corticospinal Neurons after Dorsal Column Spinal Cord Injury in Adult Mice. J Neurosci. 2016;36(14):4080-4092. [16] DANCAUSE N, BARBAY S, FROST SB, et al. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25(44):10167-10179. [17] FLORENCE SL, TAUB HB, KAAS JH. Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science. 1998;282(5391):1117-1121. [18] HAINS BC, BLACK JA, WAXMAN SG. Primary cortical motor neurons undergo apoptosis after axotomizing spinal cord injury. J Comp Neurol. 2003;462(3):328-341. [19] LEE BH, LEE KH, KIM UJ, et al. Injury in the spinal cord may produce cell death in the brain. Brain Res. 2004;1020(1-2):37-44. [20] NIELSON JL, STRONG MK, STEWARD O. A reassessment of whether cortical motor neurons die following spinal cord injury. J Comp Neurol. 2011;519(14):2852-2869. [21] WANNIER T, SCHMIDLIN E, BLOCH J, et al. A unilateral section of the corticospinal tract at cervical level in primate does not lead to measurable cell loss in motor cortex. J Neurotrauma. 2005;22(6):703-717. [22] NIELSON JL, SEARS-KRAXBERGER I, STRONG MK, et al. Unexpected survival of neurons of origin of the pyramidal tract after spinal cord injury. J Neurosci. 2010; 30(34):11516-11528. [23] MCBRIDE RL, FERINGA ER, GARVER MK, et al. Retrograde transport of fluoro-gold in corticospinal and rubrospinal neurons 10 and 20 weeks after T-9 spinal cord transection. Exp Neurol. 1990;108(1):83-85. [24] BARRON KD, DENTINGER MP, POPP AJ, et al. Neurons of layer Vb of rat sensorimotor cortex atrophy but do not die after thoracic cord transection. J Neuropathol Exp Neurol. 1988;47(1):62-74. [25] TAKATA Y, YAMANAKA H, NAKAGAWA H, et al. Morphological changes of large layer V pyramidal neurons in cortical motor-related areas after spinal cord injury in macaque monkeys. Sci Rep. 2023;13(1):82. [26] NAGENDRAN T, TAYLOR AM. Unique Axon-to-Soma Signaling Pathways Mediate Dendritic Spine Loss and Hyper-Excitability Post-axotomy. Front Cell Neurosci. 2019;13:431. [27] KIM BG, DAI HN, MCATEE M, et al. Remodeling of synaptic structures in the motor cortex following spinal cord injury. Exp Neurol. 2006;198(2):401-415. [28] GHOSH A, PEDUZZI S, SNYDER M, et al. Heterogeneous spine loss in layer 5 cortical neurons after spinal cord injury. Cereb Cortex. 2012;22(6):1309-1317. [29] NAGENDRAN T, LARSEN RS, BIGLER RL, et al. Distal axotomy enhances retrograde presynaptic excitability onto injured pyramidal neurons via trans-synaptic signaling. Nat Commun. 2017;8(1):625. [30] CHO Y, SLOUTSKY R, NAEGLE KM, et al. Injury-induced HDAC5 nuclear export is essential for axon regeneration. Cell. 2013;155(4):894-908. [31] KRUSE F, BOSSE F, VOGELAAR CF, et al. Cortical gene expression in spinal cord injury and repair: insight into the functional complexity of the neural regeneration program. Front Mol Neurosci. 2011;4:26. [32] POPLAWSKI GHD, KAWAGUCHI R, VAN NIEKERK E, et al. Injured adult neurons regress to an embryonic transcriptional growth state. Nature. 2020;581(7806):77-82. [33] BRADKE F, FAWCETT JW, SPIRA ME. Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nat Rev Neurosci. 2012;13(3):183-193. [34] DI GIOVANNI S, DE BIASE A, YAKOVLEV A, et al. In vivo and in vitro characterization of novel neuronal plasticity factors identified following spinal cord injury. J Biol Chem. 2005;280(3):2084-2091. [35] ALJOVIĆ A, JACOBI A, MARCANTONI M, et al. Synaptogenic gene therapy with FGF22 improves circuit plasticity and functional recovery following spinal cord injury. EMBO Mol Med. 2023;15(2):e16111. [36] WANG Z, MEHRA V, SIMPSON MT, et al. KLF6 and STAT3 co-occupy regulatory DNA and functionally synergize to promote axon growth in CNS neurons. Sci Rep. 2018;8(1):12565. [37] URBAN ET 3RD, BURY SD, BARBAY HS, et al. Gene expression changes of interconnected spared cortical neurons 7 days after ischemic infarct of the primary motor cortex in the rat. Mol Cell Biochem. 2012;369(1-2):267-286. [38] SANDROW-FEINBERG HR, HOULÉ JD. Exercise after spinal cord injury as an agent for neuroprotection, regeneration and rehabilitation. Brain Res. 2015;1619:12-21. [39] CHENG X, MAO GP, HU WJ, et al. Exercise combined with administration of adipose-derived stem cells ameliorates neuropathic pain after spinal cord injury. Neural Regen Res. 2023;18(8):1841-1846. [40] KUMRU H, FLORES A, RODRÍGUEZ-CAÑÓN M, et al. Non-invasive brain and spinal cord stimulation for motor and functional recovery after a spinal cord injury. Rev Neurol. 2020;70(12):461-477. [41] SONG QF, CUI Q, WANG YS, et al. Mesenchymal stem cells, extracellular vesicles, and transcranial magnetic stimulation for ferroptosis after spinal cord injury. Neural Regen Res. 2023;18(9):1861-1868. [42] LUO Y, FAN L, LIU C, et al. An injectable, self-healing, electroconductive extracellular matrix-based hydrogel for enhancing tissue repair after traumatic spinal cord injury. Bioact Mater. 2021;7:98-111. [43] LI G, ZHANG B, SUN JH, et al. An NT-3-releasing bioscaffold supports the formation of TrkC-modified neural stem cell-derived neural network tissue with efficacy in repairing spinal cord injury. Bioact Mater. 2021;6(11):3766-3781. [44] LIU H, XU X, TU Y, et al. Engineering Microenvironment for Endogenous Neural Regeneration after Spinal Cord Injury by Reassembling Extracellular Matrix. ACS Appl Mater Interfaces. 2020;12(15):17207-17219. |
| [1] |
Song Jiating, Chen Jianmin, Wang Kewen, Huang Lanying, Xu Senming, Gui Yuchang, Xu Jianwen.
Metabolomics analysis of serum and urine in patients with traumatic spinal cord injury #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(在线): 1-6.
|
| [2] | Zhou Bangyu, Li Jie, Ruan Yushang, Geng Funeng, Li Shaobo. Effects of Periplaneta americana powder on motor function and autophagic protein Beclin-1 in rats undergoing spinal cord hemisection [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1223-1228. |
| [3] | Zeng Fanzhuo, Li Yuxin, Sun Jiachen, Gu Xinyang, Wen Shan, Tian He, Mei Xifan. Efficient strategies for microglia replacement in spinal cord injury models [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1007-1014. |
| [4] | Liu Tao, Zhang Wenkai, Ma Ziqian, Zhang Yan, Chen Xueming. Riluzole interferes with the activation of NLRP3 inflammasome in microglia of rats with spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1036-1042. |
| [5] | Chen Zepeng, Hou Yonghui, Chen Shudong, Hou Yu, Lin Dingkun. Tauroursodeoxycholic acid treats spinal cord injury by reducing apoptosis of spinal cord neurons under glucose and oxygen deprivation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 528-534. |
| [6] | Tao Jingwei, Zhou Jingya, Zhao Yi, Ren Jingpei, Hu Chuanyu, Xu Lin, Mu Xiaohong, Fan Xiao. Effect and mechanism of tetramethylpyrazine regulating ferroptosis in rats with spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(26): 4158-4163. |
| [7] | Ye Jiapeng, Wang Jianwei, Wu Mao, Li Shaoshuo, Wang Guopeng, Wang Haotian, Tang Zhi, Shao Yang. miR-146a-3p regulates astrocyte proliferation, migration and apoptosis by inhibiting insulin-like growth factor 1 expression [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(25): 4048-4053. |
| [8] | Long Zhisheng, Gong Feipeng, Wen Jiabin, Min Huan, Shu Yang, Lai Zhuoxi, Chen Gang. Bone marrow mesenchymal stem cell exosomes combined with epigallocatechin-3-gallate in treatment of spinal cord ischemia/reperfusion injury in rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(19): 2953-2959. |
| [9] | He Wanyu, Cheng Leping. Strategies and advance on stem cell transplantation for repair of spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(19): 3090-3096. |
| [10] | Zhan Lifen, Ai Kun, Zeng Xuejiu, Liang Rouyun, Ding Qiangsheng, Zhang Hong. Application and prospect of reconstructing bladder micturition reflex in neurogenic bladder after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(18): 2925-2931. |
| [11] | Xu Zihan, Bi Yunfeng, Li Jiang, Zhang Zongliang, Song Chen, Dong Jie, Liu Dong. Repetitive magnetic stimulation of S3 nerve root and M1 area for treating urinary retention after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(11): 1719-1723. |
| [12] | Shang Wenya, Ren Yafeng, Li Bing, Wei Huilin, Zhang Zhilan, Huang Xiaomeng, Huang Jing. Regulatory mechanisms and therapeutic strategies for pyroptosis after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(11): 1772-1779. |
| [13] | Yang Yulin, Chang Wanpeng, Ding Jiangtao, Xu Hongli, Wu Xiao, Xiao Boheng, Ma Lihong. Transcranial direct current stimulation at different targets for Parkinson’s disease: a network Meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(11): 1797-1804. |
| [14] | Wu Yangpeng, Yang Xiaohui, Lao Kecheng, Dai Shiyou, Fan Xiao. Characterization and repair effect of supramolecular conducting hydrogel carrying ligustrazine on spinal cord injury in rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(10): 1505-1511. |
| [15] | Long Qingxi, Zhang Pingshu, Liu Qing, Ou Ya, Zhang Lili, Yuan Xiaodong. Single-cell RNA sequencing reveals the heterogeneity of astrocytes [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 139-146. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||