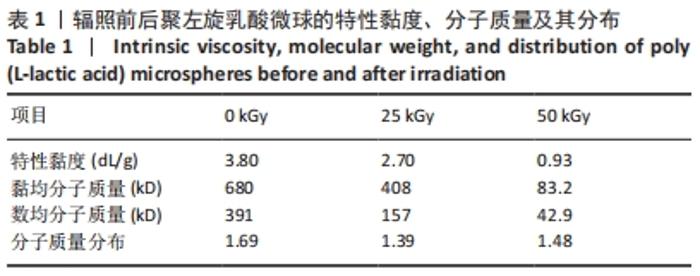
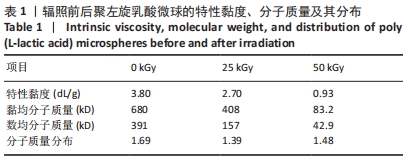
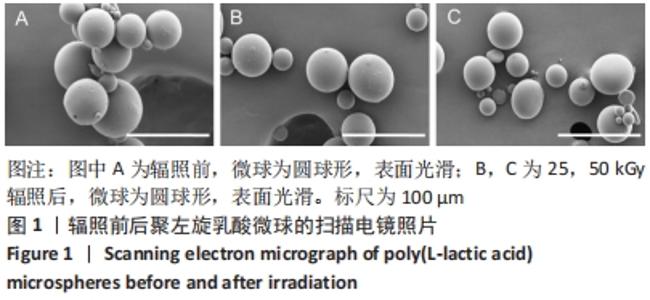
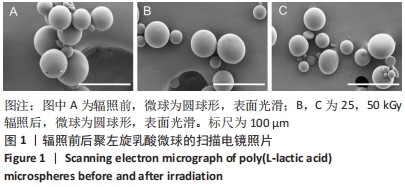
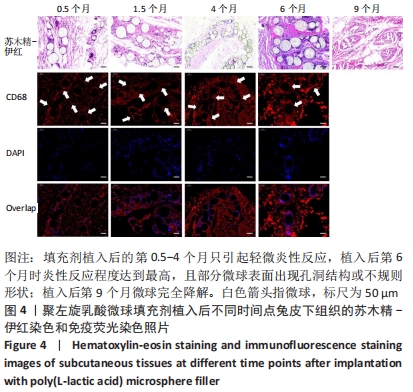
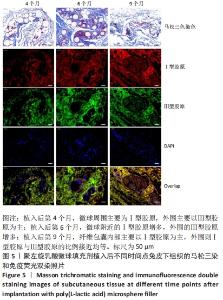
[1] KONTIS THEDA C. Contemporary Review of Injectable Facial Fillers. Jama Facial Plast Su. 2013;15(1):58.
[2] AKINBIYI T, OTHMAN S, FAMILUSI O, et al. Better Results in Facial Rejuvenation with Fillers. Plast Reconstr Surg-Glob Open. 2020;8(10): e2763.
[3] LUEBBERDING S, ALEXIADES-ARMENAKAS M. Facial Volume Augmentation in 2014: Overview of Different Filler Options. J Drugs Dermatol. 2013;12(12):1339-1344.
[4] LEE JC, LORENC ZP. Synthetic Fillers for Facial Rejuvenation. Clin Plast Surg. 2016;43(3):497-503.
[5] STEIN P, VITAVSKA O, KIND P, et al. The biological basis for poly-l-lactic acid-induced augmentation. J Dermatol Sci. 2015;78(1):26-33.
[6] FITZGERALD R, BASS LM, GOLDBERG DJ, et al. Physiochemical Characteristics of Poly-L-Lactic Acid (PLLA). Aesthet Surg J. 2018;38: 13-17.
[7] LEE JM, KIM YJ. Foreign body granulomas after the use of dermal fillers: pathophysiology, clinical appearance, histologic features, and treatment. Arch Plast Surg. 2015;42(2):232-239.
[8] VLEGGAAR D. Soft-Tissue Augmentation and the Role of Poly-L-Lactic Acid. Plast Reconstru Surg. 2006;118:S46-S54.
[9] JABBAR A, ARRUDA S, SADICK N. Off Face Usage of Poly-L-Lactic Acid for Body Rejuvenation. J drugs dermatol. 2017;16(5):489-494.
[10] FISHER GJ, VARANI J, VOORHEES JJ. Looking Older: Fibroblast Collapse and Therapeutic Implications. Arch Dermatol. 2008;144(5):666-672.
[11] GOLDBERG D, GUANA A, VOLK A, et al. Single-Arm Study for the Characterization of Human Tissue Response to Injectable Poly-L-Lactic Acid. Dermatol Surg. 2013;39(6):915-922.
[12] PRAJAPATI VD, JANI GK, KAPADIA JR. Current knowledge on biodegradable microspheres in drug delivery. Expert Opin Drug Del. 2015;12(8):1-17.
[13] WENG H, WU Z, ZHAO C, et al. Construction of multi-hollow polymer microspheres with tailored mesoporous wall. Polymer Chemistry. 2019; 10(12):1508-1518.
[14] RAMAZANI F, CHEN W, VAN NOSTRUM CF, et al. Formulation and characterization of microspheres loaded with imatinib for sustained delivery. Int J Pharm. 2015;482(1):123-130.
[15] KWON T, HAN SW, YEO IK, et al. Biostimulatory effects of polydioxanone, poly-d, l lactic acid, and polycaprolactone fillers in mouse model. J Cosmet Dermatol. 2019;18(4):1002-1008.
[16] MATLAGA BF, YASENCHARK LP, SALTHOUSE TN. Issue response to implanted polymers: the significance of sample shape. J Biomed Mater Res. 1976;10:391-397.
[17] DAVISON NOEL L, BARRèRE-DE GROOT F, GRIJPMA DW. Chapter 6 - Degradation of Biomaterials //BLITTERSWIJK CAV, DE BOER J. Tissue Engineering (2nd ed). Oxford; Academic Press, 2014:177-215.
[18] GINJUPALLI K, SHAVI GV, AVERINENI RK, et al. Poly(α-hydroxy acid) based polymers: A review on material and degradation aspects. Polym Degrad Stabil. 2017;144:520-535.
[19] GB/T 16886.5-2017.医疗器械生物学评价第五部分:体外细胞毒性试验[S].北京:中国标准出版社,2017.
[20] ENGLAND LJ, TAN MH, SHUMAKER PR, et al. Effects of monopolar radiofrequency treatment over soft-tissue fillers in an animal model. Lasers Surg Med. 2010;37(5):356-365.
[21] KIM CM, KIM BY, SUH HD, et al. The efficacy of powdered polydioxanone in terms of collagen production compared with poly‐L‐lactic acid in a murine model. J Cosmet Dermatol. 2019;18:1-6.
[22] LEMPERLE G, MORHENN V, CHARRIER U. Human Histology and Persistence of Various Injectable Filler Substances for Soft Tissue Augmentation. Aesthetic Plast Surg. 2003;27(5):354-366.
|