中国组织工程研究 ›› 2025, Vol. 29 ›› Issue (20): 4266-4275.doi: 10.12307/2025.692
• 组织构建综述 tissue construction review • 上一篇 下一篇
神经生长因子对骨骼形成及骨疾病的影响
- 吉林大学第二医院脊柱外科,吉林省长春市 130000
-
收稿日期:2024-07-27接受日期:2024-09-14出版日期:2025-07-18发布日期:2024-12-20 -
通讯作者:夏鹏,博士,副教授,博士生导师,吉林大学第二医院脊柱外科,吉林省长春市 130000 -
作者简介:魏鹤翔,男,1998年生,山东省滨州市人,汉族,吉林大学在读硕士,主要从事骨修复和骨整合研究。 -
基金资助:吉林省自然科学基金项目(YDZJ202201ZYTS505),项目负责人:夏鹏;吉林省重点研发计划项目(20230204114YY),项目负责人:夏鹏
Effects of nerve growth factor on osteogenesis and bone diseases
Wei Hexiang, Sun Bin, Liu Hao, Liu Hanqiang, Xia Peng
- Department of Orthopedics, The Second Hospital of Jilin University, Changchun 130000, Jilin Province, China
-
Received:2024-07-27Accepted:2024-09-14Online:2025-07-18Published:2024-12-20 -
Contact:Xia Peng, MD, Associate professor, Doctoral supervisor, Department of Orthopedics, The Second Hospital of Jilin University, Changchun 130000, Jilin Province, China -
About author:Wei Hexiang, Master’s candidate, Department of Orthopedics, The Second Hospital of Jilin University, Changchun 130000, Jilin Province, China -
Supported by:the Natural Science Foundation of Jilin Province, No. YDZJ202201ZYTS505 (to XP); Jilin Province Key R&D Program, No. 20230204114YY (to XP)
摘要:
文题释义:
神经生长因子:一种广泛分布于人体各组织中的蛋白质,主要作用为调控神经元生长、存活、分化,同时也在包括骨骼、血管在内的非神经组织中起关键调控作用。
骨再生:骨骼具有完全再生能力,新生成骨组织与正常骨组织没有区别。这种完全再生能力由成骨细胞、破骨细胞等多种骨组织细胞和长入愈伤组织的神经、血管共同实现。
背景:神经生长因子在骨骼的生理和病理进程中发挥了重要作用,系统性分析神经生长因子对骨组织的影响在组织工程及临床治疗两方面都具有重要意义。
目的:探究神经生长因子通过骨组织细胞和骨神经-血管耦合等途径调控骨形成的过程,同时研究神经生长因子在骨相关疾病病理进程中的作用。
方法:在中国知网、万方、PubMed数据库以“神经生长因子,TrkA,骨,软骨”为中文检索词,以“Nerve growth factor,TrkA,NGF,bone,cartilage”为英文检索词进行文献检索,共检索到2 925篇文献。经过筛选后纳入116篇文献进行归纳总结,撰写综述。
结果与结论:神经生长因子既可在骨、软骨、神经、血管等组织细胞中表达,又可作用于这些细胞发挥调控作用。通过多种分泌调控方式,神经生长因子在骨组织内部和骨、神经、血管组织间发挥信号传导作用。通过促进骨髓间充质干细胞的增殖、分化,神经生长因子可促进骨形成及骨修复。神经生长因子介导的破骨细胞生成说明其对骨组织具有多向调控作用。同时,神经生长因子与多种骨科疾病的发生发展高度相关,可能提供新的临床治疗思路。对神经生长因子的研究是了解骨骼生理及病理变化的重要方向之一。
https://orcid.org/0000-0003-0793-2217(夏鹏)
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
中图分类号:
引用本文
魏鹤翔, 孙 彬, 刘 昊, 刘含强, 夏 鹏. 神经生长因子对骨骼形成及骨疾病的影响[J]. 中国组织工程研究, 2025, 29(20): 4266-4275.
Wei Hexiang, Sun Bin, Liu Hao, Liu Hanqiang, Xia Peng. Effects of nerve growth factor on osteogenesis and bone diseases[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(20): 4266-4275.
神经生长因子通过与TrkA受体结合激活促进细胞存活、分化和生长的信号通路[6]。TrkA具有受体酪氨酸激酶活性,与神经生长因子结合后可通过磷酸化激活级联反应启动信号传导[7]。TrkA受体激活的下游信号通路已经得到了广泛研究,主要包括细胞外调节蛋白激酶/丝裂原活化蛋白激酶(mitogen-activated protein kinases/extracellular signal-regulated kinase,ERK/MAPK)通路、磷脂酰肌醇三激酶/蛋白激酶B(phosphatidylinositide 3-kinases/protein kinase B,PI3K/Akt)通路、磷酸肌醇特异性磷脂酶C/ 蛋白激酶C(phosphoinositide-specific phospholipase C/protein kinase C,PLC/PKC)通路等[8]。此外,神经细胞上与神经生长因子结合的TrkA还可形成信号内吞体,沿轴突逆向运输,并以细胞外囊泡形式被神经元释放,从而作用于体细胞[9]。
p75NTR不具有酪氨酸激酶活性,因而通常与其他跨膜蛋白结合,以实现其下游作用[10]。在缺乏TrkA受体的情况下,p75NTR受体倾向于促进细胞死亡[8]。成熟神经生长因子对TrkA受体的亲和力强于p75NTR受体,而神经生长因子前体则缺乏对TrkA受体的亲和力,主要作为p75NTR受体的有效配体诱导细胞凋亡[11]。p75NTR与细胞凋亡高度相关,被视为一种神经细胞死亡受体。然而,当TrkA受体存在时,p75NTR的作用转为增强TrkA对神经生长因子的敏感性,进而促进靶细胞存活和分化[12]。p75NTR与TrkA的共表达可产生神经生长因子的高亲和力结合位点,从而显著提升TrkA对神经生长因子的结合能力和特异性[8]。因此,p75NTR可通过激活不同的信号通路,产生不同的调节作用,这是神经生长因子作用多效性的基础[13]。
神经生长因子及其受体的表达分布于多种组织细胞,包括单核细胞、淋巴细胞、血管内皮细胞在内的多种非神经细胞同样受到神经生长因子的调控[14-15]。通过对这些非神经细胞的调控,神经生长因子可作为信号传导因子参与多种重要生理和病理过程,如炎症反应[16]、血管生长[17]、痛觉产生等[18]。因此,神经生长因子不仅作为组织工程靶点受到重视,还被认为具有广阔的临床应用前景。
2.2 神经生长因子对骨形成的调控
2.2.1 神经生长因子对骨形成的调控作用概述 神经生长因子受体广泛分布于骨组织及支配骨的神经血管中。早期文献即指出,神经生长因子可增强骨折模型中神经支配和骨再生,从而加速骨愈伤组织骨化[19]。近期研究证明,神经生长因子可通过引导神经血管长入、与骨组织细胞膜上受体结合、协同力学刺激信号等多种方式促进骨骼的形成、分化和愈合[20]。神经生长因子在骨形成领域的研究进程,见表1。
神经支配和血管生成在骨形成中起着至关重要的作用,而骨骼中大多数神经纤维和血管细胞表达TrkA受体,神经生长因子可以通过TrkA受体将感觉神经轴突和新生血管引导至初始骨化部位,通过促进血管形成和神经长入正向调控骨形成过程。LI等[21]使用免疫荧光技术对神经生长因子在骨骼中的表达进行定位,发现生理情况下成熟皮质骨中几乎不表达神经生长因子,神经生长因子
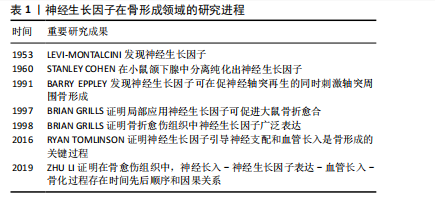
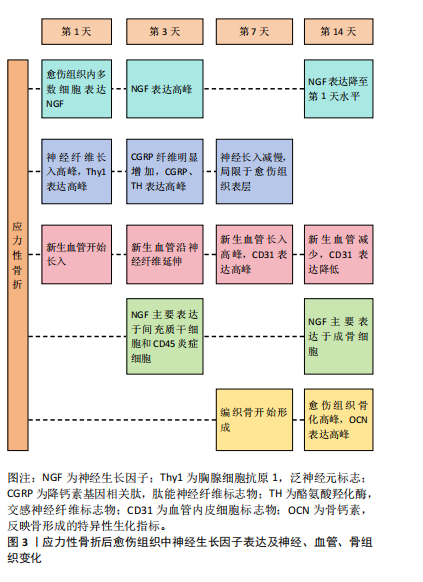
主要分布于骨膜,而骨损伤后愈伤组织内神经生长因子表达显著增强且持续升高,说明神经生长因子与骨骼的生长和修复存在显著关联。上述研究发现,在应力性骨折后形成的骨折愈伤组织中,首先发生神经纤维再支配,愈伤组织中多数细胞表达神经生长因子引导新生神经、血管长入,愈伤组织面积扩大;随着神经生长因子整体表达水平下降,神经血管长入减缓,此时神经生长因子表达主要集中于成骨细胞,引发愈伤组织骨化。而使用阻断剂抑制神经生长因子可导致神经再支配减少、血运重建减弱、总成骨细胞活性降低以及骨折愈伤组织骨化的显著延迟,提示引导新生神经和血管长入是神经生长因子影响骨骼的重要方式之一,见图3。
神经生长因子对骨组织内的各种细胞具有直接调控作用[22]。刘国铭等[23]发现神经生长因子可促进骨组织细胞中骨形态发生蛋白2表达,通过骨形态发生蛋白2/Smad通路促进骨折愈合。研究发现神经生长因子可促进Runt相关转录因子2蛋白表达,提示神经生长因子可能通过增强骨形态发生蛋白2/Smads/Runt相关转录因子2信号级联反应促进骨形成[24]。另外,王裕祥等[25]发现神经生长因子还可与转化生长因子β、成纤维细胞生长因子协同促进骨修复。通过与多种靶细胞膜上受体结合激活胞内信号通路,神经生长因子可直接影响骨组织细胞的生长、分化、代谢、凋亡,从而调节骨骼的生理进程和病理变化。
现有研究指出,力学负荷对骨组织的生长、发育和修复均存在重要影响,而骨细胞感知力学信号和传递生物信号的机制仍不清楚[26]。研究表明,长时间、剧烈的体育锻炼可引发血清神经生长因子水平升高[27]。成熟的成骨细胞在力学负荷下可产生神经生长因子,而神经生长因子表达是力学负荷实现最大骨形成的必要条件之一,神经生长因子-TrkA信号传导可能是骨细胞接受力学刺激的主要通路之一[28]。FIORAVANTI等[29]证明对小鼠四肢长骨施加力学负荷后,骨骼中神经生长因子水平显著上升。而TOMLINSON等[30]发现给予小鼠注射外源性神经生长因子增加了力学刺激诱导的骨形成速率,抑制TrkA信号传导显著降低了前肢受轴向力学刺激后的骨形成速率。神经生长因子在骨骼接受力学刺激调节过程中的作用,以及周围神经血管通过神经生长因子参与这一过程,可能是进一步研究的方向。
2.2.2 神经生长因子通过促骨髓间充质干细胞增殖分化促进骨形成 神经生长因子通过调节间充质干细胞的活性和功能对骨形成过程进行正向调节。骨髓间充质干细胞可以促进骨骼基质形成并矿化为骨组织,还可协调骨折愈合的阶段转换过程,在骨形成过程中具有重要作用[31-32]。骨髓间充质干细胞本身可分泌包括神经生长因子在内的神经营养因子,同时还能促进施万细胞分泌神经生长因子[33-34]。骨髓间充质干细胞又可受到位于骨膜的交感神经和感觉神经纤维分泌的包括神经生长因子在内的神经递质调节[35]。崔国胜等[36]发现经神经生长因子处理的骨髓间充质干细胞的成骨分化基因表达水平和钙化能力均显著增加。FANG等[37]研究进一步发现神经生长因子过表达可增强骨髓间充质干细胞的细胞活力、黏附能力和迁移能力,而敲除神经生长因子则对骨髓间充质干细胞呈现相反的效果,同时,敲除神经生长因子受体TrkA的骨髓间充质干细胞同样表现出细胞活力下降、黏附功能降低等特征。通过促进间充质干细胞成骨分化,神经生长因子对骨形成发挥重要的促进作用。
神经生长因子对骨髓间充质干细胞及其衍生细胞的促增殖分化作用已得到大量研究支持,但是神经生长因子的作用浓度范围仍需探索。YE等[38]通过复合涂层释放神经生长因子显著增强了骨髓间充质干细胞的成骨分化能力,但该释放量不能促进骨髓间充质干细胞的黏附和增殖。AN等[39]研究发现,随着神经生长因子添加量的增加,对MC3T3细胞成骨分化的促进作用先增大后减小,在水凝胶中神经生长因子浓度达到0.5%之前,成骨分化呈剂量依赖性增加,然而,神经生长因子高于0.5%浓度对成骨细胞分化反而表现出抑制作用。现有研究结果显示神经生长因子对成骨分化的作用或许存在与剂量相关的多效性,提示不同浓度神经生长因子对成骨的影响可作为未来的研究方向。
2.2.3 神经生长因子通过促破骨细胞分化成熟调控骨形成 破骨细胞是来源于单核细胞/巨噬细胞造血谱系的骨吸收多核巨细胞,也是主要的骨吸收细胞[40-41]。神经生长因子与其受体TrkA共同在多核破骨细胞中表达[42]。通过促进破骨细胞分化成熟,神经生长因子可抑制过度的骨形成并促进骨重塑[43]。而神经生长因子对破骨细胞的具体作用受到神经生长因子剂量和其他因素的影响。MELONI等[44]研究证实了神经生长因子诱导破骨细胞成熟的能力,但是该研究同时指出,较高剂量(100 ng)的神经生长因子干预后小鼠单核细胞向破骨细胞分化的能力反而不如较低剂量(10,50 ng)的神经生长因子。这种表现不能简单地用过量神经生长因子使TrkA受体饱和解释,提示与神经生长因子对骨髓间充质干细胞的作用类似,神经生长因子对破骨细胞的影响可能同样存在剂量相关的多效性。
神经生长因子在破骨细胞核因子受体激活因子κB配体(receptor activator for nuclear factor-κB ligand,RANKL)非依赖性生成过程中扮演的作用同样受到重视。生理情况下,RANKL/核因子κB受体活化因子信号转导是破骨细胞形成的主要途径[45]。但是在某些情况下,特别是在与病理性骨吸收相关的疾病中,包括神经生长因子在内的其他细胞因子可以替代RANKL促进破骨细胞的分化[46]。体外研究显示,将神经生长因子添加到单核细胞培养物中可诱导抗酒石酸盐酸性磷酸酶阳性的多核破骨细胞的形成,其效果是增殖诱导配体或B细胞活化因子诱导效果的5倍,与用白细胞介素6或转化生长因子β处理的培养物相当[47]。然而,另一体外研究证明,在没有RANKL的情况下,神经生长因子在体外显著增加了破骨细胞的数量,但是,在RANKL存在的情况下,不同浓度神经生长因子不仅没有增强RANKL的作用,反而减少了抗酒石酸盐酸性磷酸酶阳性破骨细胞的数量[48]。神经生长因子与RANKL单独使用时均对破骨细胞存在促形成作用,两种因子同时存在时反而表现出负面作用,这一反常现象的机制和原理需要进一步研究探索。神经生长因子介导的破骨细胞生成过程不仅体现了神经生长因子对骨形成的负向调节能力,还可能与骨吸收过度导致的疾病有关。
2.2.4 神经生长因子通过神经-血管耦合调控骨形成 非骨骼系统,包括血管系统和神经系统,也是骨骼生理环境的关键参与者[49]。骨骼形成、发育、再生和重塑过程中,新长入血管在提供营养和氧气方面起着至关重要的作用[50]。早期研究认为骨组织再生可以定义为一种成骨与血管生成相互影响的复杂机制[51]。近期研究已经注意到,在骨组织生长和血管长入过程中,神经调控同样扮演极为重要的角色,并在此基础上提出了骨骼中的神经-血管耦合机制[52],即神经可通过分泌神经递质和神经肽来调节骨代谢并引导血管长入[53]。
LI等[21]研究首先提出,在骨修复过程中,感觉神经对骨折愈伤组织的再支配在时间上早于新骨形成部位的血管形成,且两者的轨迹会相互影响;同时,在神经生长高峰与血管生长高峰之间,愈伤组织出现了神经生长因子表达高峰,说明神经生长因子在神经调控血管形成的过程中起到关键作用。在近期研究中,LIAN等[54]发现神经生长因子诱导骨髓间充质干细胞产生的外泌体在显著促进施万细胞增殖、迁移及营养神经功能的同时,通过施万细胞增强骨髓间充质干细胞的成骨分化能力;在体内实验中,此外泌体促进了多孔支架中的骨整合,并显著增强了血管、神经向支架内生长。关于神经生长因子对血管内皮细胞影响的研究显示,神经生长因子存在显著促血管形成的作用[55]。神经生长因子能通过TrkA和p75信号传导诱导人脐静脉内皮细胞分化和管腔形成[56-57]。神经生长因子在骨髓干细胞、施万细胞、血管内皮细胞等组织细胞中的表达使其对骨修复、周围神经再支配和血管形成等过程均具有重要的影响。同时,神经生长因子还可作为信号传递因子在血管、神经与骨之间发挥沟通作用(图4)。目前研究认为神经生长因子可能在神经-血管耦合机制中起到关键作用,但具体机制仍需进一步研究[1]。
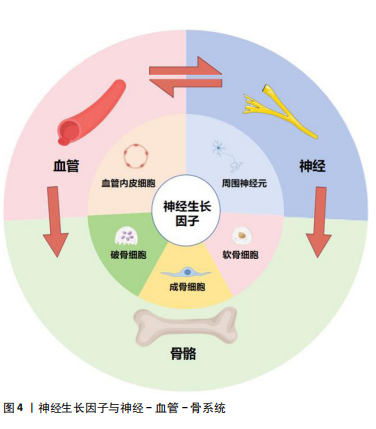
神经生长因子通过骨骼中神经-血管耦合机制对病理骨骼的影响可能与多种骨科疾病有关。Li等[21]研究指出,神经生长因子在骨痂处的表达在骨愈合后消失,并再次局限表达于骨膜。但在骨不愈合或愈合不良的情况下,神经生长因子表达可能持续存在于未愈合处,从而导致感觉神经过度支配,进而产生慢性疼痛。此类异位神经萌发已被证明与未愈合骨骼的慢性疼痛有关[58]。同时,有观点认为,神经生长因子对血管生成的抑制作用,可能是导致抗神经生长因子药物在临床试验中出现骨坏死和快速进展性骨关节病等不良反应的原因之一[59]。目前,以神经生长因子为核心的神经-血管耦合机制已经在骨组织工程研究中得到了大量的应用(表2),但是其负面影响和在病理过程中的作用仍然缺乏关注。
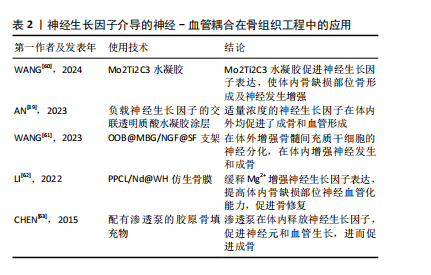
2.3 神经生长因子与骨相关疾病的关系
2.3.1 神经生长因子对软骨组织的影响 神经生长因子与多种骨疾病有关,这种关联往往表现为软骨组织中神经生长因子的高表达。GAO等[64]通过单细胞RNA测序认为正常状态下的软骨组织细胞中神经生长因子表达水平极低,这可能是因为生理状态下软骨组织的再生能力较弱,且神经血管分布较少,故神经生长因子在成熟软骨组织细胞中的表达和作用较不显著[65]。SANG等[66-67]在基于小鼠骨折模型的研究中发现,软骨愈伤组织中出现了明显的神经生长因子表达,而通过转基因技术提高神经生长因子表达则明显促进了软骨生长分化和软骨细胞分化转录因子SOX9基因的表达。因此,可以认为神经生长因子在软骨组织中的表达与在骨组织中的表达类似,在生理情况下较低,而在软骨增殖活跃的情况下才会出现短时间内的快速升高,常见于各种关节炎症和软骨损伤或病理性增生。
神经生长因子对软骨组织的影响主要通过对神经、血管的影响间接实现。CHERIEF等[68]研究指出,创伤早期的软骨愈伤组织中神经生长因子表达急剧增加,随后引发神经血管长入,使用TrkA激动剂后,血管长入和血管网进一步成熟,软骨修复能力显著增强,甚至可导致异位骨化。YU等[69]发现与经过神经生长因子预处理的软骨细胞共培养后,内皮细胞的体外血管生成活性显著增强,而敲除软骨细胞上的TrkA受体则显著消除了上述影响。研究指出神经生长因子不仅可以直接作用于周围神经和血管促进长入,还可能通过作用于软骨细胞激活PI3K/Akt信号通路的方式影响血管内皮细胞间接促进血管长入。生理情况下除部分半月板外,软骨组织内血管通常缺如,血管长入软骨组织多见于关节炎症等病理情况,因此神经生长因子对软骨神经和血管的积极影响可能与各种骨软骨疾病高度相关。
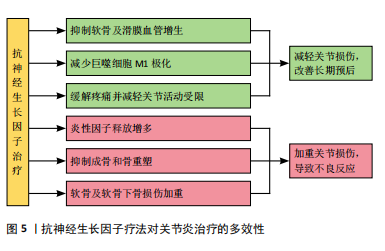
2.3.2 神经生长因子与关节炎的关系 神经生长因子不仅能通过神经血管参与骨软骨病理过程,还能作为炎症因子和疼痛递质介导多种关节炎症[70]。目前研究已经证实,在骨关节炎和类风湿性关节炎患者的关节软骨和滑液中,神经生长因子表达水平均升高[71-72]。这些关节炎症均会出现滑膜血管增生和软骨下骨重塑,而增生血管和软骨内成骨的分布与神经生长因子表达高度相关[73]。因此,神经生长因子作为潜在的关节炎治疗靶点受到广泛重视。LIN等[74]发现,在类风湿性关节炎的发病过程中,神经生长因子可以增强从幼稚巨噬细胞到M1型巨噬细胞的极化,从而加剧炎症并介导关节损伤。神经生长因子还作为重要的疼痛相关因子介导关节炎疼痛,这种疼痛极大地导致了关节功能受限和生活质量下降[75]。NWOSU等[76]发现,通过抑制TrkA阻断神经生长因子信号传导可减轻骨关节炎大鼠疼痛。因此,目前多数研究认为抑制神经生长因子和TrkA可以减轻患者痛苦并延缓疾病进展。
然而,由于神经生长因子作用存在多效性(图5),在关节炎治疗中,抑制神经生长因子-TrkA信号的效果仍然存在争议,尚不明确是否存在显著的负面影响[77]。在慢性关节炎患者中,外周血和滑液单核细胞中TrkA表达显著降低,导致神经生长因子对炎性细胞因子释放的抑制作用丧失,提示抑制神经生长因子-TrkA信号通路反而可能对关节炎患者产生不利影响[78]。同时,由于神经生长因子对成骨的促进作用,阻断神经生长因子表达的治疗方案可导致快速进展性骨关节炎等不良反应[79]。MENGES等[80]研究指出,抗神经生长因子抗体对不稳定关节中的软骨下骨和软骨等结构有负面影响,可能会加剧关节损伤。ZHAO等[81]针对骨关节炎的研究发现,抑制神经生长因子信号会导致骨关节炎关节的骨重塑受到阻碍和延迟。如何在避免不良反应的情况下发挥神经生长因子在关节炎治疗中的价值,是未来研究关注的重点问题。
2.3.3 神经生长因子与骨肿瘤的关系 ANTUNES等[82]最早通过免疫荧光证明神经生长因子及其受体在原发性骨肉瘤中表达。HOU等[83]指出在骨肉瘤组织中神经生长因子的表达显著高于其他生长因子,细胞实验证明神经生长因子通过上调基质金属蛋白酶2表达促进了骨肉瘤细胞转移。研究表明,在癌症发生早期开始持续的神经生长因子抑制治疗,可有效减少肿瘤诱导的骨破坏,延迟骨折时间,延长肢体使用时间[84]。另一方面,骨是癌症转移的首选靶器官之一,骨与肿瘤中的神经可通过相互交流促进癌症进展和转移[85]。神经生长因子作为神经源性信号因子,可在包括乳腺癌、口腔鳞状细胞癌、前列腺癌在内的多种恶性肿瘤向骨转移的过程中发挥重要作用[86-88]。
神经生长因子与骨肿瘤的关系与其高亲和力受体TrkA有关,TrkA的编码基因NTRK1可能发生融合突变,从而持续激活下游通路[89]。因此,研究认为在骨恶性肿瘤的治疗中,以拉罗替尼为代表的抗神经营养性酪氨酸受体激酶药物具有极大潜力[83]。然而,阻断神经生长因子-TrkA信号传导的治疗方案存在多种与骨破坏有关的并发症[90]。针对神经生长因子的单克隆抗体药物研究也由于其严重不良反应被多次中止[91-92]。因此,抗神经生长因子方案的临床应用仍面临诸多问题,神经生长因子对骨骼的复杂影响尚未得到充分研究是限制其临床应用的主要原因。
2.3.4 神经生长因子与骨痛的关系 在骨相关疾病的临床研究中,神经生长因子作为致痛因子诱导疼痛的能力受到特别重视[93]。神经生长因子受体在大部分骨伤害感受器中表达,外源神经生长因子可通过TrkA受体迅速激活和敏化骨伤害感受器[94]。其中,神经生长因子在肿瘤导致的骨痛中发挥的作用尤其受到关注。在骨骼原发性和继发性肿瘤中,肿瘤细胞可通过神经生长因子-TrkA信号通路诱导感觉和交感神经末梢长入肿瘤组织,从而导致癌症相关骨痛[95-96],骨痛的程度会随肿瘤发展进行性加重,其潜在机制与损伤、炎症、肿瘤侵犯以及其他多种神经病理过程有关[97-98]。抗神经生长因子治疗可以从神经和炎症两个方面减轻骨痛的程度[99]。MARTYN等[100]研究表明,即使在肿瘤组织中神经生长因子表达已经引发轴突长入和神经瘤形成的情况下,阻断神经生长因子-TrkA信号传导仍能显著减轻骨痛。目前,治疗各种疾病导致骨骼疼痛的常用药物仍为阿片类药物或非类固醇抗炎药,但是这两种药物不仅可能引发严重不良反应,还存在阻碍骨修复的风险。近期研究认为,通过使用特异性中和抗体阻断骨痛-TrkA信号通路,可以在不延迟骨愈合的情况下消除或减轻骨痛[101]。但是,KAN等[102]研究指出,神经生长因子可通过上调膜相关阿片受体增强其在癌痛模型中的镇痛作用,提示神经生长因子-TrkA信号通路也在镇痛机制中发挥上游调控作用。抗神经生长因子治疗作为传统骨痛治疗方案的替代被寄予厚望,但不良反应限制了其进一步临床应用。
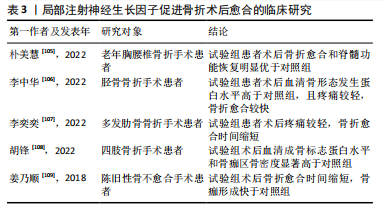
2.3.5 神经生长因子的药用潜力 利用神经生长因子促进骨形成的能力治疗骨损伤疾病的临床前景同样受到关注。尽管神经生长因子已被用于多种神经损伤的临床治疗[103],使用神经生长因子治疗骨折或骨不连依然处于临床试验阶段。由于神经生长因子作为大分子物质难以通过血液循环作用于骨损伤位置,目前研究一般采用局部注射方式给药、辅助手术治疗[104]。大量临床研究证明,使用神经生长因子可有效促进骨折术后愈合(表3)。
然而,简单的局部注射神经生长因子存在诸多问题,如:痛觉过敏[110]、异常性疼痛[111]、机械敏化[112],因快速吸收而失活,以及缺乏对局部神经生长因子浓度的控制等[113]。RIVERA等[114]关于神经生长因子促进骨折修复的研究指出,局部注射神经生长因子给药需要高剂量和重复给药才能产生治疗效果,然而,这种给药方案可能会降低患者的依从性。因此,有必要探索通过药物递送平台对神经生长因子进行可控缓释的方法[115]。YE等[38]通过构建神经生长因子-硫酸软骨素/羟基磷灰石的复合涂层显著增强了骨髓间充质干细胞在钛合金表面的成骨分化,但是,该涂层不能促进骨髓间充质干细胞的黏附和增殖,这或许是因为神经生长因子的释放量受限。探索在尽可能长的时间内缓释充足数量神经生长因子的药物递送平台仍然存在挑战。
| [1] QIN Q, LEE S, PATEL N, et al. Neurovascular coupling in bone regeneration. Exp Mol Med. 2022;54(11):1844-1849. [2] ROCCO ML, SOLIGO M, MANNI L, et al. Nerve Growth Factor: Early Studies and Recent Clinical Trials. Curr Neuropharmacol. 2018;16(10): 1455-1465. [3] ZHENG XQ, HUANG J, LIN JL, et al. Pathophysiological mechanism of acute bone loss after fracture. J Adv Res. 2023;49:63-80. [4] SUN R, BAI L, YANG Y, et al. Nervous System-Driven Osseointegration. Int J Mol Sci. 2022;23(16):8893. [5] STABILE AM, PISTILLI A, BARTOLINI D, et al. Short-Term Effects of Side-Stream Smoke on Nerve Growth Factor and Its Receptors TrKA and p75NTR in a Group of Non-Smokers. Int J Environ Res Public Health. 2022;19(16):10317. [6] STABILE AM, PISTILLI A, MORETTI E, et al. A Possible Role for Nerve Growth Factor and Its Receptors in Human Sperm Pathology. Biomedicines. 2023;11(12):3345. [7] TONI T, DUA P, VAN DER GRAAF PH. Systems Pharmacology of the NGF Signaling Through p75 and TrkA Receptors. CPT Pharmacometrics Syst Pharmacol. 2014;3(12):e150. [8] CONROY JN, COULSON EJ. High-affinity TrkA and p75 neurotrophin receptor complexes: A twisted affair. J Biol Chem. 2022;298(3):101568. [9] MASON AJ, KEELER AB, KABIR F, et al. Sympathetic neurons secrete retrogradely transported TrkA on extracellular vesicles. Sci Rep. 2023;13(1):3657. [10] KRAEMER BR, YOON SO, CARTER BD. The biological functions and signaling mechanisms of the p75 neurotrophin receptor. Handb Exp Pharmacol. 2014;220:121-164. [11] SAJANTI A, LYNE SB, GIRARD R, et al. A comprehensive p75 neurotrophin receptor gene network and pathway analyses identifying new target genes. Sci Rep. 2020;10(1):14984. [12] FRANCO ML, NADEZHDIN KD, LIGHT TP, et al. Interaction between the transmembrane domains of neurotrophin receptors p75 and TrkA mediates their reciprocal activation. J Biol Chem. 2021;297(2):100926. [13] MEEKER RB, WILLIAMS KS. The p75 neurotrophin receptor: at the crossroad of neural repair and death. Neural Regen Res. 2015;10(5): 721-725. [14] MOGI M, KONDO A, KINPARA K, et al. Anti-apoptotic action of nerve growth factor in mouse osteoblastic cell line. Life Sci. 2000;67(10): 1197-1206. [15] MONTAGNOLI C, TIRIBUZI R, CRISPOLTONI L, et al. β-NGF and β-NGF receptor upregulation in blood and synovial fluid in osteoarthritis. Biol Chem. 2017;398(9):1045-1054. [16] MINNONE G, DE BENEDETTI F, BRACCI-LAUDIERO L. NGF and Its Receptors in the Regulation of Inflammatory Response. Int J Mol Sci. 2017;18(5):1028. [17] SAMARIO-ROMÁN J, LARQUÉ C, PÁNICO P, et al. NGF and Its Role in Immunoendocrine Communication during Metabolic Syndrome. Int J Mol Sci. 2023;24(3):1957. [18] OHASHI Y, UCHIDA K, FUKUSHIMA K, et al. NGF Expression and Elevation in Hip Osteoarthritis Patients with Pain and Central Sensitization. Biomed Res Int. 2021;2021:9212585. [19] WANG L, ZHOU S, LIU B, et al. Locally applied nerve growth factor enhances bone consolidation in a rabbit model of mandibular distraction osteogenesis. J Orthop Res. 2006;24(12):2238-2245. [20] GRILLS BL, SCHUIJERS JA, WARD AR. Topical application of nerve growth factor improves fracture healing in rats. J Orthop Res. 1997;15(2):235-242. [21] LI Z, MEYERS CA, CHANG L, et al. Fracture repair requires TrkA signaling by skeletal sensory nerves. J Clin Invest. 2019;129(12):5137-5150. [22] DAMIATI LA, EL SOURY M. Bone-nerve crosstalk: a new state for neuralizing bone tissue engineering-A mini review. Front Med (Lausanne). 2024;11:1386683. [23] 刘国铭,王钦奋,林克凤,等.全身应用神经生长因子对大鼠胫骨干骨折早期愈合作用及骨形态发生蛋白2和血管内皮生长因子表达的影响[J].中国组织工程研究,2020,24(29):4680-4685. [24] PARKER RS, NAZZAL MK, MORRIS AJ, et al. Role of the Neurologic System in Fracture Healing: An Extensive Review. Curr Osteoporos Rep. 2024;22(1):205-216. [25] 王裕祥,王卫东,杨应忠,等.NGF、转化生长因子β1、成纤维细胞生长因子在骨折愈合中的作用效果分析[J].湖南师范大学学报(医学版),2018,15(3):95-98. [26] WRIGHT CS, LEWIS KJ, SEMON K, et al. Deletion of the auxiliary α2δ1 voltage sensitive calcium channel subunit in osteocytes and late-stage osteoblasts impairs femur strength and load-induced bone formation in male mice. J Bone Miner Res. 2024;39(3):298-314. [27] BONINI M, FIORETTI D, SARGENTINI V, et al. Increased nerve growth factor serum levels in top athletes. Clin J Sport Med. 2013;23(3): 228-231. [28] RAJPAR I, KUMAR G, FORTINA P, et al. Toll-like receptor 4 signaling in osteoblasts is required for load-induced bone formation in mice. iScience. 2023;26(4):106304. [29] FIORAVANTI G, HUA PQ, TOMLINSON RE. The TrkA agonist gambogic amide augments skeletal adaptation to mechanical loading. Bone. 2021;147:115908. [30] TOMLINSON RE, LI Z, LI Z, et al. NGF-TrkA signaling in sensory nerves is required for skeletal adaptation to mechanical loads in mice. Proc Natl Acad Sci U S A. 2017;114(18):E3632-E3641. [31] YANG S, LIU H, LIU Y, et al. Effect of adiponectin secreted from adipose-derived stem cells on bone-fat balance and bone defect healing. J Tissue Eng Regen Med. 2019;13(11):2055-2066. [32] ZHA K, TAN M, HU Y, et al. Regulation of metabolic microenvironment with a nanocomposite hydrogel for improved bone fracture healing. Bioact Mater. 2024;37:424-438. [33] GHASEMI M, TALEBI A, GHANBARI A, et al. Bone marrow stromal cell-conditioned medium regenerates injured sciatic nerve by increasing expression of MPZ and NGF and decreasing apoptosis. Iran J Basic Med Sci. 2024;27(5):596-602. [34] AL-MASSRI KF, AHMED LA, EL-ABHAR HS. Mesenchymal stem cells in chemotherapy-induced peripheral neuropathy: A new challenging approach that requires further investigations. J Tissue Eng Regen Med. 2020;14(1):108-122. [35] BRAZILL JM, BEEVE AT, CRAFT CS, et al. Nerves in Bone: Evolving Concepts in Pain and Anabolism. J Bone Miner Res. 2019;34(8): 1393-1406. [36] 崔国胜,曾剑玉,张婧,等.神经生长因子对2型糖尿病小鼠骨髓基质细胞体外成骨能力的影响[J].中华口腔医学杂志,2018, 53(2):97-102. [37] FANG CN, TAN HQ, SONG AB, et al. NGF/TrkA promotes the vitality, migration and adhesion of bone marrow stromal cells in hypoxia by regulating the Nrf2 pathway. Metab Brain Dis. 2022;37(6):2017-2026. [38] YE J, GONG P. NGF-CS/HA-coating composite titanium facilitates the differentiation of bone marrow mesenchymal stem cells into osteoblast and neural cells. Biochem Biophys Res Commun. 2020;531(3):290-296. [39] AN J, SHI X, ZHANG J, et al. Dual aldehyde cross-linked hyaluronic acid hydrogels loaded with PRP and NGF biofunctionalized PEEK interfaces to enhance osteogenesis and vascularization. Mater Today Bio. 2023; 24:100928. [40] HUANG M, ZHOU J, LI X, et al. Mechanical protein polycystin-1 directly regulates osteoclastogenesis and bone resorption. Sci Bull (Beijing). 2024;69(12):1964-1979. [41] KONNO T, MURACHI H, OTSUKA K, et al. Ctdnep1 phosphatase is required for negative regulation of RANKL-induced osteoclast differentiation in RAW264.7 cells. Biochem Biophys Res Commun. 2024;719:150063.
[42] DELAY L, BARBIER J, AISSOUNI Y, et al. Tyrosine kinase type A-specific signalling pathways are critical for mechanical allodynia development and bone alterations in a mouse model of rheumatoid arthritis. Pain. 2022;163(7):e837-e849. [43] ZHANG Z, WANG F, HUANG X, et al. Engineered Sensory Nerve Guides Self-Adaptive Bone Healing via NGF-TrkA Signaling Pathway. Adv Sci (Weinh). 2023;10(10):e2206155. [44] MELONI M, CESSELLI D, CAPORALI A, et al. Cardiac Nerve Growth Factor Overexpression Induces Bone Marrow-derived Progenitor Cells Mobilization and Homing to the Infarcted Heart. Mol Ther. 2015; 23(12):1854-1866. [45] XIE G, HUANG C, JIANG S, et al. Smoking and osteoimmunology: Understanding the interplay between bone metabolism and immune homeostasis. J Orthop Translat. 2024;46:33-45. [46] FENG W, GUO J, LI M. RANKL-independent modulation of osteoclastogenesis. J Oral Biosci. 2019;61(1):16-21. [47] HEMINGWAY F, TAYLOR R, KNOWLES HJ, et al. RANKL-independent human osteoclast formation with APRIL, BAFF, NGF, IGF I and IGF II. Bone. 2011;48(4):938-944. [48] XU L, NWOSU LN, BURSTON JJ, et al. The anti-NGF antibody muMab 911 both prevents and reverses pain behaviour and subchondral osteoclast numbers in a rat model of osteoarthritis pain. Osteoarthritis Cartilage. 2016;24(9):1587-1595. [49] SUN W, YE B, CHEN S, et al. Neuro-bone tissue engineering: emerging mechanisms, potential strategies, and current challenges. Bone Res. 2023;11(1):65. [50] LIU H, CHEN H, HAN Q, et al. Recent advancement in vascularized tissue-engineered bone based on materials design and modification. Mater Today Bio. 2023;23:100858. [51] DIOMEDE F, MARCONI GD, FONTICOLI L, et al. Functional Relationship between Osteogenesis and Angiogenesis in Tissue Regeneration. Int J Mol Sci. 2020;21(9):3242. [52] ZHANG Z, HAO Z, XIAN C, et al. Neuro-bone tissue engineering: Multiple potential translational strategies between nerve and bone. Acta Biomater. 2022;153:1-12. [53] LEROUX A, PAIVA DOS SANTOS B, LENG J, et al. Sensory neurons from dorsal root ganglia regulate endothelial cell function in extracellular matrix remodelling. Cell Commun Signal. 2020;18(1):162. [54] LIAN M, QIAO Z, QIAO S, et al. Nerve Growth Factor-Preconditioned Mesenchymal Stem Cell-Derived Exosome-Functionalized 3D-Printed Hierarchical Porous Scaffolds with Neuro-Promotive Properties for Enhancing Innervated Bone Regeneration. ACS Nano. 2024;18(10):7504-7520. [55] AHLUWALIA A, JONES MK, BRZOZOWSKI T, et al. Nerve growth factor is critical requirement for in vitro angiogenesis in gastric endothelial cells. Am J Physiol Gastrointest Liver Physiol. 2016;311(5):G981-G987. [56] CANTARELLA G, LEMPEREUR L, PRESTA M, et al. Nerve growth factor-endothelial cell interaction leads to angiogenesis in vitro and in vivo. FASEB J. 2002;16(10):1307-1309. [57] CAPORALI A, EMANUELI C. Cardiovascular actions of neurotrophins. Physiol Rev. 2009;89(1):279-308. [58] SUN S, DIGGINS NH, GUNDERSON ZJ, et al. No pain, no gain? The effects of pain-promoting neuropeptides and neurotrophins on fracture healing. Bone. 2020;131:115109. [59] LEE S, HWANG C, MARINI S, et al. NGF-TrkA signaling dictates neural ingrowth and aberrant osteochondral differentiation after soft tissue trauma. Nat Commun. 2021;12(1):4939. [60] WANG H, HSU YC, WANG C, et al. Conductive and Enhanced Mechanical Strength of Mo2Ti2C3 MXene-Based Hydrogel Promotes Neurogenesis and Bone Regeneration in Bone Defect Repair. ACS Appl Mater Interfaces. 2024;16(14):17208-17218. [61] WANG X, ZHENG W, BAI Z, et al. Mimicking bone matrix through coaxial electrospinning of core-shell nanofibrous scaffold for improving neurogenesis bone regeneration. Biomater Adv. 2023;145:213246. [62] LI Q, LIU W, HOU W, et al. Micropatterned photothermal double-layer periosteum with angiogenesis-neurogenesis coupling effect for bone regeneration. Mater Today Bio. 2022;18:100536. [63] CHEN WH, MAO CQ, ZHUO LL, et al. Beta-nerve growth factor promotes neurogenesis and angiogenesis during the repair of bone defects. Neural Regen Res. 2015;10(7):1159-1165. [64] GAO X, MURPHY MM, PEYER JG, et al. Leptin receptor+ cells promote bone marrow innervation and regeneration by synthesizing nerve growth factor. Nat Cell Biol. 2023;25(12):1746-1757. [65] FUJII Y, LIU L, YAGASAKI L, et al. Cartilage Homeostasis and Osteoarthritis. Int J Mol Sci. 2022;23(11):6316. [66] SANG XG, WANG ZY, CHENG L, et al. Analysis of the mechanism by which nerve growth factor promotes callus formation in mice with tibial fracture. Exp Ther Med. 2017;13(4):1376-1380. [67] HASEEB A, KC R, ANGELOZZI M, et al. SOX9 keeps growth plates and articular cartilage healthy by inhibiting chondrocyte dedifferentiation/osteoblastic redifferentiation. Proc Natl Acad Sci U S A. 2021;118(8):e2019152118. [68] CHERIEF M, NEGRI S, QIN Q, et al. TrkA+ Neurons Induce Pathologic Regeneration After Soft Tissue Trauma. Stem Cells Transl Med. 2022; 11(11):1165-1176. [69] YU X, QI Y, ZHAO T, et al. NGF increases FGF2 expression and promotes endothelial cell migration and tube formation through PI3K/Akt and ERK/MAPK pathways in human chondrocytes. Osteoarthritis Cartilage. 2019;27(3):526-534. [70] DENK F, BENNETT DL, MCMAHON SB. Nerve Growth Factor and Pain Mechanisms. Annu Rev Neurosci. 2017;40:307-325. [71] ZHANG L, LI M, LI X, et al. Characteristics of sensory innervation in synovium of rats within different knee osteoarthritis models and the correlation between synovial fibrosis and hyperalgesia. J Adv Res. 2021;35:141-151. [72] FARINA L, MINNONE G, ALIVERNINI S, et al. Pro Nerve Growth Factor and Its Receptor p75NTR Activate Inflammatory Responses in Synovial Fibroblasts: A Novel Targetable Mechanism in Arthritis. Front Immunol. 2022;13:818630. [73] WALSH DA, MCWILLIAMS DF, TURLEY MJ, et al. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology (Oxford). 2010;49(10):1852-1861. [74] LIN CY, LEE KT, LIN YY, et al. NGF facilitates ICAM-1-dependent monocyte adhesion and M1 macrophage polarization in rheumatoid arthritis. Int Immunopharmacol. 2024;130:111733. [75] YU H, HUANG T, LU WW, et al. Osteoarthritis Pain. Int J Mol Sci. 2022; 23(9):4642. [76] NWOSU LN, MAPP PI, CHAPMAN V, et al. Blocking the tropomyosin receptor kinase A (TrkA) receptor inhibits pain behaviour in two rat models of osteoarthritis. Ann Rheum Dis. 2016;75(6):1246-1254.
[77] WALSH DA, NEOGI T. A tale of two TrkA inhibitor trials: same target, divergent results. Osteoarthritis Cartilage. 2019;27(11):1575-1577.
[78] PRENCIPE G, MINNONE G, STRIPPOLI R, et al. Nerve growth factor downregulates inflammatory response in human monocytes through TrkA. J Immunol. 2014;192(7):3345-3354. [79] WISE BL, SEIDEL MF, LANE NE. The evolution of nerve growth factor inhibition in clinical medicine. Nat Rev Rheumatol. 2021;17(1):34-46. [80] MENGES S, MICHAELIS M, KLEINSCHMIDT-DÖRR K. Anti-NGF treatment worsens subchondral bone and cartilage measures while improving symptoms in floor-housed rabbits with osteoarthritis. Front Physiol. 2023;14:1201328. [81] ZHAO L, LAI Y, JIAO H, et al. Nerve growth factor receptor limits inflammation to promote remodeling and repair of osteoarthritic joints. Nat Commun. 2024;15(1):3225. [82] ANTUNES BP, BECKER RG, BRUNETTO AT, et al. Expression of neurotrophins and their receptors in primary osteosarcoma. Rev Col Bras Cir. 2019;46(2):e2094. [83] HOU CH, CHEN WL, LIN CY. Targeting nerve growth factor-mediated osteosarcoma metastasis: mechanistic insights and therapeutic opportunities using larotrectinib. Cell Death Dis. 2024;15(5):381. [84] MCCAFFREY G, THOMPSON ML, MAJUTA L, et al. NGF blockade at early times during bone cancer development attenuates bone destruction and increases limb use. Cancer Res. 2014;74(23):7014-7023. [85] YONEDA T, HIASA M, OKUI T, et al. Sensory nerves: A driver of the vicious cycle in bone metastasis? J Bone Oncol. 2021;30:100387. [86] BLOOM AP, JIMENEZ-ANDRADE JM, TAYLOR RN, et al. Breast cancer-induced bone remodeling, skeletal pain, and sprouting of sensory nerve fibers. J Pain. 2011;12(6):698-711. [87] SHAN Q, TAKABATAKE K, KAWAI H, et al. Significance of cancer stroma for bone destruction in oral squamous cell carcinoma using different cancer stroma subtypes. Oncol Rep. 2022;47(4):81. [88] ZHANG Y, LI W, GUO S, et al. FBXO22 Mediates the NGF/TRKA Signaling Pathway in Bone Metastases in Prostate Cancer. Am J Pathol. 2023;193(9):1248-1266. [89] FAN Y, ZHANG B, DU X, et al. Regulating Tumorigenicity and Cancer Metastasis through TRKA Signaling. Curr Cancer Drug Targets. 2024; 24(3):271-287. [90] CARRINO JA, MCALINDON TE, SCHNITZER TJ, et al. Characterization of adverse joint outcomes in patients with osteoarthritis treated with subcutaneous tanezumab. Osteoarthritis Cartilage. 2023;31(12): 1612-1626. [91] TAHIR S, SADIK O, EZENWA V, et al. Various Doses of Tanezumab in the Management of Chronic Low Back Pain (CLBP): A Pooled Analysis of 4,514 Patients. Cureus. 2023;15(10):e46790. [92] LIN KJ, TURNER KC, HASSAN HE, et al. Population Pharmacokinetics of Fasinumab in Healthy Volunteers and Patients With Pain Due to Osteoarthritis of the Knee or Hip. Clin Pharmacol Drug Dev. 2024; 13(6):621-630. [93] 颜冰,戴文玲,刘吉华.神经病理性疼痛中神经生长因子的作用及其相关镇痛药物研发进展[J].药学进展,2019,43(2):111-117. [94] NENCINI S, RINGUET M, KIM DH, et al. Mechanisms of nerve growth factor signaling in bone nociceptors and in an animal model of inflammatory bone pain. Mol Pain. 2017;13:1744806917697011. [95] YONEDA T, HIASA M, OKUI T, et al. Cancer-nerve interplay in cancer progression and cancer-induced bone pain. J Bone Miner Metab. 2023;41(3):415-427. [96] YANG Y, YANG W, ZHANG R, et al. Peripheral Mechanism of Cancer-Induced Bone Pain. Neurosci Bull. 2024;40(6):815-830. [97] YANG H, WANG Y, ZHEN S, et al. AMPK activation attenuates cancer-induced bone pain by reducing mitochondrial dysfunction-mediated neuroinflammation. Acta Biochim Biophys Sin (Shanghai). 2023;55(3):460-471. [98] JING D, ZHAO Q, ZHAO Y, et al. Management of pain in patients with bone metastases. Front Oncol. 2023;13:1156618. [99] SEVCIK MA, GHILARDI JR, PETERS CM, et al. Anti-NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain. 2005;115(1-2): 128-141. [100] MARTYN JAJ, MAO J, BITTNER EA. Opioid Tolerance in Critical Illness. N Engl J Med. 2019;380(4):365-378. [101] RAPP AE, KRONER J, BAUR S, et al. Analgesia via blockade of NGF/TrkA signaling does not influence fracture healing in mice. J Orthop Res. 2015;33(8):1235-1241. [102] KAN BF, LIU XY, HAN MM, et al. Nerve Growth Factor/Tyrosine Kinase A Receptor Pathway Enhances Analgesia in an Experimental Mouse Model of Bone Cancer Pain by Increasing Membrane Levels of δ-Opioid Receptors. Anesthesiology. 2024;140(4):765-785. [103] 郑湘予,文建国,王福建,等.不同给药途径生长因子对坐骨神经再生的影响[J].中华小儿外科杂志,2013,34(7):539-542. [104] 杨冰,马天宇,马维.神经生长因子促进骨折愈合的研究进展[J].中国医学科学院学报,2020,42(4):546-551. [105] 朴美慧.后路减压内固定联合mNGF对老年胸腰椎骨折伴脊髓损伤患者脊髓功能及血清mRNA-124、Neuritin的影响[J].社区医学杂志,2022,20(16):912-918. [106] 李中华,张发元,王燕青,等.闭合复位髓内钉内固定联合鼠神经生长因子对胫骨开放性骨折患者血清BMP-2、BMP-7水平的影响[J].临床和实验医学杂志,2022,21(16):1729-1733. [107] 李奕奕,刘兴涛,李旭开.鼠神经生长因子联合早期微创内固定治疗在创伤性多发性肋骨骨折患者中的应用探讨[J].现代医学与健康研究电子杂志,2022,6(3):52-54. [108] 胡锋,洪芳,杨月太.神经生长因子联合抗氧化治疗肢骨折内固定术后骨折愈合、骨代谢及氧化应激反应程度的影响[J].临床和实验医学杂志,2022,21(8):842-846. [109] 姜乃顺.神经生长因子联合组合式外固定架治疗骨折不愈合的疗效观察[J].中国现代药物应用,2018,12(12):86-88. [110] ENOMOTO M, MANTYH PW, MURRELL J, et al. Anti-nerve growth factor monoclonal antibodies for the control of pain in dogs and cats. Vet Rec. 2019;184(1):23. [111] ANDRESEN T, NILSSON M, NIELSEN AK, et al. Intradermal Injection with Nerve Growth Factor: A Reproducible Model to Induce Experimental Allodynia and Hyperalgesia. Pain Pract. 2016;16(1):12-23. [112] SVENSSON P, WANG K, ARENDT-NIELSEN L, et al. Effects of NGF-induced muscle sensitization on proprioception and nociception. Exp Brain Res. 2008;189(1):1-10. [113] ALASTRA G, ALOE L, BALDASSARRO VA, et al. Nerve Growth Factor Biodelivery: A Limiting Step in Moving Toward Extensive Clinical Application? Front Neurosci. 2021;15:695592. [114] RIVERA KO, RUSSO F, BOILEAU RM, et al. Local injections of β-NGF accelerates endochondral fracture repair by promoting cartilage to bone conversion. Sci Rep. 2020;10(1):22241. [115] RIVERA KO, CUYLEAR DL, DUKE VR, et al. Encapsulation of β-NGF in injectable microrods for localized delivery accelerates endochondral fracture repair. Front Bioeng Biotechnol. 2023;11:1190371. [116] EDMONDS ME, CLARKE MB, NEWTON S, et al. Increased uptake of bone radiopharmaceutical in diabetic neuropathy. Q J Med. 1985; 57(224):843-855. |
| [1] | 赖鹏宇, 梁 冉, 沈 山. 组织工程技术修复颞下颌关节:问题与挑战[J]. 中国组织工程研究, 2025, 29(在线): 1-9. |
| [2] | 李加根, 陈跃平, 黄柯琪, 陈尚桐, 黄川洪. 线粒体自噬视域下的类风湿关节炎:多机器学习算法构建预测模型及验证并免疫调控分析[J]. 中国组织工程研究, 2025, 29(在线): 1-15. |
| [3] | 张艺博, 卢健棋, 毛美玲, 庞 延, 董 礼, 杨尚冰, 肖 湘. 类风湿关节炎与冠状动脉粥样硬化的因果关系:GWAS数据库血清代谢物和炎症因子数据[J]. 中国组织工程研究, 2025, 29(在线): 1-9. |
| [4] | 李良奎, 黄永灿, 王鹏, 于滨生. 颈椎前路椎体骨化物可控前移融合对后纵韧带骨化物和内植物影响的有限元分析[J]. 中国组织工程研究, 2025, 29(9): 1761-1767. |
| [5] | 周金海, 李江伟, 王序全, 庄 颖, 赵 瑛, 杨渝勇, 王嘉嘉, 杨 阳, 周仕炼. 不同骨强度下全膝置换过程中发生股骨前皮质切迹的三维有限元分析[J]. 中国组织工程研究, 2025, 29(9): 1775-1782. |
| [6] | 陈 曦, 汤 涛, 陈铜兵, 李 青, 张 文. 不同内固定系统治疗股骨转子间骨折的力学稳定性[J]. 中国组织工程研究, 2025, 29(9): 1783-1788. |
| [7] | 黄浩波, 梁馨元, 叶国忠, 谢庆祥, 苏博源. 纤维带与无头加压螺钉治疗第1,2跖骨近端粉碎性骨折Lisfranc损伤[J]. 中国组织工程研究, 2025, 29(9): 1803-1809. |
| [8] | 刘 琰, 王 铠, 吴 敏. 踝关节骨折术后踝穴冠状位角度波动与关节功能恢复的关系[J]. 中国组织工程研究, 2025, 29(9): 1820-1826. |
| [9] | 张 浩, 王 清, 张 建, 李广州, 王高举. 后路C2-3固定结合顶棒置入与单纯后路C2-3固定治疗不稳定Hangman骨折的比较[J]. 中国组织工程研究, 2025, 29(9): 1848-1854. |
| [10] | 苏林涛, 江剑峰, 马 俊, 黄亮亮, 雷昌宇, 韩尧政, 康 辉. O臂导航在椎弓根发育性狭窄胸腰椎骨折中的精准应用[J]. 中国组织工程研究, 2025, 29(9): 1855-1862. |
| [11] | 马 驰, 王 宁, 陈 拥, 魏志晗, 刘逢纪, 朴成哲. 3D打印个体化截骨导板结合定制钢板在开放楔形胫骨高位截骨中的应用[J]. 中国组织工程研究, 2025, 29(9): 1863-1869. |
| [12] | 高振洋, 曾秀安, 杨其兵, 寇贤帅, 王克竞, 厉 孟. 计算机模拟复位联合骨盆复位架治疗APC-Ⅲ型骨盆骨折[J]. 中国组织工程研究, 2025, 29(9): 1870-1875. |
| [13] | 冯志萌, 孙 宁, 孙兆忠, 李岳飞, 刘昌震, 李 洒. 影像三维重建安全辅助单孔分体内镜治疗L5/S1极外侧腰椎间盘突出症[J]. 中国组织工程研究, 2025, 29(9): 1876-1882. |
| [14] | 周佳俊, 马 飞, 冷叶波, 徐世财, 何宝强, 李 洋, 廖烨晖, 唐 强, 唐 超, 王 清, 钟德君. 全脊柱MRI评估骨质疏松患者椎体骨折的分布特点及临床意义[J]. 中国组织工程研究, 2025, 29(9): 1883-1889. |
| [15] | 晋继明, 郝阳泉, 赵汝顺, 张玉婷, 姜永宏, 许 鹏, 鲁 超. 双髋关节MRI预测股骨头坏死塌陷风险[J]. 中国组织工程研究, 2025, 29(9): 1890-1896. |
神经生长因子对骨骼生长和修复的影响得到了大量研究的关注。但是,现有研究多着眼于神经生长因子对骨折模型的整体影响,或针对神经生长因子在某一种组织细胞中的作用。骨骼的生长与修复是涉及血管、神经等多种组织、器官和系统的复杂过程[3]。神经生长因子的表达和受体广泛分布于上述相关组织中,然而对于神经生长因子在骨骼与血管、神经等不同系统组织间协调发挥的作用,目前研究较为有限。文章主要介绍神经生长因子对骨、软骨组织细胞增殖、分化和功能的影响,同时从神经-血管耦合及关节炎症等方面简述神经生长因子对骨骼生理进程和病理变化的影响。
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
1.1 资料来源
1.1.1 检索人及检索时间 由第一作者在2024年8月进行检索。
1.1.2 检索文献时限 2010年1月至2024年8月。
1.1.3 检索数据库 中文数据库:万方数据库、中国知网;英文数据库:PubMed数据库。
1.1.4 检索词 中文检索词为“神经生长因子,TrkA,骨,软骨”;英文检索词为“nerve growth factor,NGF,TrkA,bone,cartilage”。
1.1.5 检索文献类型 原始论文、综述、病例报告等。
1.1.6 检索策略 以PubMed数据库为例,检索策略见图1。
1.1.7 手工检索情况 通过阅读文献,对纳入文献的相似文献和参考文献进行补充检索,不再限制发表时间,手工录入文献19篇。
1.1.8 检索文献量 中文文献2 044篇,英文文献881篇。
1.2 纳入标准 ①题目和摘要与主题词相关的文献;②内容与神经生长因子、骨形成、骨疾病联系紧密,相关度高的文献。
1.4 质量评估与数据的提取 从数据库综合检索到2 925篇文献,按照纳入、排除标准进行初筛,排除重复、无参考意义、与主题无关的文献,初筛后纳入437篇文献,随后详细阅读全文进行进一步筛选,并通过引用文献进行检索增补,最终纳入116篇文献撰写综述。文献检索流程见图2。
3.1 既往他人在该领域研究的贡献和存在的问题 自1953年LEVI-MONTALCINI发现神经生长因子以来,研究者深入研究了神经生长因子在神经系统中的作用和机制。20世纪末,对神经生长因子的研究拓展到非神经组织,随后的研究深入探索了神经生长因子在骨组织细胞增殖、分化和骨代谢中的作用,以及神经生长因子通过特定信号通路影响骨形成和骨重塑的能力。1985年,EDMONDS等[116]发现糖尿病患者足部存在继发于周围神经病变的血流增加,导致骨吸收加重、骨折风险上升,并最终导致糖尿病足神经性骨关节病。他们的研究是骨组织中神经-血管耦合的最早依据之一。尽管大量后续研究探讨了骨-神经和骨-血管的相互作用,但直到近年,骨、神经和血管三者才被作为一个整体来加以强调。虽然已有广泛的研究基础,神经-血管网络调节骨形成和修复的具体机制和通路仍需进一步探索和整合,而神经生长因子在这一过程中的影响仍不完全明确。
3.2 作者综述区别于他人他篇的特点 此综述考虑了神经生长因子在多种不同组织中的作用,重点关注神经生长因子在骨组织中的协调能力。同时,此综述论述了神经生长因子作用的多效性,指出其不仅具有促进骨形成和骨修复的积极影响,也可能在多种骨疾病中发挥重要作用,这种多效性可能是解决神经生长因子和抗神经生长因子药物临床应用问题的关键。
3.3 综述的局限性 神经生长因子在国内外均得到了长期关注和广泛研究,此综述可能无法涵盖所有相关的研究成果。同时,限于篇幅,此综述仅论述了神经生长因子在骨形成和骨疾病中的作用,没有综合考虑神经营养因子家族的其他成员。
3.4 综述的重要意义 此综述阐述了神经生长因子在骨形成和骨疾病中的重要作用。通过讨论骨组织神经-血管耦合机制,提出了未来神经生长因子的研究应向多系统、整体化发展的设想。
3.5 课题专家组对未来的建议 神经生长因子的作用广泛而多样,在组织工程和临床应用方面都具有巨大的潜力和研究价值。在体内,神经生长因子的靶细胞种类繁多且复杂。通过与不同受体结合,神经生长因子可对细胞功能进行促进和抑制的双向调节,体现了作用的多效性。然而,现有研究多集中关注单一组织细胞,忽视了神经生长因子在多种组织细胞共存情况下的协调作用。被神经生长因子激活的靶细胞内的多种信号通路,以及在不同靶组织中起主导作用的特定组织,尚难以明确,这是当前神经生长因子作用机制研究面临的主要挑战。相关研究的不足导致神经生长因子相关的临床药物面临难以解决的不良反应问题。因此,从整体视角系统性地研究神经生长因子对骨骼及相关组织的影响,可能是解决其临床应用问题的关键。
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||