Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (35): 5589-5595.doi: 10.12307/2022.912
Previous Articles Next Articles
Inhibiting NADPH oxidase alleviates hepatocellular injury and lipid metabolism disorder in a mouse model of alcoholic liver damage
Cui Wei1, Cui Di1, Ouyang Ting2, Li Xiang1, Wei Huiting1, Xue Weiyue1, Zhou Gang1, Qiu Ye2
- 1Department of Physical Education, 2College of Biology, Hunan University, Changsha 410000, Hunan Province, China
-
Received:2021-12-21Accepted:2022-01-29Online:2022-12-18Published:2022-05-17 -
Contact:Cui Di, PhD, Assistant professor, Department of Physical Education, Hunan University, Changsha 410000, Hunan Province, China -
About author:Cui Wei, Master candidate, Department of Physical Education, Hunan University, Changsha 410000, Hunan Province, China -
Supported by:the Project of Hunan Provincial Sports Bureau, No. 2020XH030 (to CW)
CLC Number:
Cite this article
Cui Wei, Cui Di, Ouyang Ting, Li Xiang, Wei Huiting, Xue Weiyue, Zhou Gang, Qiu Ye. Inhibiting NADPH oxidase alleviates hepatocellular injury and lipid metabolism disorder in a mouse model of alcoholic liver damage[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(35): 5589-5595.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
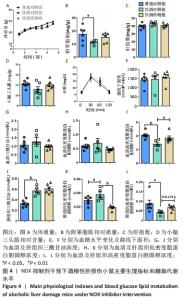
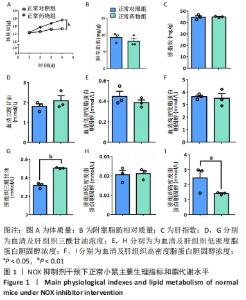
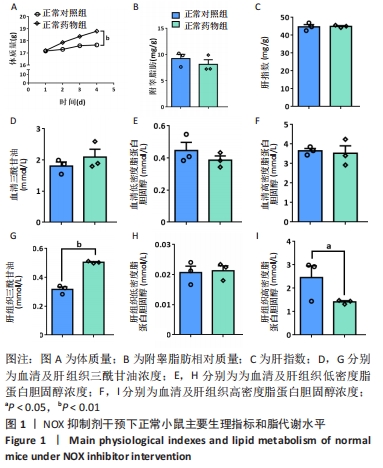
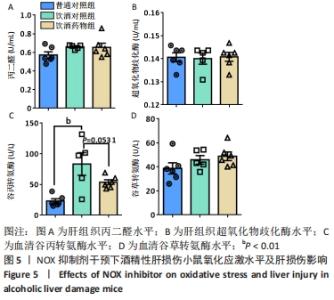
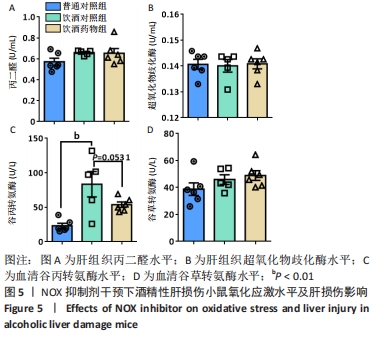
2.2 NOX抑制剂对酒精性肝损伤小鼠糖脂代谢及肝细胞损伤的影响(实验二结果) 2.2.1 实验动物数量分析 实验选用小鼠18只,分为3组,实验过程中无脱失,全部进入结果分析。 2.2.2 主要生理指标变化 与普通对照组相比,饮酒对照组小鼠体质量偏轻,附睾脂肪相对质量显著下降(P < 0.05),小腿三头肌相对含量减少且离散程度大,无显著性差异;服用葡萄糖溶液后30-60 min时间段内血糖下降明显减慢(P < 0.05);肝组织三酰甘油和低密度脂蛋白胆固醇浓度明显增加(P < 0.01,P < 0.05);血清及肝组织内高密度脂蛋白胆固醇浓度显著下降(P < 0.05,P < 0.01)。与饮酒对照组相比,饮酒药物组小鼠体质量、附睾脂肪和小腿三头肌相对含量均增加;服用葡萄糖溶液后30-60 min时间段血糖下降速度无显著性差异;肝组织内低密度脂蛋白胆固醇浓度显著下降(P < 0.01);血清内高密度脂蛋白胆固醇浓度显著增加(P < 0.01),肝组织内也有所上升(P=0.173 6),见图4。"
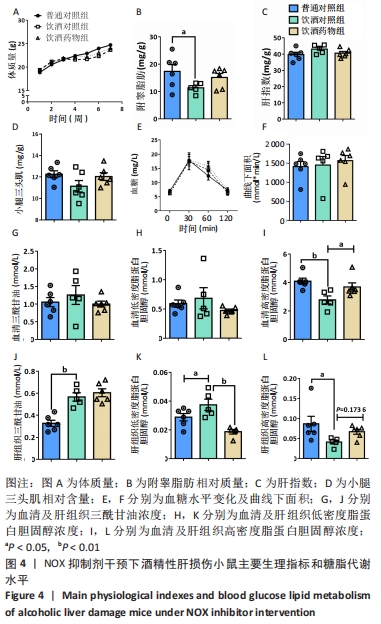
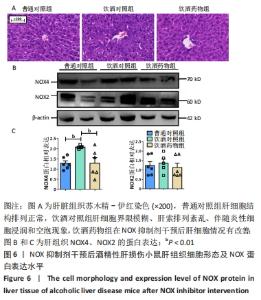
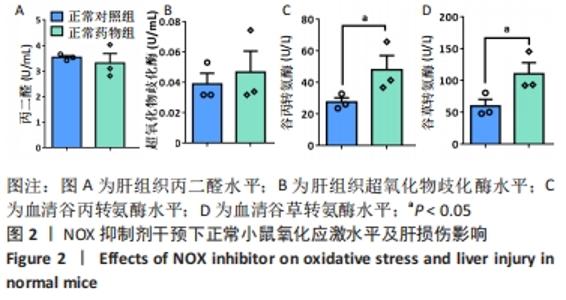
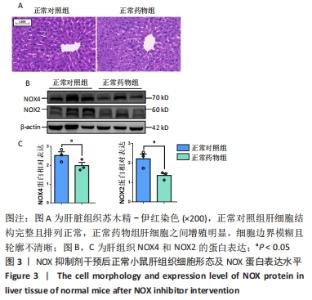
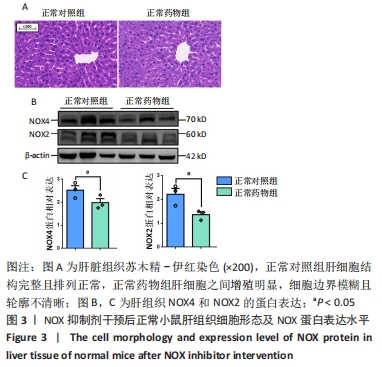
2.2.4 肝组织细胞形态及NOX蛋白表达的影响 普通对照组小鼠肝索结构清晰可见、细胞结构完整且排列整齐;与普通对照组相比,饮酒对照组小鼠肝细胞界限模糊、肝索排列紊乱、伴随炎性细胞浸润和空泡现象(P < 0.05 );NOX抑制剂干预后情况改善,饮酒药物组小鼠肝组织的小叶构造完整,炎性细胞的浸润程度缓解,相较于饮酒对照组差异无显著性意义。与普通对照组相比,饮酒对照组小鼠肝组织内NOX4和NOX2蛋白表达均有不同程度的升高,NOX4变化最显著(P < 0.01);NOX抑制剂干预后,肝组织中NOX2与NOX4蛋白表达均降低,肝组织蛋白NOX4的蛋白表达降低有显著性意义(P < 0.01),见图6。"
| [1] LI S, HONG M, TAN HY, et al. Insights into the Role and Interdependence of Oxidative Stress and Inflammation in Liver Diseases. Oxid Med Cell Longev. 2016; 2016:4234061. [2] ZHOU T, ZHANG YJ, XU DP, et al. Protective Effects of Lemon Juice on Alcohol-Induced Liver Injury in Mice. Biomed Res Int. 2017;2017:7463571. [3] LUSHUCHAK VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact. 2014;224:164-175. [4] SUMINMOTO H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008;275(13):3249-3277. [5] BRANDES RP, WEISSMANN N, SCHRODER K. Nox family NADPH oxidases: Molecular mechanisms of activation. Free Radic Biol Med. 2014;76:208-226. [6] SORCE S, KRAUSE KH, JAQUET V. Targeting NOX enzymes in the central nervous system: therapeutic opportunities. Cell Mol Life Sci. 2012;69(14):2387-2407. [7] MARTNER A, AYDIN E, HELLSTRAND K. NOX2 in autoimmunity, tumor growth and metastasis. J Pathol. 2019;247(2):151-154. [8] SINGEL KL, SEGAL BH. NOX2-dependent regulation of inflammation. Clin Sci (Lond). 2016;130(7):479-490. [9] CHAO Z, YONGJI X, LIZHI B, et al. Effects of Crocin on Nox2 Expression and ROS Level of Hypoxia/Reoxygenation-induced Injury of Cardiomyocytes. Medicinal Plant. 2020;11(5):71-75. [10] TISEO G, CAVARRETTA E, FORNITI A, et al. Interplay between Nox2 Activity and Platelet Activation in Patients with Sepsis and Septic Shock: A Prospective Study. Oxid Med Cell Longev. 2020;2020:4165358. [11] YANG Q, WU FR, WANG JN, et al. Nox4 in renal diseases: An update. Free Radic Biol Med. 2018;124:466-472. [12] CHEN C, LI L, ZHOU HJ, et al. The Role of NOX4 and TRX2 in Angiogenesis and Their Potential Cross-Talk. Antioxidants (Basel). 2017;6(2):42. [13] IIATOVSKAYA DV, BLASS G, PALYGIN O, et al. A NOX4/TRPC6 Pathway in Podocyte Calcium Regulation and Renal Damage in Diabetic Kidney Disease. J Am Soc Nephrol. 2018;29(7):1917-1927. [14] RAJARAM RD, DISSARD R, FAIVRE A, et al. Tubular NOX4 expression decreases in chronic kidney disease but does not modify fibrosis evolution. Redox Biol. 2019; 26:101234. [15] CHOUDHURY SR, BABES L, RAHN JJ, et al. Dipeptidase-1 Is an Adhesion Receptor for Neutrophil Recruitment in Lungs and Liver. Cell. 2019;178(5):1205-1221. [16] KANURI BN, REBELLO SC, PATHAK P, et al. Glucose and lipid metabolism alterations in liver and adipose tissue pre-dispose p47(phox) knockout mice to systemic insulin resistance. Free Radic Res. 2018;52(5):568-582. [17] TARAFDAR A, PULA G. The Role of NADPH Oxidases and Oxidative Stress in Neurodegenerative Disorders. Int J Mol Sci. 2018;19(12):3824. [18] QIN YY, LI M, FENG X, et al. Corrigendum to “Combined NADPH and the NOX inhibitor apocynin provides greater anti-inflammatory and neuroprotective effects in a mouse model of stroke” [Free Radic. Biol. Med. 104 (2017) 333-345].Free Radic Biol Med. 2018;115:498-499. [19] BERTOLA A, MATHEWS S, KI SH, et al. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc. 2013,8(3):627-637. [20] THOMPSON KJ, NAZARI SS, JACOBS WC, et al. Use of a crossed high alcohol preferring (cHAP) mouse model with the NIAAA-model of chronic-binge ethanol intake to study liver injury. Alcohol Alcohol. 2017;52(6):629-637. [21] 崔玮,崔迪.白细胞介素-6在酒精性肝病中的作用[J].中国生物化学与分子生物学报,2021,37(4):467-474. [22] SEITZ HK, BATALLER R, CORTEZ-PINTO H, et al. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4(1):16. [23] SZABO G. Gut-liver axis in alcoholic liver disease.Gastroenterology. 2015;148(1):30-36. [24] TORRUELLAS C, FRENCH SW, MEDICI V. Diagnosis of alcoholic liver disease. World J Gastroenterol. 2014;20(33):11684-11699. [25] SASAKI Y, DEHNAD A, FISH S, et al. NOX4 Regulates CCR2 and CCL2 mRNA Stability in Alcoholic Liver Disease. Sci Rep. 2017;7:46144. [26] DE MINICIS S, BRENNER DA. NOX in liver fibrosis. Arch Biochem Biophys. 2007; 462(2):266-272. [27] SHI Q, LEE DY, FELIERS D, et al. Interplay between RNA-binding protein HuR and Nox4 as a novel therapeutic target in diabetic kidney disease. Mol Metab. 2020;36:100968. [28] LI M, HE Y, ZHOU Z, et al. MicroRNA-223 ameliorates alcoholic liver injury by inhibiting the IL-6-p47(phox)-oxidative stress pathway in neutrophils. Gut. 2017; 66(4):705-715. [29] WANG X, ZHAO S, SU M, et al. Geraniol improves endothelial function by inhibiting NOX-2 derived oxidative stress in high fat diet fed mice. Biochem Biophys Res Commun. 2016;474(1):182-187. [30] CHENG S, GE J, ZHAO C, et al. Effect of aerobic exercise and diet on liver fat in pre-diabetic patients with non-alcoholic-fatty-liver-disease:A randomized controlled trial. Sci Rep. 2017;7(1):15952. [31] VIEIRA AF, COSTA RR, MACEDO RC, et al. Effects of aerobic exercise performed in fasted v. fed state on fat and carbohydrate metabolism in adults: a systematic review and meta-analysis. Br J Nutr. 2016;116(7):1153-1164. [32] MUSCELLA A, STEFANO E, LUNETTI P, et al. The Regulation of Fat Metabolism During Aerobic Exercise. Biomolecules. 2020;10(12):1699. [33] 欧阳婷,崔玮,陈伟恺,等. NOX介导氧化应激与阿尔兹海默病研究进展[J].生命科学,2020,32(12):1338-1345. [34] 刘姣,周刚,梅雨,等. 一次性力竭运动致大鼠骨骼肌氧化应激的机制[J].中国应用生理学杂志,2020,36(1):17-22. [35] LIANG S, KISSELEVA T, BRENNER DA. The Role of NADPH Oxidases (NOXs) in Liver Fibrosis and the Activation of Myofibroblasts. Front Physiol. 2016;7:17. [36] PAIK YH, KIM J, AOYAMA T, et al. Role of NADPH oxidases in liver fibrosis. Antioxid Redox Signal. 2014;20(17):2854-2872. [37] SANCHO P, MAINEZ J, CROSAS-MOLIST E, et al. NADPH oxidase NOX4 mediates stellate cell activation and hepatocyte cell death during liver fibrosis development. PLoS One. 2012;7(9):e45285. [38] GONCALVES I O, PASSOS E, ROCHA-RODRIGUES S, et al. Physical exercise antagonizes clinical and anatomical features characterizing Lieber-DeCarli diet-induced obesity and related metabolic disorders. Clin Nutr. 2015,34(2):241-247. |
| [1] | Cui Wei, Cui Di, Ouyang Ting, Li Xiang, Wei Huiting, Xue Weiyue, Zhou Gang, Qiu Ye. Inhibiting NOX alleviates alcoholic liver damage and lipid metabolism disorder [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(在线): 1-8. |
| [2] | Liu Yapu, Su Yuanyuan, Liu Qi, Yang Zhou, Li Rong, Huang Zucheng, Huang Zhiping, Wu Xiaoliang, Zhu Qingan. Antioxidative stress of trihydroxyethyl rutin on cervical spinal cord injury in rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(36): 5868-5874. |
| [3] | Yuan Xixi, Zhao Zirui, Zhang Yanyan, Huang Yu, Shi Kaikai, Fan Dengying, Zhu Yahui, Liu Chunyan. Effect of mandibular advancement device on mitochondrial ultrastructure of the genioglossus in the treatment of obstructive sleep apnea [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(35): 5626-5632. |
| [4] | Zhang Haiyong, Huang Jingwen, Xie Bingying, Chen Sainan, Xie Lihua, Chen Xuan, Li Shengqiang, Ge Jirong. Anti-osteoporosis effect of Gushukang in ovariectomized rats: a lumbar metabonomic analysis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(32): 5185-5190. |
| [5] | Gu Chao, Chen Weikai, Liu Tao, Yang Huilin, He Fan. Mitochondrial dysfunction affects osteogenic differentiation potential of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(31): 4921-4927. |
| [6] | Wei Hewei, Zheng Weipeng, Liu Zhijun, Zhao Guoyuan, Fang Weihua, Chen Sheng, Liao Zhihao, Wan Lei. Expression of autophagy in rotator cuff tendon stem cells induced by oxidative stress [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(31): 4954-4961. |
| [7] | Li Suyao, Guo Minfang, Yu Jingwen, Meng Tao, Mu Bingtao, Li Mengdi, Li Na, Song Lijuan, Ma Cungen, Yu Jiezhong. Effect of eriodictyol on the imbalance of mitochondrial dynamics and apoptosis in SH-SY5Y cells induced by hydrogen peroxide [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(31): 4975-4981. |
| [8] | Liao Rongzhen, Chen Dejun, He Mincong, Huang Xingru, Fang Jian, Zhu Genfu. Correlation between TCM syndrome types and ERK in lipid metabolism of senile osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(29): 4704-4708. |
| [9] | Mi Jianguo, Qiao Rongqin, Liu Shaojin. Bushen Jianpi Huoxue Recipe improves bone metabolism, oxidative stress, and autophagy in osteoporotic rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(26): 4147-4152. |
| [10] | Zhao Zheyi, Wang Zhengrong, Fang Xingyan, Wang Tian, Xie Tingting. Effects of sodium arsenite on oxidative stress, apoptosis and Hippo signaling pathway in AML12 hepatocytes [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(26): 4186-4191. |
| [11] | Deng Bowen, Li Xiaoye, Jiang Shengyuan, Liu Gan, Zhao Yi, Zhang Houjun, He Feng, Mu Xiaohong. Mechanisms of Huangqi Guizhi Wuwu Decoction in the treatment of cervical spondylosis and anxiety disorder based on the principle of “treating different diseases with the same method”: a network pharmacology analysis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(23): 3650-3656. |
| [12] | Wang Sihan, Chen Junji, Mo Weibin. Effects of Dendrobium officinale flavonoid on oxidative stress and autophagy in the liver of an exhaustive exercise rat model [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(20): 3212-3219. |
| [13] | Li Anan, Jiang Tao, Zhan Min, Cai Yuning, Song Min, Li Congcong, Lin Wenzheng, Zhang Jiayuan, Liu Wengang. Pharmacological mechanism of Shenling Baizhu San in the treatment of knee osteoarthritis based on network pharmacology and molecular docking [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(2): 197-204. |
| [14] | Li Wanhai, Dong Yuhang, Shi Chao, Jiang Yiyao, Liu Ge, Diao Wenjie, Wu Zhen. Ligustrazine furoxan complex protects against renal ischemia-reperfusion injury in mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(2): 205-210. |
| [15] | Gu Qingfang, Guo Minfang, Liu Xiaoqin, Mu Bingtao, Li Weimei, Song Lijuan, Chai Zhi, Ma Cungen, Yu Jiezhong. Mechanism by which bone marrow mesenchymal stem cells improve cognitive function in APP/PS1 double transgenic mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(19): 2964-2969. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||