中国组织工程研究 ›› 2025, Vol. 29 ›› Issue (31): 6811-6820.doi: 10.12307/2025.548
• 干细胞综述 stem cell review • 上一篇
不同来源间充质干细胞治疗炎症性肠病
袁 潇1,梁松林1,谢亚楠1,管冬梅1,樊龙雨1,殷晓轩2
- 1山东中医药大学,山东省济南市 250355;2 兖矿新里程总医院,山东省济宁市 273599
-
收稿日期:2024-08-05接受日期:2024-09-14出版日期:2025-11-08发布日期:2025-02-28 -
通讯作者:殷晓轩,博士,主任医师,兖矿新里程总医院,山东省济宁市 273599 -
作者简介:袁潇,女,1999年生,湖北省咸宁市人,汉族,2024年山东中医药大学毕业,硕士,医师,主要从事中西医结合内科疾病研究。 -
基金资助:山东省中医药科技项目(2020Z41),项目负责人:殷晓轩
Mesenchymal stem cells from different sources in treatment of inflammatory bowel disease
Yuan Xiao1, Liang Songlin1, Xie Yanan1, Guan Dongmei1, Fan Longyu1, Yin Xiaoxuan2
- 1Shandong University of Traditional Chinese Medicine, Jinan 250355, Shandong Province, China; 2Yankuang New Journey General Hospital, Jining 273599, Shandong Province, China
-
Received:2024-08-05Accepted:2024-09-14Online:2025-11-08Published:2025-02-28 -
Contact:Yin Xiaoxuan, MD, Chief physician, Yankuang New Journey General Hospital, Jining 273599, Shandong Province, China -
About author:Yuan Xiao, MS, Physician, Shandong University of Traditional Chinese Medicine, Jinan 250355, Shandong Province, China -
Supported by:Shandong Province Traditional Chinese Medicine Science and Technology Project, No. 2020Z41 (to YXX)
摘要:
文题释义:
间充质干细胞:是一类具有多向分化潜能的干细胞,能够分化成各种类型细胞,如脂肪细胞、成骨细胞、软骨细胞等。间充质干细胞在体内分布广泛,可以从骨髓、脂肪、脐带等多种组织中分离得到。间充质干细胞已被研究用于治疗多种疾病,在组织修复和再生、免疫调节、抗炎等多方面发挥重要作用。炎症性肠病:是一种长期的慢性肠道炎性疾病,主要包括溃疡性结肠炎和克罗恩病,前者主要损害结肠和直肠,后者可损害从口腔到肛门之间的任意胃肠道部位,以小肠末端和结肠多见,主要临床表现为腹痛、腹泻、血便、体质量减轻等。
摘要
背景:炎症性肠病发病率逐渐升高,且暂无良好的治疗方案,研究表明与传统治疗方法相比,间充质干细胞治疗炎症性肠病展现出较佳的疗效。
目的:综述不同来源间充质干细胞治疗炎症性肠病的作用机制,为后续研究提供思路。
方法:以“间充质干细胞,外泌体,炎症性肠病,溃疡性结肠炎,克罗恩病”和“mesenchymal stem cells,MSCs,exosomes,extracellular vesicles,EVs,inflammatory bowel disease,IBD,ulcerative colitis,UC,Crohn’s disease,CD”分别作为中、英文关键词,在中国知网、PubMed数据库进行检索,最终纳入符合标准的89篇文献进行综述。
结果与结论:目前用于治疗炎症性肠病的间充质干细胞共有6类,分别为骨髓间充质干细胞、脂肪间充质干细胞、围产期组织来源间充质干细胞、诱导多能干细胞衍生的间充质干细胞、胚胎干细胞衍生的间充质干细胞、牙龈间充质干细胞。移植方式共有5种,静脉注射和腹腔注射为最常用方式,此外还有局部注射、肠系膜注射、灌肠3种方式。上述间充质干细胞减轻炎症性肠病症状或延缓其进展的主要机制包括分化、再生、抗炎、免疫调节、神经保护、抗氧化应激、归巢、调节肠道菌群、自噬、铁死亡、内质网应激等。不同来源间充质干细胞在功能上有一定相似性,但又具有不同特点,这些特点使它们具有独特优势和治疗潜力。然而,目前对不同来源间充质干细胞特性的研究仍然有限,需要对不同来源间充质干细胞之间的细微差别进行更深入研究,以优化其治疗炎症性肠病的潜力,同时,临床使用安全性也有待进一步观察。
中图分类号:
引用本文
袁 潇, 梁松林, 谢亚楠, 管冬梅, 樊龙雨, 殷晓轩. 不同来源间充质干细胞治疗炎症性肠病[J]. 中国组织工程研究, 2025, 29(31): 6811-6820.
Yuan Xiao, Liang Songlin, Xie Yanan, Guan Dongmei, Fan Longyu, Yin Xiaoxuan. Mesenchymal stem cells from different sources in treatment of inflammatory bowel disease[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6811-6820.
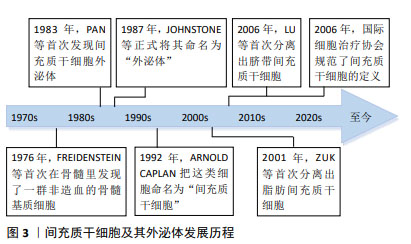
2.2 骨髓间充质干细胞 骨髓间充质干细胞是被发现的首个间充质干细胞类型,因其易于分离培养和卓越的体外多谱系分化的特性,在实验模型中占主导地位。
2.2.1 分化 骨髓间充质干细胞移植到炎症性肠病大鼠体内后,可定植于受损的肠上皮各层细胞中,通过激活Wnt/β-catenin信号通路抑制骨髓间充质干细胞的自我增殖,促使其分化为肠上皮细胞,补充受损的肠上皮组织,修复炎症区域[8]。
2.2.2 再生 骨髓间充质干细胞具有较强的再生作用,由造模引起的隐窝丢失、脓肿形成和局灶性溃疡在移植骨髓间充质干细胞后消失,取而代之的是上皮分岔、不规则隐窝和杯状细胞增多等肠黏膜修复表现,表明骨髓间充质干细胞可促进实验性肠损伤后黏膜细胞再生,对受损结肠有较强的再生和保护作用[9-10]。另有研究表明移植骨髓间充质干细胞来源外泌体也可通过刺激上皮再生修复和减少上皮细胞凋亡来改善结肠炎[11]。
2.2.3 抗炎 研究表明,使用骨髓间充质干细胞能够减少中性粒细胞向炎症结肠的浸润,降低炎症因子表达,提示骨髓间充质干细胞可能通过直接影响体内炎症细胞来发挥抗炎作用[12],使用白细胞介素6预处理可增强这种抗炎功能[13]。骨髓间充质干细胞外泌体通过抑制体内炎症反应、环氧化酶2和核因子κB激活及p38丝裂原活化蛋白激酶磷酸化缓解小鼠溃疡性结肠炎[14]。此外,工程化干细胞也被证实在抗炎方面治疗炎症性肠病有显著疗效。过表达程序性死亡配体1的骨髓间充质干细胞外泌体通过阻断磷脂酰肌醇3-激酶/蛋白激酶B/哺乳动物雷帕霉素靶蛋白(phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin,PI3K/AKT/mTOR)通路的激活和调节Th17/Treg细胞的平衡来减轻结肠炎症[15]。另外,使用慢病毒转染制备过表达H19的骨髓间充质干细胞用于治疗溃疡性结肠炎小鼠,通过下调miR-139和miR-141的表达,从而上调其靶基因细胞间黏附分子1和趋化因子受体4的表达,减弱硫酸葡聚糖钠盐诱导的结肠炎症反应[16]。
另一项研究中,骨髓间充质干细胞来源外泌体被血红素加氧酶1修饰后,增加了炎症环境中miR-200b的表达,通过靶向高迁移率族蛋白B3减轻肠上皮细胞的炎性损伤[17]。
2.2.4 免疫调节 腹腔注射骨髓间充质干细胞后观察到小鼠腹腔内形成含有巨噬细胞、B细胞和T细胞的聚集体,而敲除骨髓间充质干细胞中的血小板反应蛋白1可显著减弱其诱导白细胞介素10阳性B细胞的作用,提示骨髓间充质干细胞可能通过旁分泌血小板反应蛋白1诱导产生表达白细胞介素10的B细胞,从而有助于结肠炎治疗[18]。Foxp3为控制Treg细胞发育和功能的关键转录因子,骨髓间充质干细胞治疗可以提高溃疡性结肠炎小鼠脾中Foxp3的表达,诱导Treg细胞分化[19]。此外,过表达程序性死亡受体配体1的骨髓间充质干细胞外泌体可通过抑制急性结肠炎小鼠结肠中T细胞的增殖,调节Treg细胞的应答,靶向和修复组织病变[20]。
2.2.5 神经保护 与肠道炎症相关的肠神经系统损伤可能是肠道功能持续改变的基础,在治疗中发现骨髓间充质干细胞可通过产生营养因子来防止轴突损伤并促进神经纤维再生,其作用机制可能是减少结肠炎相关的肌肠神经元丢失和神经纤维结构损伤[21]。在随后的实验中观察到骨髓间充质干细胞对结肠炎的这种神经保护作用在一定范围内呈剂量依赖性[22],在结肠炎诱导后3 h给药1×106个细胞时,骨髓间充质干细胞有能力防止对肠神经系统损伤,而更高剂量没有进一步的益处。另外,移植骨髓间充质干细胞可降低小鼠肌肠神经元氧化应激和超氧化物生成标志物水平,表明骨髓间充质干细胞有望通过保护肠神经系统免受氧化应激诱导的损伤,作为缓解慢性结肠炎的治疗干预手段[23]。
2.2.6 抗氧化应激 氧化应激已被证实在炎症性肠病的进展中发挥作用,移植骨髓间充质干细胞可降低超氧化物歧化酶和过氧化氢酶水平,发挥抗氧化应激作用,达到治疗炎症性肠病的目的[24]。在缺氧环境下,骨髓间充质干细胞外泌体通过限制肠上皮细胞活性氧积累和减少缺氧诱导因子1α的DNA损伤来减轻溃疡性结肠炎损伤[25]。许多研究表明低浓度过氧化氢预处理可以保护细胞免受氧化损伤,并在调节干细胞生理过程中发挥类似生长因子的作用[26]。LIU等[27]通过实验证实25 μmol/L是过氧化氢预处理间充质干细胞的最佳浓度,且间充质干细胞外泌体主要是通过增强Nrf2/Keap1/ARE信号通路[28],减轻氧化应激诱导的细胞死亡。
2.2.7 归巢 在机体受到损伤或炎症刺激时,间充质干细胞能够定向迁移到受损或炎症部位,这种归巢功能被证实是间充质干细胞治疗疾病的一大特点[29]。使用CXCR2 mRNA修饰的骨髓间充质干细胞治疗炎症性肠病时,可观察到间充质干细胞特异性地迁移到发炎的结肠部位,以响应从结肠受损组织释放的巨噬细胞炎症蛋白2和巨噬细胞炎症蛋白5,显著降低促炎因子水平,减轻小鼠炎症性肠病的症状[30]。
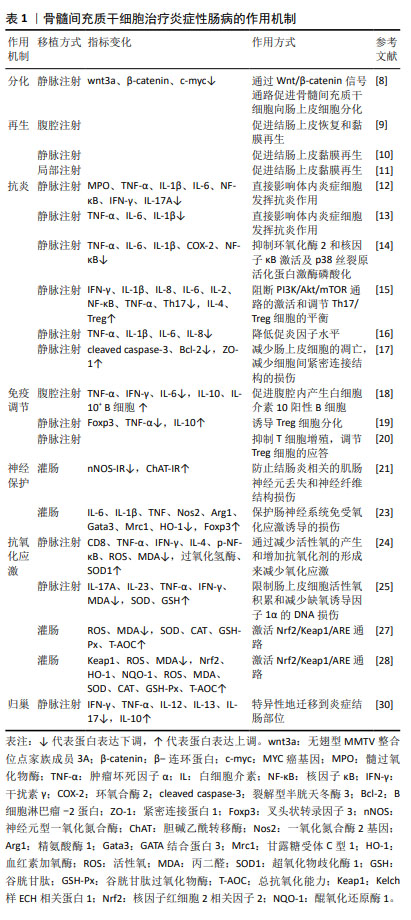
骨髓间充质干细胞治疗炎症性肠病的作用机制,见表1。
2.3 脂肪间充质干细胞 脂肪间充质干细胞在2001年由Zuk利用负压吸引技术从人脂肪组织中鉴定和分离出来。相较于其他来源的间充质干细胞,脂肪间充质干细胞易于获取、分离程序简单、增殖能力较强,这些特征使脂肪间充质干细胞成为目前治疗炎症性肠病最有前途的方法之一。
2.3.1 抗炎 脂肪间充质干细胞主要是通过减少炎症细胞在肠道的浸润和下调各种炎症递质的产生,抑制黏膜增厚及黏膜下细胞浸润等事件,以发挥其抗炎作用[31]。类似的结果在另一项关于脂肪间充质干细胞外泌体的实验中被证实,实验组促炎因子的表达下调,抑炎因子的表达上调,结肠炎性损伤得到有效修复[32]。此外,有研究选用肠系膜注射脂肪间充质干细胞的方法,发现肠系膜注射脂肪间充质干细胞除了调节炎症因子水平外,还可通过抑制炎症性Th17细胞反应改善三硝基苯磺酸诱导的结肠炎[33]。
2.3.2 免疫调节 脂肪间充质干细胞外泌体可通过调节Treg细胞种群,减少肠黏膜细胞浸润,使炎症性肠病小鼠的临床症状和组织损伤减轻[34]。除Treg细胞外,脂肪间充质干细胞还可调节免疫细胞亚群如肠固有层的树突状细胞和巨噬细胞,从而降低总体临床评分和组织损伤,减轻炎症反应[35],这些对免疫失衡的纠正作用可持续存在,并对长期维持肠道稳态至关重要[36]。另外,M1和M2巨噬细胞在免疫调节中发挥着重要作用。YUAN等[37]发现脂肪间充质干细胞可通过抑制琥珀酸盐积累,增加脯氨酰羟化酶2来阻止M1巨噬细胞过表达缺氧诱导因子1α,从而重新编程M1巨噬细胞的糖酵解途径,逆转其促炎作用。研究证明巨噬细胞极性可在早期注射或缺氧环境诱导下转变为抗炎M2表型[38-39],移植的脂肪间充质干细胞大多数在腹腔内形成聚集体,并通过分泌因子在远离炎症结肠的部位诱导为M2巨噬细胞,从而缓解结肠炎症状[40]。
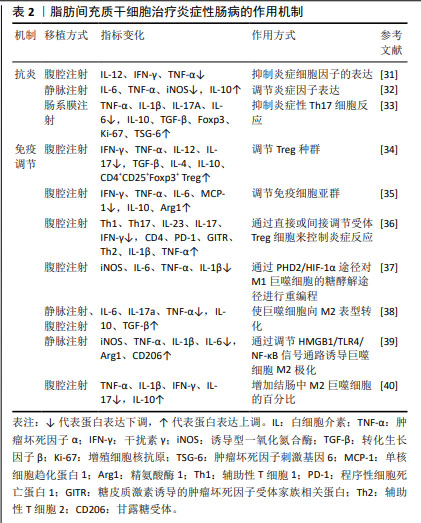
脂肪间充质干细胞治疗炎症性肠病的作用机制,见表2。
2.4 围产期组织来源间充质干细胞 自脐带间充质干细胞于2006年被首次分离得出后,研究人员先后从围产期组织中分离出不同间充质干细胞用于治疗炎症性肠病,它们既拥有与骨髓间充质干细胞相似的基因谱,也具有更强的增殖能力,且在获取渠道上更不受阻碍,因此成为了一种新型的替代方案。
2.4.1 再生 工程化间充质干细胞是细胞治疗的重要发展方向,过表达白细胞介素10基因的人脐带间充质干细胞可促进肠组织细胞增殖和血管再生进而促进结肠组织修复[41]。此外,脐带间充质干细胞来源外泌体被证实同样具有优异的再生能力,富含亮氨酸重复序列的G蛋白偶联受体5是肠道干细胞的特异性标志物,脐带间充质干细胞来源外泌体可上调其表达水平,赋予肠道类器官更优异的生长和出芽能力,加速肠道干细胞和上皮再生,促进实验性结肠炎的黏膜愈合[42]。
2.4.2 抗炎 研究表明脐带间充质干细胞可抑制细胞外调节蛋白激酶磷酸化,从而增强中性粒细胞从N1表型到N2表型的极化,使小鼠肠道炎症得到缓解[43]。另有其他从围产期组织中分离得到的间充质干细胞也发挥了较强的抗炎能力,如从羊膜中分离得到的间充质干细胞可抑制炎症递质在大鼠结肠中的表达水平,对大鼠结肠炎有显著改善作用[44]。将羊水来源间充质干细胞外泌体应用于脂多糖诱导的人肠道上皮下肌成纤维细胞炎症模型,外泌体处理组促炎反应显著降低,表现出外泌体具有治疗炎症性肠病的潜力[45]。而胎盘来源间充质干细胞衍生外泌体通过调节结肠炎小鼠的促炎和抗炎细胞因子之间的平衡来减轻结肠炎[46],将小檗碱工程化载入胎盘间充质干细胞外泌体中,除了可观察到结肠中炎症因子的表达降低,还可观察到巨噬细胞向M2表型极化增强,从而改善小鼠结肠炎[47]。
2.4.3 免疫调节 脐带间充质干细胞可减少炎症结肠的T细胞浸润,增加肠系膜淋巴结中Treg细胞的数量,且干扰素γ刺激可通过分泌更多的前列腺素E2和吲哚胺2,3-双加氧酶增强这种免疫抑制作用[48]。QI等[49]证明脐带间充质干细胞在输注至体内后,可阻止Tr1细胞凋亡,促进Tr1细胞增殖,增加脾脏和肠系膜淋巴结中Tr1细胞比例,在结肠炎中发挥免疫调节作用。此外,脐带间充质干细胞及其外泌体在诱导Treg细胞的同时还可抑制Th1的生成,调节Th1/Th17/Treg细胞和相关细胞因子的再平衡,有效缓解小鼠结肠炎症状[50-52]。还有研究表明脐带间充质干细胞来源外泌体主要通过肿瘤坏死因子α诱导基因6蛋白修复黏膜屏障,维持Th2和Th17细胞之间的平衡,达到治疗炎症性肠病的目的[53]。
2.4.4 调节肠道菌群 肠道菌群失调是肠道屏障功能和免疫稳态失衡的重要因素,下调导致肠道菌群失调的优势差异菌群,可重塑肠道菌群的结构和多样性,加速整体肠道健康和愈合[54]。进一步实验中使用广谱抗生素消耗小鼠肠道菌群,发现随着肠道菌群的消耗,实验组与对照组的炎症反应呈现出相同的趋势,表明肠道菌群的存在对于结肠炎肠道免疫稳态的保护作用是不可或缺的[55]。另外,通过降低硫代谢和核黄素代谢活性,逆转被三硝基苯磺酸破坏的氨基酸、赖氨酸和鞘脂代谢以及次生胆汁酸的生物合成,也可使异常菌群恢复正常[56]。
2.4.5 自噬 自噬在维持肠道稳态、调节肠道生态和抗微生物保护中起着关键作用[57],可通过诱导溶酶体降解紧密连接蛋白调节肠道屏障功能,从而降低肠上皮通透性[58]。PAN等[59]研究发现脐带间充质干细胞可促进肠紧密连接蛋白的表达,下调结肠组织中自噬标志物LC3A/B的蛋白表达,从而延缓肠上皮细胞自噬死亡,维持肠壁的机械屏障。
2.4.6 铁死亡 研究发现,炎症性肠病患者受损的肠道可表现出铁沉积、谷胱甘肽耗竭和脂质过氧化等铁死亡的基本特征[60]。WANG等[61]证明脐带间充质干细胞通过上调铁死亡相关基因,抑制肠上皮细胞铁死亡,促进肠道干细胞和上皮细胞再生从而达到改善炎症性肠病的目的。miR-129-5p修饰脐带间充质干细胞来源外泌体,可靶向酰基辅酶A合成酶长链家族成员4抑制脂质过氧化和铁死亡,通过减少肠道炎症和修复损伤来缓解炎症性肠病[62]。
2.4.7 内质网应激 肠道上皮细胞是内质网应激的主要感受器之一,当肠道受到炎症刺激时,上皮细胞内的内质网应激反应会被激活[63]。BANERJEE等[64]使用NOD.CB17-Prkdcscid/J小鼠进行实验,其特征是具有严重的免疫缺陷,发现内质网应激相关蛋白的表达显著下降,且基质金属蛋白酶2和基质金属蛋白酶9的表达也下降,从而维持结肠黏膜的完整性,防止组织降解和炎症细胞向结肠迁移。
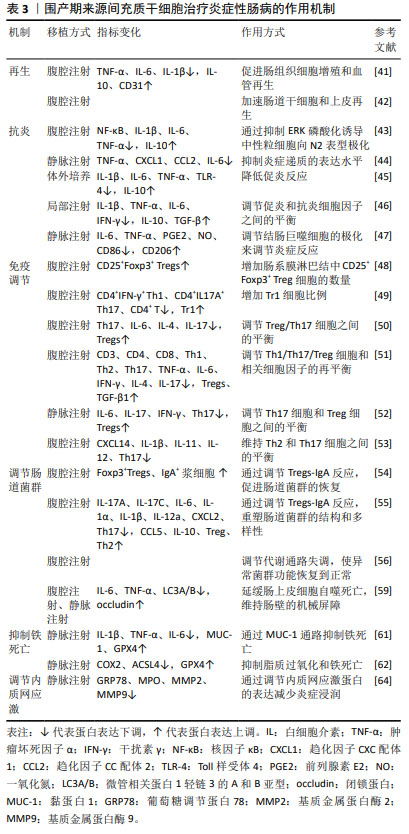
围产期组织来源间充质干细胞治疗炎症性肠病的作用机制见表3。
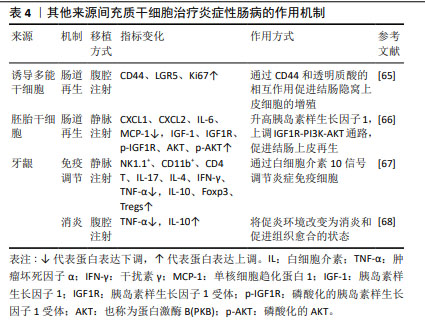
2.5 其他来源的间充质干细胞 除上述三大主要来源的间充质干细胞外,还有其他来源的间充质干细胞在治疗炎症性肠病方面展现了较佳疗效,见表4。如诱导多能干细胞衍生的间充质干细胞可促进结肠炎小鼠的黏膜愈合,表现为实验组溃疡边缘每个隐窝的CD44+上皮细胞和增殖上皮细胞(Ki67+)的数量增加,即通过CD44和透明质酸的相互作用促进结肠隐窝上皮细胞的增殖[65]。研究发现,胚胎干细胞衍生的间充质干细胞可提高结肠炎小鼠的循环胰岛素样生长因子1水平,上调下游IGF1R-PI3K-AKT通路,维持结肠上皮的完整性[66]。牙龈来源间充质干细胞可通过白细胞介素10信号调节炎症免疫细胞,使用抗白介素10受体抗体治疗逆转了这种作用[67],证实白细胞介素10可能是介导牙龈来源间充质干细胞在炎症性肠病中发挥保护作用的关键细胞因子。除此之外,过表达缺氧诱导因子的牙龈来源间充质干细胞外泌体可以与免疫细胞、内皮细胞和成纤维细胞相互作用,并将炎症性肠病中促炎环境改变为消炎和促进组织愈合的状态[68]。
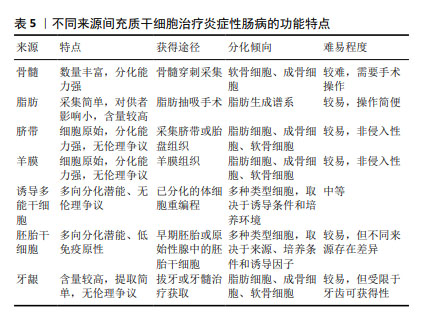
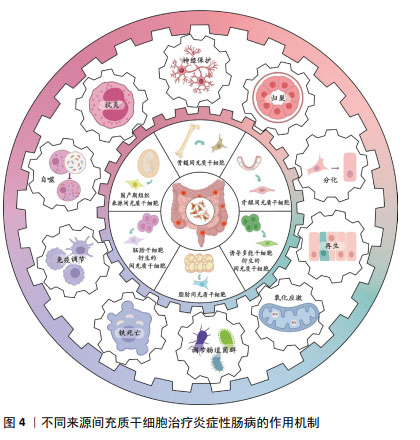
2.6 不同来源的间充质干细胞功能对比 骨髓间充质干细胞在实验中占主导地位,而大多数临床试验选用的是脂肪间充质干细胞,因此在间充质干细胞的最佳组织来源方面并没有达成共识。不同组织来源间充质干细胞之间的功能差异突出了它们的不同特性,从而适用于特定的治疗应用,它们治疗炎症性肠病的功能特点见表5,作用机制见图4。有证据表明脐带间充质干细胞具有较高的增殖率,其次是脂肪间充质干细胞,效力均超过骨髓间充质干细胞[69];在治疗炎症性肠病时,脂肪间充质干细胞产生的集落形成单位数量是骨髓间充质干细胞2倍以上[70]。而不同的间充质干细胞可能分化产生不同的细胞系,骨髓间充质干细胞表现出更倾向软骨细胞和成骨细胞分化[71],而与骨髓间充质干细胞相比,脐带间充质干细胞更倾向于成骨分化[72],脂肪间充质干细胞则表现出明显更强的脂肪生成谱系分化倾向以及更高的血管生成潜力[73]。对抗炎功能进行比较时,可观察到脐带间充质干细胞和骨髓间充质干细胞在体内展现出比胚胎干细胞和诱导多能干细胞衍生间充质干细胞更佳的抗炎效果[74],而骨髓间充质干细胞也比脂肪间充质干细胞更具治疗性[75]。另外,在免疫调节方面,有研究认为脂肪间充质干细胞发挥免疫调节作用的速度比骨髓间充质干细胞慢[70],这可能是不同来源间充质干细胞之间存在细微而独特的免疫调节特性,使得在不同实验条件下展现出不同的结果,而现有的实验尚未对这些细微之处进行深入的比较。
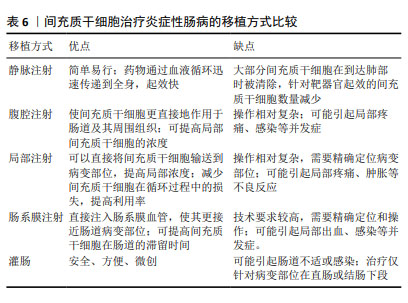
2.7 不同移植方式对间充质干细胞治疗炎症性肠病的影响 目前静脉注射与腹腔注射是治疗炎症性肠病最常用的移植方式,有研究表明在改善炎症性肠病小鼠生存率方面腹腔注射优于静脉注射,而溃疡修复效果方面静脉注射优于腹腔注射[76],意味着2种给药方式发挥治疗作用的机制有差异,可能各有优劣之处。根据炎症性肠病的症状特点,近来又出现局部注射、肠系膜注射、灌肠等针对性的给药方式。现有的间充质干细胞不同给药方式治疗炎症性肠病的优缺点见表6。
2.8 不同来源间充质干细胞治疗炎症性肠病的临床药物转化 目前关于间充质干细胞及其外泌体的临床药物转化是研究热点,得到国家药品监督管理局批准的用于治疗炎症性肠病的间充质干细胞临床默示许可现有3项[77],但国内未有相关药物获批上市。Darvadstrocel是同种异体脂肪来源间充质干细胞,可用于克罗恩病难治性肛瘘的治疗,分别于2018年和2021年在欧盟和日本获批上市。Darvadstrocel已被证实在多个国家和地区的临床试验中取得较佳疗效[78-80],与安慰剂组相比,Darvadstrocel组的临床缓解比例具有统计学上的显著优势(50% vs. 34%)[81],且治疗后的临床缓解可长达104周[82]。Cupistem于2012年在韩国获批上市,是自体脂肪来源间充质干细胞产品[83]。
目前在部分国家已获批上市的两种产品均为脂肪来源,但其他来源的间充质干细胞如骨髓[84-86]、脐带[87]、羊膜[88]、胎盘等[89],也被证实在临床试验中发挥疗效。然而,这些临床研究具有单中心、样本量小、缺乏随机对照设计等缺点,且缺乏长期随访数据的积累,如何优化给药途径或提高疗效及安全性,仍面临诸多挑战。
| [1] ANIWAN S, SANTIAGO P, LOFTUS EV JR, et al. The epidemiology of inflammatory bowel disease in Asia and Asian immigrants to Western countries. United European Gastroenterol J. 2022;10(10):1063-1076. [2] 吴开春,梁洁,冉志华,等.炎症性肠病诊断与治疗的共识意见(2018年·北京)[J].中国实用内科杂志,2018,38(9):796-813. [3] GIRAUD EL, THOMAS PWA, VAN LINT JA, et al. Adverse Drug Reactions from Real-World Data in Inflammatory Bowel Disease Patients in the IBDREAM Registry. Drug Saf. 2021;44(5):581-588. [4] WANG Y, FANG J, LIU B, et al. Reciprocal regulation of mesenchymal stem cells and immune responses. Cell Stem Cell. 2022;29(11): 1515-1530. [5] WANG Y, HUANG B, JIN T, et al. Intestinal Fibrosis in Inflammatory Bowel Disease and the Prospects of Mesenchymal Stem Cell Therapy. Front Immunol. 2022;13:835005. [6] LU Y, WANG L, ZHANG M, et al. Mesenchymal Stem Cell-Derived Small Extracellular Vesicles: A Novel Approach for Kidney Disease Treatment. Int J Nanomedicine. 2022;17:3603-3618. [7] XU H, WANG B, LI A, et al. Mesenchymal Stem Cells-based Cell-free Therapy Targeting Neuroinflammation. Aging Dis. 2024;15(3):965-976. [8] 邢艳粉,解绪红,苑召虎,等.骨髓间充质干细胞移植炎症性肠上皮组织中Wnt/β-catenin信号分子的表达[J].中国组织工程研究, 2015,19(1):49-53. [9] PERVIN B, GIZER M, ŞEKER ME, et al. Bone marrow mesenchymal stromal cells support regeneration of intestinal damage in a colitis mouse model, independent of their CXCR4 expression. Clin Transl Sci. 2024;17(5):e13821. [10] 孟炜程,陈要臻,安宁,等.骨相关间充质干细胞CD166~+亚群治疗炎性肠病模型小鼠的研究[J].临床输血与检验,2022,24(6): 693-697. [11] BARNHOORN MC, PLUG L, JONGE ESMM, et al. Mesenchymal Stromal Cell-Derived Exosomes Contribute to Epithelial Regeneration in Experimental Inflammatory Bowel Disease. Cell Mol Gastroenterol Hepatol. 2020;9(4):715-717.e8. [12] PARK JS, YI TG, PARK JM, et al. Therapeutic effects of mouse bone marrow-derived clonal mesenchymal stem cells in a mouse model of inflammatory bowel disease. J Clin Biochem Nutr. 2015;57(3):192-203. [13] DING Q, FANG H, JIN P, et al. Pretreating mesenchymal stem cells with IL-6 regulates the inflammatory response of DSS-induced ulcerative colitis in rats. Transpl Immunol. 2023;76:101765. [14] 刘继攀,孙伟,宋默,等.间充质干细胞外泌体通过抑制COX-2和NF-κB激活及p38 MAPK磷酸化缓解小鼠溃疡性结肠炎[J].中国免疫学杂志,2021,37(14):1688-1692+1700. [15] HE H, CHEN Q, FAN H, et al. Extracellular vesicles produced by bone marrow mesenchymal stem cells overexpressing programmed death-ligand 1 ameliorate dextran sodium sulfate-induced ulcerative colitis in rats by regulating Th17/Treg cell balance through PTEN/PI3K/AKT/mTOR axis. J Gastroenterol Hepatol. 2022;37(12): 2243-2254. [16] ZHAO ML, CHEN T, ZHANG TH, et al. H19 Overexpression Improved Efficacy of Mesenchymal Stem Cells in Ulcerative Colitis by Modulating the miR-141/ICAM-1 and miR-139/CXCR4 Axes. Dis Markers. 2021; 2021:7107705. [17] SUN D, CAO H, YANG L, et al. MiR-200b in heme oxygenase-1-modified bone marrow mesenchymal stem cell-derived exosomes alleviates inflammatory injury of intestinal epithelial cells by targeting high mobility group box 3. Cell Death Dis. 2020;11(6):480. [18] LIU J, LAI X, BAO Y, et al. Intraperitoneally Delivered Mesenchymal Stem Cells Alleviate Experimental Colitis Through THBS1-Mediated Induction of IL-10-Competent Regulatory B Cells. Front Immunol. 2022;13:853894. [19] 王艳国,李得春,张坤,等.骨髓间充质干细胞对溃疡性结肠炎模型小鼠的研究[J].中国临床药理学杂志,2022,38(13):1496-1500. [20] XU F, FEI Z, DAI H, et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles with High PD-L1 Expression for Autoimmune Diseases Treatment. Adv Mater. 2022;34(1):e2106265. [21] ROBINSON AM, SAKKAL S, PARK A, et al. Mesenchymal stem cells and conditioned medium avert enteric neuropathy and colon dysfunction in guinea pig TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2014;307(11):G1115-G1129. [22] ROBINSON AM, RAHMAN AA, MILLER S, et al. The neuroprotective effects of human bone marrow mesenchymal stem cells are dose-dependent in TNBS colitis. Stem Cell Res Ther. 2017;8(1):87. [23] STAVELY R, ROBINSON AM, FRASER S, et al. Bone marrow-derived mesenchymal stem cells mitigate chronic colitis and enteric neuropathy via anti-inflammatory and anti-oxidative mechanisms. Sci Rep. 2024; 14(1):6649. [24] JUNG KJ, LEE GW, PARK CH, et al. Mesenchymal Stem Cells Decrease Oxidative Stress in the Bowels of Interleukin-10 Knockout Mice. Gut Liver. 2020;14(1):100-107. [25] ZHU F, WEI C, WU H, et al. Hypoxic mesenchymal stem cell-derived exosomes alleviate ulcerative colitis injury by limiting intestinal epithelial cells reactive oxygen species accumulation and DNA damage through HIF-1α. Int Immunopharmacol. 2022;113(Pt A):109426. [26] ZHANG YY, HU ZL, QI YH, et al. Pretreatment of nucleus pulposus mesenchymal stem cells with appropriate concentration of H2O2 enhances their ability to treat intervertebral disc degeneration. Stem Cell Res Ther. 2022;13(1):340. [27] LIU P, XIE XR, WU H, et al. Conditioned medium of mesenchymal stem cells pretreated with H2O2 promotes intestinal mucosal repair in acute experimental colitis. Sci Rep. 2022;12(1):20772. [28] LIU P, XIE XR, WU H, et al. Mesenchymal Stem Cells Promote Intestinal Mucosal Repair by Positively Regulating the Nrf2/Keap1/ARE Signaling Pathway in Acute Experimental Colitis. Dig Dis Sci. 2023;68(5): 1835-1846. [29] YU S, YU S, LIU H, et al. Enhancing mesenchymal stem cell survival and homing capability to improve cell engraftment efficacy for liver diseases. Stem Cell Res Ther. 2023;14(1):235. [30] LI Q, LIAN Y, DENG Y, et al. mRNA-engineered mesenchymal stromal cells expressing CXCR2 enhances cell migration and improves recovery in IBD. Mol Ther Nucleic Acids. 2021;26:222-236. [31] JUNG WY, KANG JH, KIM KG, et al. Human adipose-derived stem cells attenuate inflammatory bowel disease in IL-10 knockout mice. Tissue Cell. 2015;47(1):86-93. [32] LEE JH, LÖTVALL J, CHO BS. The Anti-Inflammatory Effects of Adipose Tissue Mesenchymal Stem Cell Exosomes in a Mouse Model of Inflammatory Bowel Disease. Int J Mol Sci. 2023;24(23): 16877. [33] FU ZW, ZHANG ZY, GE HY. Mesenteric injection of adipose-derived mesenchymal stem cells relieves experimentally-induced colitis in rats by regulating Th17/Treg cell balance. Am J Transl Res. 2018;10(1): 54-66. [34] HEIDARI N, ABBASI-KENARSARI H, NAMAKI S, et al. Adipose-derived mesenchymal stem cell-secreted exosome alleviates dextran sulfate sodium-induced acute colitis by Treg cell induction and inflammatory cytokine reduction. J Cell Physiol. 2021;236(8):5906-5920. [35] DE AGUIAR CF, CASTOLDI A, ANDRADE-OLIVEIRA V, et al. Mesenchymal stromal cells modulate gut inflammation in experimental colitis. Inflammopharmacology. 2018;26(1):251-260. [36] ALVES VBF, DE SOUSA BC, FONSECA MTC, et al. A single administration of human adipose tissue-derived mesenchymal stromal cells (MSC) induces durable and sustained long-term regulation of inflammatory response in experimental colitis. Clin Exp Immunol. 2019;196(2): 139-154. [37] YUAN Y, NI S, ZHUGE A, et al. Adipose-Derived Mesenchymal Stem Cells Reprogram M1 Macrophage Metabolism via PHD2/HIF-1α Pathway in Colitis Mice. Front Immunol. 2022;13:859806. [38] KAWATA Y, TSUCHIYA A, SEINO S, et al. Early injection of human adipose tissue-derived mesenchymal stem cell after inflammation ameliorates dextran sulfate sodium-induced colitis in mice through the induction of M2 macrophages and regulatory T cells. Cell Tissue Res. 2019;376(2):257-271. [39] QIAN W, HUANG L, XU Y, et al. Hypoxic ASCs-derived Exosomes Attenuate Colitis by Regulating Macrophage Polarization via miR-216a-5p/HMGB1 Axis. Inflamm Bowel Dis. 2023;29(4):602-619. [40] SONG WJ, LI Q, RYU MO, et al. TSG-6 Secreted by Human Adipose Tissue-derived Mesenchymal Stem Cells Ameliorates DSS-induced colitis by Inducing M2 Macrophage Polarization in Mice. Sci Rep. 2017;7(1):5187. [41] 冯乙芮,高天芸,王亚萍,等.白细胞介素10工程化修饰人脐带间充质干细胞优效治疗炎症性肠病[J].中国组织工程研究,2025, 29(23):4878-4887. [42] LIANG X, LI C, SONG J, et al. HucMSC-Exo Promote Mucosal Healing in Experimental Colitis by Accelerating Intestinal Stem Cells and Epithelium Regeneration via Wnt Signaling Pathway. Int J Nanomedicine. 2023;18:2799-2818. [43] WANG G, JOEL MDM, YUAN J, et al. Human umbilical cord mesenchymal stem cells alleviate inflammatory bowel disease by inhibiting ERK phosphorylation in neutrophils. Inflammopharmacology. 2020;28(2):603-616. [44] MIYAMOTO S, OHNISHI S, ONISHI R, et al. Therapeutic effects of human amnion-derived mesenchymal stem cell transplantation and conditioned medium enema in rats with trinitrobenzene sulfonic acid-induced colitis. Am J Transl Res. 2017;9(3):940-952. [45] KATIFELIS H, FILIDOU E, PSARAKI A, et al. Amniotic Fluid-Derived Mesenchymal Stem/Stromal Cell-Derived Secretome and Exosomes Improve Inflammation in Human Intestinal Subepithelial Myofibroblasts. Biomedicines. 2022;10(10):2357. [46] DUAN L, HUANG H, ZHAO X, et al. Extracellular vesicles derived from human placental mesenchymal stem cells alleviate experimental colitis in mice by inhibiting inflammation and oxidative stress. Int J Mol Med. 2020;46(4):1551-1561. [47] DENG C, ZHANG H, LI Y, et al. Exosomes derived from mesenchymal stem cells containing berberine for ulcerative colitis therapy. J Colloid Interface Sci. 2024;671:354-373. [48] LI Y, MA K, ZHANG L, et al. Human Umbilical Cord Blood Derived-Mesenchymal Stem Cells Alleviate Dextran Sulfate Sodium-Induced Colitis by Increasing Regulatory T Cells in Mice. Front Cell Dev Biol. 2020;8:604021. [49] QI L, WU J, ZHU S, et al. Mesenchymal Stem Cells Alleviate Inflammatory Bowel Disease Via Tr1 Cells. Stem Cell Rev Rep. 2022; 18(7):2444-2457. [50] HEIDARI N, ABBASI-KENARSARI H, NAMAKI S, et al. Regulation of the Th17/Treg balance by human umbilical cord mesenchymal stem cell-derived exosomes protects against acute experimental colitis. Exp Cell Res. 2022;419(1):113296. [51] FU Y, ZHANG C, XIE H, et al. Human umbilical cord mesenchymal stem cells alleviated TNBS-induced colitis in mice by restoring the balance of intestinal microbes and immunoregulation. Life Sci. 2023;334:122189. [52] YAN Y, LI K, JIANG J, et al. Perinatal tissue-derived exosomes ameliorate colitis in mice by regulating the Foxp3 + Treg cells and gut microbiota. Stem Cell Res Ther. 2023;14(1):43. [53] YANG S, LIANG X, SONG J, et al. A novel therapeutic approach for inflammatory bowel disease by exosomes derived from human umbilical cord mesenchymal stem cells to repair intestinal barrier via TSG-6. Stem Cell Res Ther. 2021;12(1):315. [54] LIU A, WANG X, LIANG X, et al. Human umbilical cord mesenchymal stem cells regulate immunoglobulin a secretion and remodel the diversification of intestinal microbiota to improve colitis. Front Cell Infect Microbiol. 2022;12:960208. [55] LIU A, LIANG X, WANG W, et al. Human umbilical cord mesenchymal stem cells ameliorate colon inflammation via modulation of gut microbiota-SCFAs-immune axis. Stem Cell Res Ther. 2023;14(1):271. [56] YANG F, NI B, LIU Q, et al. Human umbilical cord-derived mesenchymal stem cells ameliorate experimental colitis by normalizing the gut microbiota. Stem Cell Res Ther. 2022;13(1):475. [57] LARABI A, BARNICH N, NGUYEN HTT. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy. 2020;16(1):38-51. [58] NIGHOT PK, HU CA, MA TY. Autophagy enhances intestinal epithelial tight junction barrier function by targeting claudin-2 protein degradation. J Biol Chem. 2015;290(11):7234-7246. [59] PAN XH, LI QQ, ZHU XQ, et al. Mechanism and therapeutic effect of umbilical cord mesenchymal stem cells in inflammatory bowel disease. Sci Rep. 2019;9(1):17646. [60] 蒲瑜,张吉翔,董卫国.铁死亡与炎症性肠病的研究进展[J].中国全科医学,2023,26(29):3698-3703. [61] WANG H, SUN Y, XIAO FJ, et al. Mesenchymal Stem Cells Ameliorate DSS-Induced Experimental Colitis by Modulating the Gut Microbiota and MUC-1 Pathway. J Inflamm Res. 2023;16:2023-2039. [62] WEI Z, HANG S, WIREDU OCANSEY DK, et al. Human umbilical cord mesenchymal stem cells derived exosome shuttling mir-129-5p attenuates inflammatory bowel disease by inhibiting ferroptosis. J Nanobiotechnology. 2023;21(1):188. [63] DUAN J, MATUTE JD, UNGER LW, et al. Endoplasmic reticulum stress in the intestinal epithelium initiates purine metabolite synthesis and promotes Th17 cell differentiation in the gut. Immunity. 2023; 56(5):1115-1131.e9. [64] BANERJEE A, BIZZARO D, BURRA P, et al. Umbilical cord mesenchymal stem cells modulate dextran sulfate sodium induced acute colitis in immunodeficient mice. Stem Cell Res Ther. 2015;6(1):79. [65] YANG H, FENG R, FU Q, et al. Human induced pluripotent stem cell-derived mesenchymal stem cells promote healing via TNF-α-stimulated gene-6 in inflammatory bowel disease models. Cell Death Dis. 2019; 10(10):718. [66] XU J, WANG X, CHEN J, et al. Embryonic stem cell-derived mesenchymal stem cells promote colon epithelial integrity and regeneration by elevating circulating IGF-1 in colitis mice. Theranostics. 2020;10(26):12204-12222. [67] LU Y, XU Y, ZHANG S, et al. Human gingiva-derived mesenchymal stem cells alleviate inflammatory bowel disease via IL-10 signalling-dependent modulation of immune cells. Scand J Immunol. 2019; 90(3):e12751. [68] GÓMEZ-FERRER M, AMARO-PRELLEZO E, DORRONSORO A, et al. HIF-Overexpression and Pro-Inflammatory Priming in Human Mesenchymal Stromal Cells Improves the Healing Properties of Extracellular Vesicles in Experimental Crohn’s Disease. Int J Mol Sci. 2021;22(20):11269. [69] SHI L, CHEN L, GAO X, et al. Comparison of different sources of mesenchymal stem cells: focus on inflammatory bowel disease. Inflammopharmacology. 2024;32(3):1721-1742. [70] STAVELY R, ROBINSON AM, MILLER S, et al. Human adult stem cells derived from adipose tissue and bone marrow attenuate enteric neuropathy in the guinea-pig model of acute colitis. Stem Cell Res Ther. 2015;6:244. [71] MOHAMED-AHMED S, FRISTAD I, LIE SA, et al. Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Res Ther. 2018;9(1):168. [72] HAN I, KWON BS, PARK HK, et al. Differentiation Potential of Mesenchymal Stem Cells Is Related to Their Intrinsic Mechanical Properties. Int Neurourol J. 2017;21(Suppl 1):S24-S31. [73] AMABLE PR, TEIXEIRA MV, CARIAS RB, et al. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem Cell Res Ther. 2014;5(2):53. [74] KAGIA A, TZETIS M, KANAVAKIS E, et al. Therapeutic Effects of Mesenchymal Stem Cells Derived From Bone Marrow, Umbilical Cord Blood, and Pluripotent Stem Cells in a Mouse Model of Chemically Induced Inflammatory Bowel Disease. Inflammation. 2019;42(5):1730-1740. [75] STAVELY R, ROBINSON AM, MILLER S, et al. Allogeneic guinea pig mesenchymal stem cells ameliorate neurological changes in experimental colitis. Stem Cell Res Ther. 2015;6:263. [76] 葛翠翠,王慧娜,杜丽欣,等.人脐带间充质干细胞对炎症性肠病小鼠模型的治疗作用[J].生物技术通讯,2014,25(6):813-816. [77] 杨婧雯,陈芊,单云龙,等.间充质干细胞产品及其外泌体在炎症性肠病治疗中的研究进展[J].中国药科大学学报,2024,55(1): 103-114. [78] FURUKAWA S, MIZUSHIMA T, NAKAYA R, et al. Darvadstrocel for Complex Perianal Fistulas in Japanese Adults with Crohn’s Disease: A Phase 3 Study. J Crohns Colitis. 2023;17(3):369-378. [79] HERREROS MD, RAMIREZ JM, OTERO-PIÑEIRO AM, et al. Use of Darvadstrocel (Allogenic Stem Cell Therapy) for Crohn’s Fistulas in Real Clinical Practice: The National Project to Implement Mesenchymal Stem Cell for the Treatment of Perianal Crohn’s Fistula (the PRIME Study). Dis Colon Rectum. 2024;67(7):960-967. [80] DAWOUD C, WIDMANN KM, CZIPIN S, et al. Efficacy of cx601 (darvadstrocel) for the treatment of perianal fistulizing Crohn’s disease-A prospective nationwide multicenter cohort study. Wien Klin Wochenschr. 2024;136(9-10):289-294. [81] PANÉS J, GARCÍA-OLMO D, VAN ASSCHE G, et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388(10051):1281-1290. [82] GARCIA-OLMO D, GILABERTE I, BINEK M, et al. Follow-up Study to Evaluate the Long-term Safety and Efficacy of Darvadstrocel (Mesenchymal Stem Cell Treatment) in Patients With Perianal Fistulizing Crohn’s Disease: ADMIRE-CD Phase 3 Randomized Controlled Trial. Dis Colon Rectum. 2022;65(5):713-720. [83] CHO YB, PARK KJ, YOON SN, et al. Long-term results of adipose-derived stem cell therapy for the treatment of Crohn’s fistula. Stem Cells Transl Med. 2015;4(5):532-537. [84] REENAERS C, GILLARD RP, COIMBRA C, et al. Clinical and MRI Evolution After Local Injection of Bone Marrow-Derived Mesenchymal Stem Cells in Perianal Fistulae in Crohn’s Disease: Results From a Prospective Monocentric Study. J Crohns Colitis. 2023;17(5):728-737. [85] VIEUJEAN S, LOLY JP, BOUTAFFALA L, et al. Mesenchymal Stem Cell Injection in Crohn’s Disease Strictures: A Phase I-II Clinical Study. J Crohns Colitis. 2022;16(3):506-510. [86] LIGHTNER AL, REESE JS, REAM J, et al. A phase IB/IIA study of ex vivo expanded allogeneic bone marrow-derived mesenchymal stem cells for the treatment of rectovaginal fistulizing Crohn’s disease. Surgery. 2024;175(2):242-249. [87] WEI J, ZHANG Y, CHEN C, et al. Efficacy and safety of allogeneic umbilical cord-derived mesenchymal stem cells for the treatment of complex perianal fistula in Crohn’s disease: a pilot study. Stem Cell Res Ther. 2023;14(1):311. [88] KEUNG C, NGUYEN TC, LIM R, et al. Local fistula injection of allogeneic human amnion epithelial cells is safe and well tolerated in patients with refractory complex perianal Crohn’s disease: a phase I open label study with long-term follow up. EBioMedicine. 2023;98:104879. [89] PAK H, HADIZADEH A, HEIRANI-TABASI A, et al. Safety and efficacy of injection of human placenta mesenchymal stem cells derived exosomes for treatment of complex perianal fistula in non-Crohn’s cases: Clinical trial phase I. J Gastroenterol Hepatol. 2023;38(4):539-547. |
| [1] | 周盼盼, 崔应麟, 张文涛, 王姝瑞, 陈佳慧, 杨 潼. 细胞自噬在脑缺血损伤中的作用及中药调控机制[J]. 中国组织工程研究, 2025, 29(8): 1650-1658. |
| [2] | 杨治航, 孙祖延, 黄文良, 万 喻, 陈仕达, 邓 江. 神经生长因子促进兔骨髓间充质干细胞软骨分化并抑制肥大分化[J]. 中国组织工程研究, 2025, 29(7): 1336-1342. |
| [3] | 胡涛涛, 刘 兵, 陈 诚, 殷宗银, 阚道洪, 倪 杰, 叶凌霄, 郑祥兵, 严 敏, 邹 勇. 过表达神经调节蛋白1的人羊膜间充质干细胞促进小鼠皮肤创面愈合[J]. 中国组织工程研究, 2025, 29(7): 1343-1349. |
| [4] | 金 凯, 唐 婷, 李美乐, 谢裕安. 人脐带间充质干细胞条件培养基及外泌体对肝癌细胞增殖、迁移、侵袭和凋亡的影响[J]. 中国组织工程研究, 2025, 29(7): 1350-1355. |
| [5] | 李帝均, 酒精卫, 刘海峰, 闫 磊, 李松岩, 王 斌. 明胶三维微球装载人脐带间充质干细胞修复慢性肌腱病[J]. 中国组织工程研究, 2025, 29(7): 1356-1362. |
| [6] | 刘 琪, 李林臻, 李玉生, 焦泓焯, 杨 程, 张君涛. 淫羊藿苷含药血清促进3种细胞共培养体系中软骨细胞增殖和干细胞成软骨分化[J]. 中国组织工程研究, 2025, 29(7): 1371-1379. |
| [7] | 章镇宇, 梁秋健, 杨 军, 韦相宇, 蒋 捷, 黄林科, 谭 桢. 新橙皮苷治疗骨质疏松症的靶点及对骨髓间充质干细胞成骨分化的作用[J]. 中国组织工程研究, 2025, 29(7): 1437-1447. |
| [8] | 李佳林, 张耀东, 娄艳茹, 于 洋, 杨 蕊. 间充质干细胞分泌组发挥作用的分子机制[J]. 中国组织工程研究, 2025, 29(7): 1512-1522. |
| [9] | 何 波, 陈 文, 马岁录, 何志军, 宋 渊, 李金鹏, 刘 涛, 魏晓涛, 王威威, 谢 婧. 皮瓣缺血再灌注损伤的发病机制及治疗进展[J]. 中国组织工程研究, 2025, 29(6): 1230-1238. |
| [10] | 赵晓璇, 刘帅祎, 李 奇, 邢 政, 李庆雯, 褚晓蕾. 不同运动方式促进周围神经损伤后的功能恢复[J]. 中国组织工程研究, 2025, 29(6): 1248-1256. |
| [11] | 张文华, 李 荀, 张伟超, 李欣颖, 马帼澳, 王孝强. SphK1/S1P/S1PR2信号通路促进肌生成:运动改善骨骼肌健康的新视角[J]. 中国组织工程研究, 2025, 29(6): 1265-1275. |
| [12] | 吴广涛, 秦 刚, 何凯毅, 范以东, 李威材, 朱宝刚, 曹 英. 免疫细胞与膝骨关节炎之间因果作用:一项两样本双向孟德尔随机化分析[J]. 中国组织工程研究, 2025, 29(5): 1081-1090. |
| [13] | 孙现娟, 王秋花, 张锦艺, 杨杨杨, 王文双, 张晓晴. 不同静电纺丝膜上骨髓间充质干细胞的黏附、增殖与成血管平滑肌分化[J]. 中国组织工程研究, 2025, 29(4): 661-669. |
| [14] | 郑伊桐, 汪永新, 刘 文, 阿木吉特, 秦 虎. 神经内镜下人脐带间充质干细胞外泌体鞘内移植修复脊髓损伤的作用机制[J]. 中国组织工程研究, 2025, 29(36): 7743-7751. |
| [15] | 周绍兰, 袁媛园, 潘 露, 徐 文, 瞿姝熳. miR-26b对人脱落乳牙牙髓干细胞向神经及血管分化的影响[J]. 中国组织工程研究, 2025, 29(36): 7769-7775. |
间充质干细胞是一类具有自我更新能力和多向分化潜能的多能成体干细胞,广泛存在于机体多种组织中,如骨髓、脂肪、脐带、胎盘等[4]。自被发现以来,间充质干细胞卓越的再生和分化能力便引发了广泛的临床前研究和临床试验,已被证实在包括炎症性肠病在内的多种慢性炎症疾病中发挥治疗作用[5-6]。除强大的再生作用外,间充质干细胞还通过旁分泌生物活性因子和细胞间物质传递发挥抗炎、促血管生成、抗氧化应激、抗凋亡等多种作用[7],而不同来源间充质干细胞在分化倾向上存在区别,功能上也有细微差异,因此了解不同来源间充质干细胞之间的差异可为选择治疗方案提供更确切的信息。文章旨在总结不同来源间充质干细胞治疗炎症性肠病的作用机制,分析它们在功能上的细微区别,为进一步深入研究间充质干细胞治疗炎症性肠病的临床诊疗决策提供理论依据。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
1.1.1 检索人及检索时间 第一作者在2024年7月进行检索。
1.1.2 检索文献时限 2014年7月至2024年7月。
1.1.3 检索数据库 中国知网、PubMed数据库。
1.1.4 检索词 中文检索词为“间充质干细胞,外泌体,炎症性肠病,溃疡性结肠炎,克罗恩病”;英文检索词为“mesenchymal stem cells,MSCs,exosomes,extracellular vesicles,EVs,inflammatory bowel disease,IBD,ulcerative colitis,UC,Crohn’s disease,CD”。
1.1.5 检索文献类型 基础研究、临床研究等。
1.1.6 检索策略 以PubMed数据库为例,检索策略见图1。
1.1.7 检索文献量 共检索到582篇文献,包括中文文献92篇,英文文献490篇。
1.2 入选标准
1.2.1 纳入标准 ①各类间充质干细胞治疗炎症性肠病的研究;②各类间充质干细胞外泌体治疗炎症性肠病的研究。
1.2.2 排除标准 ①研究内容或目的与文章主旨无关的文献;②重复性研究;③未能阐明具体作用机制的研究。
1.3 文献筛选过程及质量评估 共检索到582篇文献,包括中文文献92篇、英文文献490篇。排除研究目的不符及内容重复、陈旧的文献,纳入符合标准的89篇文献进行综述,包括中文文献9篇、英文文献80篇。文献筛选流程,见图2。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
3.2 作者综述区别于他人他篇的特点 目前已有文献对间充质干细胞治疗炎症性肠病进行综述,但尚未有文章总结归纳不同来源间充质干细胞治疗炎症性肠病有何区别,此文章将目前为止的细胞、动物实验汇总,以不同来源间充质干细胞发挥何种作用机制分类,对不同来源间充质干细胞在治疗机制上的侧重点进行总结分析,且对不同移植方式进行总结归纳,为临床上根据不同功能需要选择不同来源间充质干细胞,以及根据不同的病情特点选择移植方式提供了有力依据。
3.3 综述的局限性 近年来关于间充质干细胞治疗炎症性肠病的研究越来越多,文章主要总结了骨髓、脂肪、围产期组织来源间充质干细胞的多种作用机制,但关于牙龈、胚胎干细胞、诱导多能干细胞等其他来源的间充质干细胞,目前研究较少,未能对进一步的机制进行深入研究,而且将不同来源间充质干细胞进行横向对比的相关研究较少,在对比功能差异时难免有所偏颇。
3.4 综述的重要意义 文章对现有间充质干细胞治疗炎症性肠病的文献进行了全面而详细的综述,按照其来源不同进行归纳总结,并根据作用机制进行分类,对不同来源间充质干细胞治疗炎症性肠病的作用机制侧重点进行分析。大多数研究聚焦于骨髓、脂肪、围产期组织来源,主要作用机制体现在抗炎、免疫调节、分化、再生等方面。骨髓间充质干细胞优势在分化、再生、神经保护等方面,脂肪间充质干细胞优势在增殖、归巢、免疫调节等方面,围产期组织来源间充质干细胞优势在调节肠道菌群、调节自噬、降低铁死亡等方面。在移植方式上,目前研究大多使用静脉注射与腹腔注射两种方式,且腹腔注射效果略强于静脉注射,新兴的局部注射、灌肠与肠系膜注射也在研究中发挥了各自优势。此外,新兴的工程化干细胞在治疗炎症性肠病方面展现出了较佳优势,对间充质干细胞进行预处理或载入优势蛋白,可有助于发挥间充质干细胞的治疗优势。这些为后续研究提供了理论依据,可根据病情特点挑选更佳来源的间充质干细胞及更有优势的移植方式,加速药物的临床转化。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
文题释义:
间充质干细胞:是一类具有多向分化潜能的干细胞,能够分化成各种类型细胞,如脂肪细胞、成骨细胞、软骨细胞等。间充质干细胞在体内分布广泛,可以从骨髓、脂肪、脐带等多种组织中分离得到。间充质干细胞已被研究用于治疗多种疾病,在组织修复和再生、免疫调节、抗炎等多方面发挥重要作用。 #br# 炎症性肠病:是一种长期的慢性肠道炎性疾病,主要包括溃疡性结肠炎和克罗恩病,前者主要损害结肠和直肠,后者可损害从口腔到肛门之间的任意胃肠道部位,以小肠末端和结肠多见,主要临床表现为腹痛、腹泻、血便、体质量减轻等。#br##br#
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
目前已有文献对间充质干细胞治疗炎症性肠病进行综述,但尚未有文章总结归纳不同来源间充质干细胞治疗炎症性肠病有何区别,此文章将目前为止的细胞、动物实验汇总,以不同来源间充质干细胞发挥何种作用机制分类,对不同来源间充质干细胞在治疗机制上有何侧重点进行总结分析,且对不同移植方式进行总结归纳,为临床上根据不同功能需要选择不同来源间充质干细胞,以及根据不同的病情特点选择移植方式提供了有力依据。
#br#
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||