中国组织工程研究 ›› 2025, Vol. 29 ›› Issue (20): 4305-4315.doi: 10.12307/2025.697
• 组织构建综述 tissue construction review • 上一篇 下一篇
永恒蛋白基因/周期蛋白基因介导多条通路在昼夜节律中的作用
王士杰,文登台,孙国琪,王京峰,高颖晖
- 鲁东大学体育学院,山东省烟台市 264025
-
收稿日期:2024-07-31接受日期:2024-09-26出版日期:2025-07-18发布日期:2024-12-23 -
通讯作者:文登台,博士,副教授,硕士生导师,鲁东大学体育学院,山东省烟台市 264025 -
作者简介:王士杰,男,1997年生,山东省烟台市人,汉族,鲁东大学在读硕士,主要从事体育教育训练学研究。 -
基金资助:国家自然科学基金项目(32000832),项目名称:心肌salt基因介导运动训练与高盐摄入调控心脏衰老的机制研究,项目负责人:文登台;山东省高等学校青年创新团队发展计划项目(2023RW057),项目名称:脂肪组织Gαq/Gγ1/bmm通路诱导的遗传性肥胖及其并发症对运动适应的研究,项目负责人:文登台
The role of Timeless/Period gene mediated multiple pathways in circadian rhythm
Wang Shijie, Wen Dengtai, Sun Guoqi, Wang Jingfeng, Gao Yinghui
- School of Physical Education, Ludong University, Yantai 264025, Shandong Province, China
-
Received:2024-07-31Accepted:2024-09-26Online:2025-07-18Published:2024-12-23 -
Contact:Wen Dengtai, PhD, Associate professor, Master’s supervisor, School of Physical Education, Ludong University, Yantai 264025, Shandong Province, China -
About author:Wang Shijie, Master candidate, School of Physical Education, Ludong University, Yantai 264025, Shandong Province, China -
Supported by:National Natural Science Foundation of China, No. 32000832 (to WDT); Youth Innovation Team Development Program of Shandong Province Higher Education Institutions, No. 2023RW057 (to WDT)
摘要:
文题释义:
永恒蛋白基因:是编码永恒蛋白基因-周期蛋白基因复合体的关键成分,是产生昼夜节律所必需的,参与交配行为、DNA复制和幼虫趋光性。
周期蛋白基因:对生物钟功能至关重要,决定昼夜节律和超昼夜节律的周期长度。周期蛋白基因过表达会导致昼夜节律缩短,周期蛋白基因敲减会导致昼夜节律延长。
背景:昼夜节律与大多数哺乳动物及昆虫的生命活动息息相关。永恒蛋白基因作为编码永恒蛋白基因-周期蛋白基因复合物的关键成分在产生昼夜节律过程中起到十分关键的作用,但其在昼夜节律中的作用机制仍不清楚。
目的:通过整理永恒蛋白基因、周期蛋白基因、昼夜节律、环境及隐花色素基因之间的关系,更加全面地认识昼夜循环的入核、积累机制及环境对昼夜节律的影响。
方法:应用计算机在Web of Science核心合集、PubMed数据库及CNKI中进行检索,以“永恒蛋白基因,周期蛋白基因,昼夜节律,环境”为中文检索词,以“Timeless,Period,circadian rhythm,environment”为英文检索词,通过全文阅读逐步排除非相关文献,最终纳入126篇文献进行综述。
结果与结论:在昼夜时钟中,昼夜自发输出周期蛋白kaput和CYCLE激活永恒蛋白基因/周期蛋白基因,永恒蛋白基因调控周期蛋白基因的入核机制及稳定性,而周期蛋白基因也可以通过一些机制单独入核。酪蛋白激酶2、Shaggy蛋白激酶及双倍时间基因都可以通过磷酸化永恒蛋白基因/周期蛋白基因的方式来调节昼夜节律,参与转录。隐花色素基因介导的永恒蛋白基因降解对转录的完整性有十分重要的作用。环境因素、膳食方式等外界因素均可以通过永恒蛋白基因/周期蛋白基因对昼夜节律产生影响,而限时进食可作为一种改善昼夜节律紊乱的有效方法。
https://orcid.org/0000-0002-8969-9027(王士杰)
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
中图分类号:
引用本文
王士杰, 文登台, 孙国琪, 王京峰, 高颖晖. 永恒蛋白基因/周期蛋白基因介导多条通路在昼夜节律中的作用[J]. 中国组织工程研究, 2025, 29(20): 4305-4315.
Wang Shijie, Wen Dengtai, Sun Guoqi, Wang Jingfeng, Gao Yinghui. The role of Timeless/Period gene mediated multiple pathways in circadian rhythm[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(20): 4305-4315.
2.2 昼夜节律的机制
2.2.1 永恒蛋白基因/周期蛋白基因介导的入核/核积累机制 见图3。
为了保持健康,生物钟必须与当地时间同步。环境变化(如每日的明暗或温度循环)会引起生物钟的相应改变[18]。周期蛋白基因和永恒蛋白基因的表达由昼夜节律转录因子CLK和CYC在中午启动,CLK/CYC的周期性激活和抑制与周期性磷酸化水平同时存在[19]。磷酸化可以调节周期蛋白基因和永恒蛋白基因的入核时间及永恒蛋白基因的降解[20],这些翻译后修饰似乎可以调节昼夜节律时钟的周期、相位和振幅。周期蛋白基因和永恒蛋白基因的mRNA水平在夜间早些时候积累,几小时后达到峰值[21],它们形成异二聚体(周期蛋白基因-永恒蛋白基因)在夜间中途进入细胞核,通过阻断转录激活因子CLK和CYC活性来负向调节周期蛋白基因和永恒蛋白基因的转录[22]。周期蛋白基因和永恒蛋白基因的累积从细胞质中开始,午夜时在细胞核中积累,早晨永恒蛋白基因的降解促进周期蛋白基因逐渐磷酸化,并最终降解,以达到重置转录周期并重新启动循环的目的[3]。没有与永恒蛋白基因结合的周期蛋白基因是不稳定且无法表达的。永恒蛋白基因中的核定位信号具有调节永恒蛋白基因核定位的作用,当其突变时会增加永恒蛋白基因和周期蛋白基因的细胞

质积累,但会减少它们的核积累,导致转基因果蝇的昼夜节律延长[23],这似乎再次证实了永恒蛋白基因和周期蛋白基因联合作用的重要性。最近的研究表明,永恒蛋白基因除了要稳定细胞质中的周期蛋白基因外,还需要将周期蛋白基因转运到细胞核中[5]。此外,永恒蛋白基因的过度表达可能会增加周期蛋白基因的表达和负反馈,从而干扰系统并阻止进展到周期的下一个阶段[3],但周期蛋白基因的丧失似乎对永恒蛋白基因入核没有显著影响[24]。
最近的报道显示,永恒蛋白基因在周期蛋白基因敲减的情况下几乎可立即进入细胞核,这表明在没有周期蛋白基因的情况下永恒蛋白基因的核输入不会延迟[25],具体机制可能是周期蛋白基因将永恒蛋白基因隔离在细胞质中,直到周期蛋白基因本身被主动运输到细胞核中[26]。有趣的是,失去周期蛋白基因的永恒蛋白基因会失去转录抑制能力[27],但入核后两者之间的关联似乎并不密切。对果蝇的研究表明,在夜间的大部分时间里(直到黎明时永恒蛋白基因水平下降)永恒蛋白基因水平的改变对周期蛋白基因功能几乎没有影响[28]。因此,永恒蛋白基因和周期蛋白基因的核转位过程似乎与昼夜转换密切关联。周期蛋白基因需要与永恒蛋白基因结合才能实现入核、增加表达水平等,而永恒蛋白基因的入核似乎不需要周期蛋白基因的参与。
周期蛋白基因和双倍时间基因(哺乳动物酪蛋白激酶1同源物)在细胞质中互相捕获,而周期蛋白基因/永恒蛋白基因的相互作用促进了周期蛋白基因/永恒蛋白基因/双倍时间基因蛋白的核定位(蛋白质被运输到细胞核内部的过程)[29]。对果蝇的研究表明,在永恒蛋白基因激活前磷酸化可能会破坏细胞质中的周期蛋白基因,并导致蛋白质峰值表达延迟[30]。在永恒蛋白基因稳定表达后周期蛋白基因会积累,然后进入细胞核,在核中它的渐进磷酸化导致了其最终衰退[31]。周期蛋白基因和永恒蛋白基因的共表达促进了它们的核积累并影响了双倍时间基因的活性,双倍时间基因磷酸化会在不影响周期蛋白基因稳定性的前提下破坏周期蛋白基因,但永恒蛋白基因会改善这一现象[32]。周期蛋白基因的核定位受永恒蛋白基因调控[33]。有研究发现,果蝇永恒蛋白基因拮抗双倍时间基因活性以抑制周期蛋白基因的入核机制,双倍时间基因的磷酸化导致周期蛋白基因不稳定,永恒蛋白基因通过减轻双倍时间基因的这种影响来稳定周期蛋白基因[34]。因此,永恒蛋白基因的积累决定了周期蛋白基因的上升。另外,蛋白磷酸酶2A对周期蛋白基因的去磷酸化也可使周期蛋白基因在细胞核中稳定下来[35]。而另一项研究发现,果蝇永恒蛋白基因还充当输入蛋白α1入核机制识别的主要货物,从而将周期蛋白基因转运到细胞核中[36]。果蝇永恒蛋白基因对于维持节律性的周期蛋白基因表达和活性至关重要。研究表明,在果蝇永恒蛋白基因无效突变体和永恒蛋白基因入核缺陷的突变体中,周期蛋白基因节律表达和行为节律被消除[37]。早期研究表明,果蝇永恒蛋白基因结合并阻断周期蛋白基因的胞质定位结构域,从而减少周期蛋白基因胞质保留[38]。一旦进入细胞核,果蝇永恒蛋白基因似乎会与周期蛋白基因组成性结合并促进周期蛋白基因抑制,周期蛋白基因-果蝇永恒蛋白基因复合物会进行磷酸化依赖的核输出,从而为控制核积累提供了额外手段[39];此外,蛋白磷酸酶也参与调节周期蛋白基因-果蝇永恒蛋白基因核积
累[38]。部分学者认为果蝇永恒蛋白基因可能充当促进周期蛋白基因-CLK相互作用的支架,或可能促进周期蛋白基因-果蝇永恒蛋白基因阻遏物复合物中尚未表征的CLK激酶磷酸化CLK并灭活转录活性[40]。周期蛋白基因似乎可以在缺乏永恒蛋白基因的情况下独立进入细胞核。对两者入核时间的分析表明,当永恒蛋白基因仅在细胞质中可检测到时周期蛋白基因就在细胞核中[41],但这有可能是由永恒蛋白基因进出细胞核或在细胞核中不稳定导致的[42]。
永恒蛋白基因具有自由进入细胞核的特性,当永恒蛋白基因/周期蛋白基因复合物入核后,为招募更多周期蛋白基因,部分永恒蛋白基因可能会与周期蛋白基因分离,从而重新回到细胞质中[43]。当然,这种现象也可能是由永恒蛋白基因降解导致的[44]。因此,周期蛋白基因在核中的积累总是快于永恒蛋白基因。最近的研究显示,当双倍时间基因活性被永恒蛋白基因以外的因素暂时抑制时,周期蛋白基因可以在没有永恒蛋白基因的情况下进入细胞核[45]。在蚕蛾和蟑螂中,用抗周期蛋白基因和抗永恒蛋白基因进行免疫组织染色检测表明,周期蛋白基因和永恒蛋白基因始终停留在细胞质中,从不进入细胞核[46-47]。
一种解释可能是,哺乳动物隐花色素基因代替周期蛋白基因-永恒蛋白基因进入细胞核并发挥转录抑制作用[48]。因此,永恒蛋白基因承担了激活及稳定周期蛋白基因的作用,其还能调控周期蛋白基因的入核时间,而永恒蛋白基因和周期蛋白基因的表达/敲减均对昼夜节律产生了极大影响。此外,在一些情况下周期蛋白基因似乎也可以单独入核。
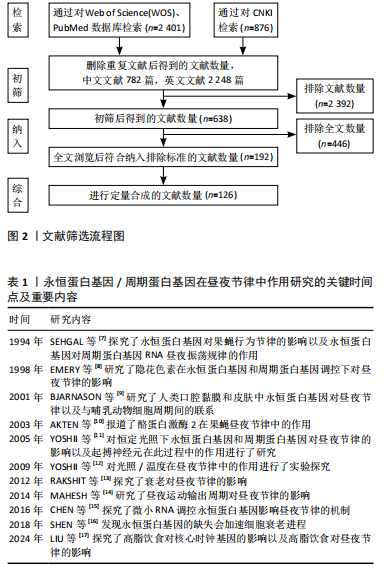
2.2.2 酪蛋白激酶2与Shaggy蛋白激酶介导的永恒蛋白基因/周期蛋白基因通路 酪蛋白激酶2和Shaggy蛋白激酶(哺乳动物糖原合酶激酶3β的同源物)可磷酸化周期蛋白基因和果蝇永恒蛋白基因并促进其进入核,而Shaggy
蛋白激酶/哺乳动物糖原合酶激酶3β的过表达都有利于周期蛋白基因的核积累[40]。酪蛋白激酶2定位于可能存在周期蛋白基因/永恒蛋白基因异二聚体的细胞质中,它可能在夜间早期作用于细胞质周期蛋白基因和/或永恒蛋白基因[49]。酪蛋白激酶2对周期蛋白基因水平的调控需要永恒蛋白基因的参与。尽管在体内无法单独影响周期蛋白基因,但永恒蛋白基因和周期蛋白基因在体外均被酪蛋白激酶2单独磷酸化[50],这些磷酸化可能与永恒蛋白基因/酪蛋白激酶2的稳定性及核进入调控息息相关[51]。
永恒蛋白基因可能是酪蛋白激酶2在体内的底物,即酪蛋白激酶2在体内磷酸化永恒蛋白基因[50],并且酪蛋白激酶2可直接影响永恒蛋白基因。CK2 Tik(酪蛋白激酶2同源物)可以在没有周期蛋白基因参与的前提下增加永恒蛋白基因水平,并且这种增加并不伴随转录本的增加[52],表明酪蛋白激酶2对于永恒蛋白基因的调节是在转录后进行的。Shaggy蛋白激酶是通过修饰周期蛋白基因和永恒蛋白基因来调节阻遏物复合物核积累的关键调节剂[53],它主要是通过影响周期蛋白基因/永恒蛋白基因异二聚体的核转位来调节分子循环周期[54]。永恒蛋白基因可以在体外被哺乳动物糖原合酶激酶3β直接磷酸化,整个过程可能是通过体内Shaggy蛋白激酶/哺乳动物糖原合酶激酶3β和永恒蛋白基因直接相互作用的机制来实现的[55]。Shaggy蛋白激酶诱导的核转位时间变化可以反映永恒蛋白基因磷酸化的变化,而永恒蛋白基因磷酸化的变化又与周期蛋白基因和永恒蛋白基因水平的变化有关[53]。在Shaggy蛋白激酶活性较低时周期蛋白基因和永恒蛋白基因会过量产生,因此,Shaggy蛋白激酶介导的永恒蛋白基因磷酸化可能会加速周期蛋白基因/永恒蛋白基因异二聚化,或直接促进周期蛋白基因/永恒蛋白基因复合物的核转位[56]。所以,Shaggy蛋白激酶突变体中永恒蛋白基因磷酸化的降低往往会延缓核转移。结合遗传和生化研究发现,Shaggy蛋白激酶和酪蛋白激酶2磷酸化ST区的2个残基(S297、T301、T305、S309和S313)以促进果蝇永恒蛋白基因核积累[57]。Shaggy蛋白激酶和酪蛋白激酶2似乎仅在部分时钟神经元中调节周期蛋白基因-果蝇永恒蛋白基因,这可能导致昼夜节律神经回路中特定神经元群的不同功能[51]。因此,酪蛋白激酶2和Shaggy蛋白激酶对永恒蛋白基因及周期蛋白基因的磷酸化,似乎验证了永恒蛋白基因/周期蛋白基因通过被磷酸化入核及调节昼夜节律的另一条有效通路。
影响永恒蛋白基因与周期蛋白基因入核/积累的因素,见图4。
2.3 环境因素对永恒蛋白基因/周期蛋白基因的影响
2.3.1 光照对永恒蛋白基因/周期蛋白基因的影响 系统
发育分析结果表明,隐花色素基因存在于多种物种中,在其中一些物种中隐花色素基因被证实是昼夜节律所必需的组成部分[58]。有研究发现,果蝇隐花色素基因具有2种功能,即作为光感受器与视觉色素一起调节昼夜节律钟以及作为昼夜节律振荡器蛋白复合物的组成部分,这也是哺乳动物隐花色素基因的特征[59]。关于果蝇的研究表明,果蝇永恒蛋白基因具有光敏感性,从而将分子时钟与来自环境的光输入相耦合,所以,果蝇永恒蛋白基因是光依赖性生物钟的组成部分[60]。隐花色素基因作为光感受器可以介导永恒蛋白基因光依赖性降解。光诱导果蝇隐花色素基因构象变化,从而使果蝇隐花色素基因能够与果蝇永恒蛋白基因结合[61];此后,E3泛素连接酶JETLAG与隐花色素基因一起在尚未表征的永恒蛋白基因酪氨酸磷酸化后促进永恒蛋白基因蛋白酶体快速降解[62]。光响应蛋白QUASIMODO主要在隐花色素基因负时钟神经元中表达,它也会在光照下触发果蝇永恒蛋白基因降解[63]。
果蝇永恒蛋白基因降解促进周期蛋白基因更新,从而重置昼夜节律时钟[60]。哺乳动物的生物钟由哺乳动物永恒蛋白基因、哺乳动物周期蛋白基因和哺乳动物隐花色素基因组成,它们的负反馈主要依赖于哺乳动物周期蛋白基因和哺乳动物隐花色素基因,哺乳动物隐花色素基因主要是由核蛋白组成,但在细胞质也广泛存在[64]。与果蝇隐花色素基因一样,哺乳动物隐花色素基因在调节昼夜节律钟方面也发挥光依赖性功能。一些研究结果表明,哺乳动物隐花色素基因蛋白具有光依赖性作用,缺少1个或2个隐花色素基因的基因敲除小鼠在光刺激下诱导基因(如周期蛋白基因和原癌基因c-fos)表达的能力降低或消失[65];此外,缺少隐花色素基因1和隐花色素基因2的突变小鼠瞳孔对光的反射反应降低[66]。因此,果蝇隐花色素基因在果蝇生物钟神经元中充当蓝光感器,可通过与永恒蛋白基因结合来触发光介导的永恒蛋白基因降解,而隐花色素基因在部分哺乳动物中似乎也起到光感器的作用,它通过光依赖作用在昼夜节律中发挥重要作用。
隐花色素基因不仅作为介导生物钟光控制的光感受器,其在哺乳动物中枢昼夜节律振荡器中起着必不可少的、不依赖于光的功能。对小鼠的研究表明,隐花色素基因1、隐花色素基因2均敲减的小鼠在明暗循环条件下表现出正常的节律性,但在自由运行(始终黑暗)条件下会瞬间完全丧失节律性[67-68]。与果蝇的情况有些类似,小鼠隐花色素基因突变体保留了介导光输入的能力,但当小鼠视觉色素的功能也受到破坏时,小鼠在明暗循环条件下出现心律失常[68],这些结果表明哺乳动物隐花色素基因蛋白确实参与了光对昼夜节律时钟的调节,但它们在昼夜节律时钟光同步中的作用可以被其他光感受器替代。最新的研究表明,与隐花色素基因一起作用于哺乳动物昼夜节律振荡器同步的其他光感受器是视觉杆锥视蛋白和相关蛋白黑视蛋白[69]。与果蝇隐花色素基因一样,哺乳动物隐花色素基因与时钟蛋白发生相互作用,包括启动子结合转录调节因子周期蛋白基因、CLK和芳香烃受体核转运蛋白类似蛋白1[70]。与果蝇的隐花色素基因相反,哺乳动物的隐花色素基因蛋白是昼夜节律时钟负反馈回路的组成部分[71]。隐花色素基因与其他时钟成分的物理相互作用影响它们的活性、相互作用、降解或核运输,从而改变时钟基因的转录调控[72]。但隐花色素基因与其他时钟蛋白(如周期蛋白基因、CLK和芳香烃受体核转运蛋白类似蛋白1)之间的相互作用似乎不受光的影响[73],表明此类相互作用可能不是光诱导昼夜节律钟的机制,就像果蝇一样。除了通过与启动子结合转录调节因子的物理相互作用直接调控转录外,隐花色素基因还可能通过参与调控组蛋白修饰来影响昼夜节律钟[74],但其具体机制仍不清楚。有趣的是,哺乳动物隐花色素基因似乎在体外抑制了CLK-CYC的转录活性[75]。有关昆虫的分子进化研究表明,基因重复和丢失导致隐花色素基因表达的3种模式;在一些昆虫中果蝇隐花色素基因或哺乳动物隐花色素基因表达,而在其他昆虫中果蝇隐花色素基因和哺乳动物隐花色素基因均表达[76]。这似乎说明了隐花色素基因在昼夜节律的光介导作用并非不可替代的,而隐花色素基因在无光情况下可能也参与了昼夜节律中的某些机制,目前已知隐花色素基因在昆虫中具有3种表达模式。隐花色素介导的永恒蛋白基因/周期蛋白基因通路,见图5。
周期蛋白基因对于光周期的影响可能是微乎其微的。有研究发现,在临界日照不同的前提下,周期蛋白基因敲减个体可以在12 ℃进入光周期依赖性休眠期[77]。而有研究提供相反的结果,周期蛋白基因在果蝇的其他光周期依赖特征中很重要,因为周期蛋白基因敲减果蝇会失去光周期依赖的耐寒性[78]。在豆虫中,降低周期蛋白基因可促进发育,即使在休眠诱导条件下也是如此[79];在寄生蜂中也观察到了类似结果,其中周期蛋白基因表达的减少使雌性寄生蜂即使在短日照下也能保持活跃的生殖状态[80]。同样对家蚕蛾的研究表明,敲除周期蛋白基因会

阻止其对短日照的反应[46]。而永恒蛋白基因敲减的果蝇在光周期中的作用可能与周期蛋白基因敲减果蝇相似[81]。
哺乳动物永恒蛋白基因具有调节周期蛋白基因水平和活性的作用。永恒蛋白基因水平受光调节,光在白天促进永恒蛋白基因的降解,这种降解的原理与果蝇隐花色素基因以光依赖方式结合永恒蛋白基因有关,从而促进周期蛋白基因的降解并允许昼夜节律转录增加[82]。随后,永恒蛋白基因积累是稳定周期蛋白基因和促进其核积累所必需的。因此,在存在光/暗循环的情况下,光会延迟永恒蛋白基因-周期蛋白基因的积累,从而导致抑制滞后[83],这些时间关系在持续黑暗中基本得以保留,并且无论有无光提示,温度循环也会使这些关系发生变化[81]。尽管这些条件下的机制尚不清楚,但似乎都说明了永恒蛋白基因/周期蛋白基因对于光周期的重要作用,时钟通路的本身及其影响因素似乎都是光周期反应不可或缺的部分。但温度反应是否是季节适应机制的组成部分,周期蛋白基因的作用是否归因于独立于其昼夜节律时钟功能的多效性,目前仍不清楚。
2.3.2 温度对永恒蛋白基因/周期蛋白基因的影响 见图6。
环境温度影响数百种基因的表达水平,温度也是同步时钟的重要时间线索,尤其是在黑暗的地方,例如地下产的卵或在黑暗中化蛹的昆虫[84]。因此,温度循环或温度阶跃会导致时钟相移,以适应环境循环的时间。对果蝇中的研究发现,在恒定光照下,温度升高会上调CLK,而温度降低会下调CLK[84];相反,周期蛋白基因、永恒蛋白基因分别因温度升高和降低而下调CLK或上调CLK[85]。温度降低后所有这些昼夜节律相关基因在恒定条件下均表现出振荡。这些温度响应在Jrk(CLK的同源基因)突变果蝇中几乎消失,这表明CLK是温度同步的主要成分[86]。与此同时,昼夜节律钟还具有温度补偿的特性,虽然生物钟可能受生理范围内的温度控制或调节,但生物分子机制和行为节律本身周期长度受到的影响有限,例如在持续黑暗的环境中,行为节律既不会因高温而加快,也不会因低温而减慢[87]。然而,超出或低于生理范围的温度可能会产生相移或破坏时钟功能,因为它直接作用于时钟的分子成分及分子间的相互作用。研究表明,短暂的有害热脉冲(约37 ℃)会导致周期蛋白基因和永恒蛋白基因蛋白丰度急剧下降,以及运动活动节律的相移[88]。而周期蛋白基因也参与了这一过程,周期蛋白基因中重复的苏氨酸-甘氨酸(Thr-Gly)束在较高温度下表现出更灵活的构象,在果蝇中表达周期蛋白基因但苏氨酸-甘氨酸束缺失的个体,昼夜节律钟的温度补偿受损[89],这似乎说明了苏氨酸-甘氨酸束对不同温度环境的多样适应性变化,可能解释了果蝇能够在不同温度范围环境中保持生物钟的步调原理。有关果蝇的研究证明,果蝇永恒蛋白基因在时钟的温度补偿过程中发挥了积极作用,在转录后水平,操纵果蝇永恒蛋白基因热敏剪接会导致温度补偿缺陷[90]。正如前人的研究所述,在翻译后水平带有大量氨基酸取代的突变系TIM rit(P1116A)和TIM blind表现出温度补偿受损[84]。
温度会调节周期蛋白基因和永恒蛋白基因表达的幅度和相位,包括产生可变剪接的mRNA。不同的热敏剪接产生不同的永恒蛋白基因异构体,在温暖环境下,永恒蛋白基因 RNA经历可变剪接产生转录本(永恒蛋白基因tiny或永恒蛋白基因-M),而在寒冷条件下会产生一种比全长永恒蛋白基因短33个氨基酸的异构体(永恒蛋白基因-cold)[91]。由于在不同的生物钟神经元中观察到了替代的周期蛋白基因-mRNA剪接模式,包括Shaggy蛋白激酶mRNA,推测这可能导致关键生物钟蛋白的细胞类型特异性翻译后修饰程序[92-93]。在较低温度下,果蝇周期蛋白基因内含子8中周期蛋白基因内含子的剪接会增加,并且与白天活动增加有关;而在较高温度下,来自周期蛋白基因内含子8剪接减少,这与夜间活动开始时间延迟和白天不活动时间延长有关[94]。进一步的研究表明,这种周期蛋白基因剪接事件与行为适应炎热漫长的夏日有关,剪接减少会导致白天不活动时间增加,并降低干燥风险[95]。
虽然这些研究集中于周期蛋白基因剪接对周期蛋白基因mRNA和周期蛋白基因积累的潜在影响以解释行为适应,但最近的一项研究提出了一种不同的分子机制:在寒冷环境下周期蛋白基因内含子8剪接增强,这种现象与周期蛋白基因转录水平及白天觉醒基因的增强有关[96]。白天觉醒基因编码一种保幼激素结合蛋白,可抑制白天不活动行为[96]。白天觉醒基因的3′端与周期蛋白基因3′端结合并稳定周期蛋白基因mRNA的因子,在这个过程中白天觉醒基因RNA也得到稳定[97],其剪接机制与温度和日照长度有关,周期蛋白基因不参与其中[98]。目前,果蝇永恒蛋白基因调节温度补偿的机制仍不清楚,一种推测是温度直接调节周期蛋白基因-果蝇永恒蛋白基因相互作用,另一种观点是温度可能通过间接调节位点特异性磷酸化来调节周期蛋白基因-果蝇永恒蛋白基因的相特异性功能,并实现温度补偿[99-100]。在哺乳动物系统中,温度已被证明可以决定竞争磷酸化位点的优先级,以调节周期蛋白基因周转率[101]。因此,温度的变化与永恒蛋白基因、周期蛋白基因的激活/敲减密切联系,温度变化与剪接的形式和机制也息息相关。隐花色素基因在温度循环中似乎起到了消极作用。对果蝇中枢神经系统的研究发现,隐花色素基因表达神经元中永恒蛋白基因的周期性表达往往与明暗循环保持同步,而隐花色素基因表达阴性细胞中永恒蛋白基因的表达与温度循环保持同
步[102]。有关隐花色素基因突变体的研究表明,在缺乏隐花色素基因的突变体中,所有起搏神经元都显示出与温度循环同步的周期性永恒蛋白基因表达[103]。在行为实验中获得了一致的结果,当光照和温度循环不同步时,行为在很大程度上适应明暗循环;但当隐花色素基因表达神经元被消融时,行为很容易适应温度循环[104]。无论温度是升高还是降低,似乎都会通过不同途径对昼夜节律产生影响,如当温度升高时CLK上调,从而激活永恒蛋白基因和周期蛋白基因,最终可能导致永恒蛋白基因及周期蛋白基因的入核,影响昼夜节律。此外,白天觉醒基因在寒冷环境下增强的剪切作用也是一个十分有趣的现象,值得更加深入的研究。
2.4 其他因素在昼夜节律过程中的作用
2.4.1 起搏神经元在昼夜节律过程中的作用 电活动被认为是激活大脑起搏神经元中分子钟的基本要素。最近的研究表明,在果蝇中时钟神经元的电沉默会导致昼夜运动节律在持续黑暗下停止自由运行[105]。免疫组织化学检测结果表明这种现象是由于时钟本身停止所导致的,因为缺乏夜间从细胞质中向细胞核运输的永恒蛋白基因蛋白[9]。在幼虫中也发现了类似的时钟停止现象[106]。然而在时钟神经元电沉默的果蝇中,分子振荡在明暗循环下仍然存在,这表明分子钟存在一种光依赖性驱动,至少可以部分补偿明暗循环中的电活动[107]。起搏神经元对维持时钟基因振荡具有十分重要的作用,但时钟基因之间在不同的亚群内部和亚群间的连接性、基因表达和功能也存在差异,例如小腹外侧神经元和大腹外侧神经元表达色素分散因子,从而达到同步个体昼夜节律时钟的作用,这是昼夜节律系统的主要神经肽输出[108]。小腹外侧神经元是昼夜节律系统的主要振荡器,因为它本身就具有在持续黑暗情况下维持昼夜运动节律的功能[109]。小腹外侧神经元和背外侧神经元组负责支持晚间活动高峰[109]。背神经元1可能以环境温度依赖的方式支持早晨或晚上的活动高峰[110]。但并非所有起搏神经元都具有内在的光敏感性,因为只有心脏和大脑腹外侧神经元、背外侧神经元组和背神经元1的子集表达隐花色素基因,而侧后神经元、背神经元2/背神经元3中根本不存在光感受器[111]。背神经元和背外侧神经元组起搏神经元能够在恒定黑暗条件下支持行为适应温度循环,但在恒定光照条件下不能[112]。然而在隐花色素基因突变时,背神经元和背外侧神经元组足以支持恒定光照条件下行为适应温度循环[113]。以上研究证明,隐花色素基因在部分神经元中以光依赖的方式拮抗温度调节。因此当两者进入冲突阶段时,果蝇通常会适应明暗循环而不是温度循环,但在隐花色素基因敲减的情况下,果蝇会很容易地适应温度循环[114]。最近的研究表明,一些外周组织可能具有与起搏神经元类似的昼夜节律振荡分子机制,尽管两者功能可能不完全相同[115]。在果蝇隐花色素基因敲减的小腹外侧神经元中表现出完整的周期蛋白基因和永恒蛋白基因分子振荡,但通过Mts细胞增殖检测发现其失去了分子振荡[116],这表明果蝇隐花色素基因不是中枢起搏器中的光感受器,而是这些外周振荡器的核心组成部分。然而最近在表皮沉积的研究中发现,另一种外周节律在隐花色素基因突变果蝇中持续存在[97]。因此,外周振荡器中至少有2种昼夜节律分子机制:一种需要果蝇隐花色素基因作为必需组成部分,另一种仅将其作为光感受器。
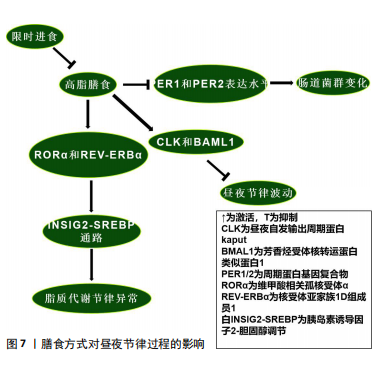
2.4.2 膳食方式对昼夜节律过程的影响 高脂膳食可能导致昼夜节律紊乱。对小鼠的研究表明,高脂膳食改变了小鼠体内核心生物钟基因CLK、芳香烃受体核转运蛋白类似蛋白1、周期蛋白基因2及隐花色素基因2的节律表达模式[117]。高脂膳食可能通过上调CLK和芳香烃受体核转运蛋白类似蛋白1的表达水平破坏昼夜节律[118];此外,高脂膳食通过维甲酸相关孤核受体α和核受体亚家族1D组成员1调控的胰岛素诱导因子2-胆固醇调节通路诱导代谢异常,进而影响脂质代谢节律,导致肝脏脂肪含量上升[119]。二十二碳六烯酸替代似乎可通过节律性核受体调控的代谢通路改善这一现象[117]。另一项研究发现,高脂膳食影响了小鼠肠道菌群代谢产物的稳态,扰乱昼夜节律[120]。这可能是由于高脂膳食降低了小鼠周期蛋白基因1和周期蛋白基因2表达水平,导致生物钟紊乱,进而导致肠道菌群的变化[121]。这种与昼夜节律紊乱相关的变化可能会导致心血管疾病、免疫系统受损、肾功能受损及代谢失调[122],说明生物钟基因在肠道菌群昼夜节律变化中的重要性。目前对高脂膳食与昼夜节律相关的研究似乎只集中于肝脏和肠道。有趣的是,限时进食可以改善高脂膳食导致的昼夜节律紊乱[123]。
限时进食对维持稳定的昼夜节律具有积极的作用,减少或消除夜间进食并延长夜间禁食间隔的饮食模式可能会持续改善人类健康。以往的研究表明,限时进食可以通过下调永恒蛋白基因激活自噬,从而有效抑制由昼夜节律紊乱引起的肿瘤发展[123]。周期蛋白基因1、周期蛋白2敲减导致的昼夜节律紊乱和饮食问题引起的某些细菌种群节律性波动,也可以通过限时进食来恢复[124]。限时进食中的禁食阶段通过激活腺苷酸活化蛋白激酶抑制哺乳动物雷帕霉素靶蛋白及其下游通路,影响昼夜节律进
程[125];此外,腺苷酸活化蛋白激酶会磷酸化隐花色素基因并促进其分解来影响昼夜节律[126],这也是禁食阶段影响昼夜节律的途径之一。当处于进食阶段时,哺乳动物雷帕霉素靶蛋白通路会磷酸化酪蛋白激酶1和糖原合酶激酶3,从而促进昼夜节律钟基因周期蛋白基因的表达,改变其稳定性[125],说明不同膳食方式都可以对昼夜节律进程产生影响。膳食方式对昼夜节律过程的影响,见图7。
| [1] LI T, BAI Y, JIANG Y, et al. The potential impacts of circadian rhythm disturbances on male fertility. Front Endocrinol (Lausanne). 2022;13: 1001316. [2] DONG D, YANG D, LIN L, et al. Circadian rhythm in pharmacokinetics and its relevance to chronotherapy. Biochem Pharmacol. 2020;178:114045. [3] CAI YD, CHIU JC. Timeless in animal circadian clocks and beyond. FEBS J. 2022;289(21):6559-6575. [4] SHAKHMANTSIR I, NAYAK S, GRANT GR, et al. Spliceosome factors target timeless (tim) mRNA to control clock protein accumulation and circadian behavior in Drosophila. Elife. 2018;7:e39821. [5] KOTWICA-ROLINSKA J, CHODÁKOVÁ L, SMÝKAL V, et al. Loss of Timeless Underlies an Evolutionary Transition within the Circadian Clock. Mol Biol Evol. 2022;39(1):msab346. [6] DUBOWY C, SEHGAL A. Circadian Rhythms and Sleep in Drosophila melanogaster. Genetics. 2017;205(4):1373-1397. [7] SEHGAL A, PRICE JL, MAN B, et al. Loss of circadian behavioral rhythms and per RNA oscillations in the Drosophila mutant timeless. Science. 1994;263(5153):1603-1606. [8] EMERY P, SO WV, KANEKO M, et al. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell. 1998;95(5):669-679. [9] BJARNASON GA, JORDAN RC, WOOD PA, et al. Circadian expression of clock genes in human oral mucosa and skin: association with specific cell-cycle phases. Am J Pathol. 2001;158(5):1793-1801. [10] AKTEN B, JAUCH E, GENOVA GK, et al. A role for CK2 in the Drosophila circadian oscillator. Nat Neurosci. 2003;6(3):251-257. [11] YOSHII T, HESHIKI Y, IBUKI-ISHIBASHI T, et al. Temperature cycles drive Drosophila circadian oscillation in constant light that otherwise induces behavioural arrhythmicity. Eur J Neurosci. 2005;22(5):1176-1184. [12] YOSHII T, VANIN S, COSTA R, et al. Synergic entrainment of Drosophila’s circadian clock by light and temperature. J Biol Rhythms. 2009;24(6): 452-564. [13] RAKSHIT K, KRISHNAN N, GUZIK EM, et al. Effects of aging on the molecular circadian oscillations in Drosophila. Chronobiol Int. 2012; 29(1):5-14.
[14] MAHESH G, JEONG E, NG FS, et al. Phosphorylation of the transcription activator CLOCK regulates progression through a 24-h feedback loop to influence the circadian period in Drosophila. J Biol Chem. 2014; 289(28):19681-19693. [15] CHEN X, ROSBASH M. mir-276a strengthens Drosophila circadian rhythms by regulating timeless expression. Proc Natl Acad Sci U S A. 2016;113(21):E2965-2972. [16] SHEN X, LI M, MAO Z, et al. Loss of circadian protein TIMELESS accelerates the progression of cellular senescence. Biochem Biophys Res Commun. 2018;503(4):2784-2791. [17] LIU L, LIU L, DENG S, et al. Circadian Rhythm Alteration of the Core Clock Genes and the Lipid Metabolism Genes Induced by High-Fat Diet (HFD) in the Liver Tissue of the Chinese Soft-Shelled Turtle (Trionyx sinensis). Genes (Basel). 2024;15(2):157. [18] ZHANG G, LI Y. Temperature compensation and entrainment in cyanobacteria circadian rhythm. Chronobiol Int. 2023;40(6):795-802. [19] KLEMZ S, WALLACH T, KORGE S, et al. Protein phosphatase 4 controls circadian clock dynamics by modulating CLOCK/BMAL1 activity. Genes Dev. 2021;35(15-16):1161-1174. [20] MONAGAS-VALENTIN P, BRIDGER R, CHANDEL I, et al. Protein tyrosine phosphatase 69D is a substrate of protein O-mannosyltransferases 1-2 that is required for the wiring of sensory axons in Drosophila. J Biol Chem. 2023;299(3):102890. [21] DEPPISCH P, PRUTSCHER JM, PEGORARO M, et al. Adaptation of Drosophila melanogaster to Long Photoperiods of High-Latitude Summers Is Facilitated by the ls-Timeless Allele. J Biol Rhythms. 2022; 37(2):185-201. [22] VAZE KM, MANOLI G, HELFRICH-FÖRSTER C. Drosophila ezoana uses morning and evening oscillators to adjust its rhythmic activity to different daylengths but only the morning oscillator to measure night length for photoperiodic responses. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024;210(4):535-548. [23] CHEN S, QIAO H, FU H, et al. Molecular cloning, characterization, and temporal expression of the clock genes period and timeless in the oriental river prawn Macrobrachium nipponense during female reproductive development. Comp Biochem Physiol A Mol Integr Physiol. 2017;207:43-51. [24] GRIMA B, PAPIN C, MARTIN B, et al. PERIOD-controlled deadenylation of the timeless transcript in the Drosophila circadian clock. Proc Natl Acad Sci U S A. 2019;116(12):5721-5726. [25] MARTIN ANDUAGA A, EVANTAL N, PATOP IL, et al. Thermosensitive alternative splicing senses and mediates temperature adaptation in Drosophila. Elife. 2019;8:e44642. [26] LIU W, CAO H, LIAO S, et al. Dibutyl phthalate disrupts conserved circadian rhythm in Drosophila and human cells. Sci Total Environ. 2021;783:147038. [27] TOBITA H, KIUCHI T. Knockout of cryptochrome 1 disrupts circadian rhythm and photoperiodic diapause induction in the silkworm, Bombyx mori. Insect Biochem Mol Biol. 2024;172:104153. [28] HUANG G, DIERICK HA. The need for unbiased genetic screens to dissect aggression in Drosophila melanogaster. Front Behav Neurosci. 2022;16:901453. [29] NOLAN RB, BONTRAGER C, BOWSER A, et al. Visual and circadian regulation of Drosophila BDBT and BDBT effects on DBT and PER localization. iScience. 2023;26(4):106343. [30] WANG J, FAN JY, ZHAO Z, et al. DBT affects sleep in both circadian and non-circadian neurons. PLoS Genet. 2022;18(2):e1010035. [31] MA D, OJHA P, YU AD, et al. Timeless noncoding DNA contains cell-type preferential enhancers important for proper Drosophila circadian regulation. Proc Natl Acad Sci USA. 2024;121(15):e2321338121. [32] SEGU A, KANNAN NN. The duration of caffeine treatment plays an essential role in its effect on sleep and circadian rhythm. Sleep Adv. 2023;4(1):zpad014. [33] TOBITA H, KIUCHI T. Knockouts of positive and negative elements of the circadian clock disrupt photoperiodic diapause induction in the silkworm, Bombyx mori. Insect Biochem Mol Biol. 2022;149:103842. [34] HWANG RD, LU Y, TANG Q, et al. DBT is a metabolic switch for maintenance of proteostasis under proteasomal impairment. bioRxiv [Preprint]. 2024:2023.09.12.556394. doi: 10.1101/2023.09.12.556394. [35] PELHAM JF, MOSIER AE, ALTSHULER SC, et al. Conformational changes in the negative arm of the circadian clock correlate with dynamic interactomes involved in post-transcriptional regulation. Cell Rep. 2023;42(4):112376. [36] TABULOC CA, CAI YD, KWOK RS, et al. CLOCK and TIMELESS regulate rhythmic occupancy of the BRAHMA chromatin-remodeling protein at clock gene promoters. PLoS Genet. 2023;19(2):e1010649. [37] XIAO Y, YUAN Y, JIMENEZ M, et al. Clock proteins regulate spatiotemporal organization of clock genes to control circadian rhythms. Proc Natl Acad Sci U S A. 2021;118(28):e2019756118. [38] ULGHERAIT M, CHEN A, MCALLISTER SF, et al. Circadian regulation of mitochondrial uncoupling and lifespan. Nat Commun. 2020;11(1):1927. [39] 张益梦,邹博亮,李源,等.昼夜节律蛋白Timeless在肿瘤生长与侵袭中的作用[J].生理科学进展,2021,52(6):461-465. [40] CAI YD, XUE Y, TRUONG CC, et al. CK2 Inhibits TIMELESS Nuclear Export and Modulates CLOCK Transcriptional Activity to Regulate Circadian Rhythms. Curr Biol. 2021;31(3):502-514.e7. [41] KUWANO R, KATSURA M, IWATA M, et al. Pigment-dispersing factor and CCHamide1 in the Drosophila circadian clock network. Chronobiol Int. 2023;40(3):284-299. [42] YOSHII T, SAITO A, YOKOSAKO T. A four-oscillator model of seasonally adapted morning and evening activities in Drosophila melanogaster. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024;210(4): 527-534. [43] AU DD, LIU JC, PARK SJ, et al. Drosophila photoreceptor systems converge in arousal neurons and confer light responsive robustness. Front Neurosci. 2023;17:1160353. [44] LANDSKRON J, CHEN KF, WOLF E, et al. A role for the PERIOD:PERIOD homodimer in the Drosophila circadian clock. PLoS Biol. 2009;7(4):e3. [45] TOP D, O’NEIL JL, MERZ GE, et al. CK1/Doubletime activity delays transcription activation in the circadian clock. Elife. 2018;7:e32679. [46] IKEDA K, DAIMON T, SEZUTSU H, et al. Involvement of the Clock Gene period in the Circadian Rhythm of the Silkmoth Bombyx mori. J Biol Rhythms. 2019;34(3):283-292. [47] COLIZZI FS, MARTÍNEZ-TORRES D, HELFRICH-FÖRSTER C. The circadian and photoperiodic clock of the pea aphid. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024;210(4):627-639. [48] SOLOVEV I, DOBROVOLSKAYA E, SHAPOSHNIKOV M, et al. Neuron-specific overexpression of core clock genes improves stress-resistance and extends lifespan of Drosophila melanogaster. Exp Gerontol. 2019; 117:61-71. [49] ZHANG H, ZHOU Z, GUO J. The Function, Regulation, and Mechanism of Protein Turnover in Circadian Systems in Neurospora and Other Species. Int J Mol Sci. 2024;25(5):2574. [50] MAHESH G, RIVAS GBS, CASTER C, et al. Proteomic analysis of Drosophila CLOCK complexes identifies rhythmic interactions with SAGA and Tip60 complex component NIPPED-A. Sci Rep. 2020;10(1):17951. [51] WANG B, STEVENSON EL, DUNLAP JC. Functional analysis of 110 phosphorylation sites on the circadian clock protein FRQ identifies clusters determining period length and temperature compensation. G3 (Bethesda). 2023;13(2):jkac334.
[52] MALIK MZ, DASHTI M, FATIMA Y, et al. Disruption in the regulation of casein kinase 2 in circadian rhythm leads to pathological states: cancer, diabetes and neurodegenerative disorders. Front Mol Neurosci. 2023;16:1217992.
[53] KHATIB L, SUBASI BS, FISHMAN B, et al. Unveiling Subtle Geographical Clines: Phenotypic Effects and Dynamics of Circadian Clock Gene Polymorphisms. Biology (Basel). 2023;12(6):858. [54] 于静,徐沛沛,羊金玲,等.生物钟基因对代谢综合征各组分的调控作用及作用机制研究进展[J].山东医药,2024,64(21):112-115. [55] DE J, CHATTERJEE A. Perception of Daily Time: Insights from the Fruit Flies. Insects. 2021;13(1):3. [56] SHIRAKAWA R, KURATA Y, SAKAI T. Regulation of long-term memory by a few clock neurons in Drosophila. Biophys Physicobiol. 2024; 21(Supplemental):e211002. [57] MENEGAZZI P, BEER K, GREBLER V, et al. A Functional Clock Within the Main Morning and Evening Neurons of D. melanogaster Is Not Sufficient for Wild-Type Locomotor Activity Under Changing Day Length. Front Physiol. 2020;11:229. [58] LEVY K, BARNEA A, TAUBER E, et al. Crickets in the spotlight: exploring the impact of light on circadian behavior. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024;210(2):267-279. [59] TAKEUCHI K, MATSUKA M, SHINOHARA T, et al. Fbxl4 Regulates the Photic Entrainment of Circadian Locomotor Rhythms in the Cricket Gryllus bimaculatus. Zoolog Sci. 2023;40(1):53-63. [60] BEER K, HELFRICH-FÖRSTER C. Model and Non-model Insects in Chronobiology. Front Behav Neurosci. 2020;14:601676. [61] NIKHIL KL, ABHILASH L, SHARMA VK. Molecular Correlates of Circadian Clocks in Fruit Fly Drosophila melanogaster Populations Exhibiting early and late Emergence Chronotypes. J Biol Rhythms. 2016;31(2):125-141. [62] CHEN C, TAMAI TK, XU M, et al. Functional Analyses of Four Cryptochromes From Aquatic Organisms After Heterologous Expression in Drosophila melanogaster Circadian Clock Cells. J Biol Rhythms. 2024;39(4):365-378. [63] CHEN KF, PESCHEL N, ZAVODSKA R, et al. QUASIMODO, a Novel GPI-anchored zona pellucida protein involved in light input to the Drosophila circadian clock. Curr Biol. 2011;21(9):719-729. [64] DEOLIVEIRA CC, CRANE BR. A structural decryption of cryptochromes. Front Chem. 2024;12:1436322. [65] VANDERSTRAETEN J, GAILLY P, MALKEMPER EP. Light entrainment of retinal biorhythms: cryptochrome 2 as candidate photoreceptor in mammals. Cell Mol Life Sci. 2020;77(5):875-884. [66] LEE YY, CAL-KAYITMAZBATIR S, FRANCEY LJ, et al. Duper is a null mutation of Cryptochrome 1 in Syrian hamsters. Proc Natl Acad Sci U S A. 2022;119(18):e2123560119. [67] BAHIRU MS, BITTMAN EL. Adult Neurogenesis Is Altered by Circadian Phase Shifts and the Duper Mutation in Female Syrian Hamsters. eNeuro. 2023;10(3):ENEURO.0359-22.2023. [68] HIRANO A, BRAAS D, FU YH, et al. FAD Regulates CRYPTOCHROME Protein Stability and Circadian Clock in Mice. Cell Rep. 2017;19(2):255-266. [69] XIE JB, ZHUANG W, ZHU Y, et al. Association between PER and CRY gene polymorphisms and the response to caffeine citrate treatment in infants with apnea of prematurity. Front Pediatr. 2024;12:1414185. [70] ZHANG Y, MARKERT MJ, GROVES SC, et al. Vertebrate-like CRYPTOCHROME 2 from monarch regulates circadian transcription via independent repression of CLOCK and BMAL1 activity. Proc Natl Acad Sci U S A. 2017;114(36):E7516-E7525. [71] CAO X, YANG Y, SELBY CP, et al. Molecular mechanism of the repressive phase of the mammalian circadian clock. Proc Natl Acad Sci U S A. 2021;118(2):e2021174118. [72] ONO M, ANDO H, DAIKOKU T, et al. The Circadian Clock, Nutritional Signals and Reproduction: A Close Relationship. Int J Mol Sci. 2023; 24(2):1545. [73] YANG Y, WU G, SANCAR A, et al. Mutations of the circadian clock genes Cry, Per, or Bmal1 have different effects on the transcribed and nontranscribed strands of cycling genes. Proc Natl Acad Sci U S A. 2024;121(8):e2316731121. [74] DEPPISCH P, KIRSCH V, HELFRICH-FÖRSTER C, et al. Contribution of cryptochromes and photolyases for insect life under sunlight. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2023;209(3):373-389. [75] YUAN Y, PADILLA MA, CLARK D, et al. Streamlined single-molecule RNA-FISH of core clock mRNAs in clock neurons in whole mount Drosophila brains. Front Physiol. 2022;13:1051544. [76] HELFRICH-FÖRSTER C. Light input pathways to the circadian clock of insects with an emphasis on the fruit fly Drosophila melanogaster. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2020;206(2): 259-272. [77] NICOU CM, PASSAGLIA CL. Effect of Ambient Lighting on Intraocular Pressure Rhythms in Rats. Invest Ophthalmol Vis Sci. 2024;65(10):16. [78] MUSCOGIURI G, POGGIOGALLE E, BARREA L, et al. Exposure to artificial light at night: A common link for obesity and cancer? Eur J Cancer. 2022;173:263-275. [79] LIN C, FENG S, DEOLIVEIRA CC, et al. Cryptochrome-Timeless structure reveals circadian clock timing mechanisms. Nature. 2023; 617(7959):194-199. [80] TENG Z, HUO M, ZHOU Y, et al. Circadian Activity and Clock Genes in Pachycrepoideus vindemmiae: Implications for Field Applications and Circadian Clock Mechanisms of Parasitoid Wasps. Insects. 2023; 14(5):486. [81] TATAROGLU O, EMERY P. The molecular ticks of the Drosophila circadian clock. Curr Opin Insect Sci. 2015;7:51-57. [82] NAVE C, ROBERTS L, HWU P, et al. Weekend Light Shifts Evoke Persistent Drosophila Circadian Neural Network Desynchrony. J Neurosci. 2021; 41(24):5173-5189. [83] ABHILASH L, SHAFER OT. Parametric effects of light acting via multiple photoreceptors contribute to circadian entrainment in Drosophila melanogaster. Proc Biol Sci. 2023;290(2006):20230149. [84] WANG G, VEGA-RODRÍGUEZ J, DIABATE A, et al. Clock genes and environmental cues coordinate Anopheles pheromone synthesis, swarming, and mating. Science. 2021;371(6527):411-415. [85] YILDIRIM E, CURTIS R, HWANGBO DS. Roles of peripheral clocks: lessons from the fly. FEBS Lett. 2022;596(3):263-293. [86] CHIANG MH, LIN YC, WU T, et al. Thermosensation and Temperature Preference: From Molecules to Neuronal Circuits in Drosophila. Cells. 2023;12(24):2792. [87] 孙萍璐,刘志华.以果蝇为模型探究温度感知的昼夜节律[J].湖北大学学报(自然科学版),2024,46(3):314-319. [88] SINGH S, GIESECKE A, DAMULEWICZ M, et al. New Drosophila Circadian Clock Mutants Affecting Temperature Compensation Induced by Targeted Mutagenesis of Timeless. Front Physiol. 2019;10:1442. [89] GIESECKE A, JOHNSTONE PS, LAMAZE A, et al. A novel period mutation implicating nuclear export in temperature compensation of the Drosophila circadian clock. Curr Biol. 2023;33(2):336-350.e5. [90] JOSHI R, CAI YD, XIA Y, et al. PERIOD Phosphoclusters Control Temperature Compensation of the Drosophila Circadian Clock. Front Physiol. 2022;13:888262. [91] Helfrich-Förster C, Bertolini E, Menegazzi P. Flies as models for circadian clock adaptation to environmental challenges. Eur J Neurosci. 2020;51(1):166-181. [92] KAURANEN H, ALA-HONKOLA O, KANKARE M, et al. Circadian clock of Drosophila montana is adapted to high variation in summer day lengths and temperatures prevailing at high latitudes. J Insect Physiol. 2016;89:9-18. [93] SHAW B, FOUNTAIN M, WIJNEN H. Control of Daily Locomotor Activity Patterns in Drosophila suzukii by the Circadian Clock, Light, Temperature and Social Interactions. J Biol Rhythms. 2019;34(5):463-481. [94] ZHANG Y, LIU S, DE MEYER M, et al. Genomes of the cosmopolitan fruit pest Bactrocera dorsalis (Diptera: Tephritidae) reveal its global invasion history and thermal adaptation. J Adv Res. 2023;53:61-74. [95] KING AN, SEHGAL A. Molecular and circuit mechanisms mediating circadian clock output in the Drosophila brain. Eur J Neurosci. 2020; 51(1):268-281. [96] HOMMA S, MURATA A, IKEGAMI M, et al. Circadian Clock Genes Regulate Temperature-Dependent Diapause Induction in Silkworm Bombyx mori. Front Physiol. 2022;13:863380. [97] YANG Y, EDERY I. Daywake, an Anti-siesta Gene Linked to a Splicing-Based Thermostat from an Adjoining Clock Gene. Curr Biol. 2019; 29(10):1728-1734.e4. [98] LOW KH, CHEN WF, YILDIRIM E, et al. Natural variation in the Drosophila melanogaster clock gene period modulates splicing of its 3’-terminal intron and mid-day siesta. PLoS One. 2012;7(11):e49536. [99] DELVENTHAL R, BARBER AF. Sensory integration: Time and temperature regulate fly siesta. Curr Biol. 2022;32(19):R1020-R1022. [100] PARASRAM K, BACHETTI D, CARMONA-ALCOCER V, et al. Fluorescent Reporters for Studying Circadian Rhythms in Drosophila melanogaster. Methods Mol Biol. 2022;2482:353-371. [101] HARA T, KOH K, COMBS DJ, et al. Post-translational regulation and nuclear entry of TIMELESS and PERIOD are affected in new timeless mutant. J Neurosci. 2011;31(27):9982-9990. [102] QIU J, DAI T, TAO H, et al. Inhibition of Expression of the Circadian Clock Gene Cryptochrome 1 Causes Abnormal Glucometabolic and Cell Growth in Bombyx mori Cells. Int J Mol Sci. 2023;24(6):5435. [103] BELLEMER A. Thermotaxis, circadian rhythms, and TRP channels in Drosophila. Temperature (Austin). 2015;2(2):227-243. [104] SHIEH BH, SUN W, FERNG D. A conventional PKC critical for both the light-dependent and the light-independent regulation of the actin cytoskeleton in Drosophila photoreceptors. J Biol Chem. 2023; 299(6):104822. [105] CAVIERES-LEPE J, EWER J. Reciprocal Relationship Between Calcium Signaling and Circadian Clocks: Implications for Calcium Homeostasis, Clock Function, and Therapeutics. Front Mol Neurosci. 2021;14:666673. [106] LIU J, WANG Y, LIU X, et al. Spatiotemporal changes in Netrin/Dscam1 signaling dictate axonal projection direction in Drosophila small ventral lateral clock neurons. Elife. 2024;13:RP96041. [107] AKPOGHIRAN O, AFONSO DJS, ZHANG Y, et al. TARANIS Interacts with VRILLE and PDP1 to Modulate the Circadian Transcriptional Feedback Mechanism in Drosophila. J Neurosci. 2024;44(5):e0922232023. [108] LEE GG, ZENG K, DUFFY CM, et al. In vivo characterization of the maturation steps of a pigment dispersing factor neuropeptide precursor in the Drosophila circadian pacemaker neurons. Genetics. 2023;225(1):iyad118. [109] LIU T, MAHESH G, HOUL JH, et al. Circadian Activators Are Expressed Days before They Initiate Clock Function in Late Pacemaker Neurons from Drosophila. J Neurosci. 2015;35(22):8662-8671. [110] NETTNIN EA, SALLESE TR, NASSERI A, et al. Dorsal clock neurons in Drosophila sculpt locomotor outputs but are dispensable for circadian activity rhythms. iScience. 2021;24(9):103001. [111] DAPERGOLA E, MENEGAZZI P, RAABE T, et al. Light Stimuli and Circadian Clock Affect Neural Development in Drosophila melanogaster. Front Cell Dev Biol. 2021;9:595754. [112] FOLEY LE, EMERY P. Drosophila Cryptochrome: Variations in Blue. J Biol Rhythms. 2020;35(1):16-27. [113] AU DD, FODEN AJ, PARK SJ, et al. Mosquito cryptochromes expressed in Drosophila confer species-specific behavioral light responses. Curr Biol. 2022;32(17):3731-3744.e4. [114] CAVIERES-LEPE J, AMINI E, ZABEL M, et al. Timed receptor tyrosine kinase signaling couples the central and a peripheral circadian clock in Drosophila. Proc Natl Acad Sci U S A. 2024;121(11):e2308067121. [115] AHMAD M, LI W, TOP D. Integration of Circadian Clock Information in the Drosophila Circadian Neuronal Network. J Biol Rhythms. 2021; 36(3):203-220. [116] LORBER C, LELEUX S, STANEWSKY R, et al. Light triggers a network switch between circadian morning and evening oscillators controlling behaviour during daily temperature cycles. PLoS Genet. 2022;18(11): e1010487. [117] FRAZIER K, KAMBAL A, ZALE EA, et al. High-fat diet disrupts REG3γ and gut microbial rhythms promoting metabolic dysfunction. Cell Host Microbe. 2022;30(6):809-823.e6. [118] LLABRE JE, TRUJILLO R, SROGA GE, et al. Circadian rhythm disruption with high-fat diet impairs glycemic control and bone quality. FASEB J. 2021;35(9):e21786. [119] SATO T, SASSONE-CORSI P. Nutrition, metabolism, and epigenetics: pathways of circadian reprogramming. EMBO Rep. 2022;23(5):e52412. [120] 江芷晴,邓国良,曾凡航,等.高蛋白日粮对肥胖小鼠脂代谢昼夜节律的调节作用[J].食品科学,2023,44(23):95-103. [121] 杨旭清,鲁聪,刘婷婷,等.时钟基因在生殖系统中的研究进展[J].中国计划生育学杂志,2023,31(3):725-731. [122] 法焕超,蔡蒙蒙,董培海,等.运动调控昼夜节律改善阿尔茨海默病的研究进展[J].生理科学进展,2024,55(3):272-277. [123] 缪明永.生物钟、代谢节律和饮食干预[J]. 肿瘤代谢与营养电子杂志,2024,11(1):20-27. [124] 丁煜堃,祝翠玲,张晓东.限时饮食对高脂饮食诱导肥胖大鼠髌下脂肪垫的影响及作用机制[J].中国组织工程研究,2024,28(34): 5425-5431. [125] DEOTA S, LIN T, CHAIX A, et al. Diurnal transcriptome landscape of a multi-tissue response to time-restricted feeding in mammals. Cell Metab. 2023;35(1):150-165.e4. [126] DONG TA, SANDESARA PB, DHINDSA DS, et al. Intermittent Fasting: A Heart Healthy Dietary Pattern? Am J Med. 2020;133(8):901-907. |
| [1] | 艾克帕尔·艾尔肯, 陈晓涛, 吾凡别克·巴合提. 成骨诱导人牙周膜干细胞来源外泌体促进炎症微环境下人牙周膜干细胞成骨分化[J]. 中国组织工程研究, 2025, 29(7): 1388-1394. |
| [2] | 刘 璐, 钟 畅, 余 欣, 任晨媛, 巩杨杨, 周 平, 王迎斌. 体外合成微环境促进人多能干细胞来源心肌细胞成熟的学术进展及临床应用[J]. 中国组织工程研究, 2025, 29(36): 7856-7862. |
| [3] | 李中正, 陈政豪, 唐子又, 娄凯阳, 张 睿, 刘 琪, 赵 娜, 杨 琨. 支架材料结合生物因素对牙囊细胞增殖及骨向分化生物学特性的影响[J]. 中国组织工程研究, 2025, 29(34): 7405-7414. |
| [4] | 张溥链, 刘宝茹, 杨 敏. 间充质干细胞治疗再生障碍性贫血:抑制或激活其病理演变过程中的相关靶点[J]. 中国组织工程研究, 2025, 29(31): 6800-6810. |
| [5] | 于庆贺, 蔡子鸣, 吴锦涛, 马鹏飞, 张 鑫, 周龙千, 王亚坤, 林晓钦, 林文平. 香草酸抑制终板软骨细胞炎症反应和细胞外基质降解[J]. 中国组织工程研究, 2025, 29(30): 6391-9397. |
| [6] | 樊佳欣, 贾 祥, 徐田杰, 刘凯楠, 郭小玲, 张 辉, 王 茜. 二甲双胍抑制铁死亡改善骨关节炎模型大鼠的软骨损伤[J]. 中国组织工程研究, 2025, 29(30): 6398-6408. |
| [7] | 周 颖, 田 勇, 钟芝梅, 古雍翔, 方 昊. 抑制TRAF6调节mTORC1/ULK1信号通路促进自噬改善脓毒症小鼠的心肌损伤[J]. 中国组织工程研究, 2025, 29(30): 6434-6440. |
| [8] | 王万春, 易 军, 严张仁, 杨 悦, 董德刚, 李玉梅. 717解毒合剂重塑细胞外基质稳态促进蝮蛇伤大鼠局部损伤组织的修复[J]. 中国组织工程研究, 2025, 29(30): 6457-6465. |
| [9] | 张 鑫, 郭宝娟, 徐慧鑫, 沈玉珍, 杨晓帆, 杨旭芳, 陈 培 . 丁苯酞对帕金森病细胞模型的保护作用及机制[J]. 中国组织工程研究, 2025, 29(30): 6466-6473. |
| [10] | 张松江, 李龙洋, 周春光, 高剑峰. 茶多酚干预运动疲劳模型小鼠的中枢抗炎作用与机制[J]. 中国组织工程研究, 2025, 29(30): 6474-6481. |
| [11] | 胡淑娟, 刘 当, 丁一庭, 刘 璇, 夏若寒, 汪献旺. 核桃油和花生油对动脉粥样硬化的改善作用[J]. 中国组织工程研究, 2025, 29(30): 6482-6488. |
| [12] | 张 健, 蔡 峰, 李婷文, 任鹏博. 基于鱼群算法对运动者疲劳步态的动作识别[J]. 中国组织工程研究, 2025, 29(30): 6489-6498. |
| [13] | 张子寒, 王加新, 杨文意, 朱 磊. 运动促进骨骼肌线粒体生物合成的调控机制[J]. 中国组织工程研究, 2025, 29(30): 6499-6508. |
| [14] | 王建旭, 董恣豪, 黄子帅, 李思颖, 杨 光. 免疫微环境与骨衰老的相互作用及治疗策略[J]. 中国组织工程研究, 2025, 29(30): 6509-6519. |
| [15] | 张博淳, 李 威, 李广政, 丁浩秦, 李 刚, 梁学振, . 神经影像学变化与骨坏死的关联:UK Biobank及FinnGen数据库的大样本分析[J]. 中国组织工程研究, 2025, 29(30): 6574-6582. |
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
1.1 资料来源
1.1.1 检索人及检索时间 由第一作者在 2024年7月进行检索。
1.1.2 检索文献时限 各数据库建库至2024-07-20。
1.1.3 检索数据库 CNKI及Web of Science(WOS)、PubMed数据库。
1.1.4 检索词 以“永恒蛋白基因,周期蛋白基因,昼夜节律,环境”为中文检索词,以“Timeless,Period,circadian rhythm,environment”为英文检索词。
1.1.5 检索文献类型 研究原著、综述、述评、经验交流及荟萃分析等。
1.1.6 手工检索情况 无。
1.1.7 检索策略 中英文数据库检索策略,见图1。
1.1.8 检索文献量 共检索到文献3 277篇。
1.2 入选标准
1.2.1 纳入标准 对应搜索检索词的近年文献,研究对象需具有代表性、确保有足够的样本量以支持研究,研究对象与研究目的紧密相关,能够为研究提供有价值的信息等文献。
1.2.2 排除标准 与核心检索词有明显出入的文献,老旧、样本量过小的文献及数据缺失严重、数据报告不清晰的文献
1.3 数据提取 数据库共检索到文献3 277篇,严格按照纳入和排除标准进行筛选,最终纳入文献126篇,包括英文文献118篇、中文文献8篇。文献筛选流程图见图2。
在昼夜时钟中,CLK和CYC激活永恒蛋白基因/周期蛋白基因,永恒蛋白基因调控周期蛋白基因的入核机制及稳定性,而周期蛋白基因也可以通过一些机制单独入核。酪蛋白激酶2、Shaggy蛋白激酶及酪蛋白激酶1同源物都可以通过磷酸化永恒蛋白基因/周期蛋白基因的方式来调节昼夜节律,参与转录,而隐花色素基因介导的永恒蛋白基因降解对转录的完整性有十分重要的作用。在不同环境中昼夜节律也受到了不同影响,如寒冷环境下周期蛋白基因转录水平增强。此外,膳食方式对于昼夜节律的影响也是个有趣的现象。该文重点探讨了不同基因及环境干预下永恒蛋白基因/周期蛋白基因的入核机制、蛋白质累积以及环境对昼夜节律的影响,但永恒蛋白基因结构变化对时钟蛋白行为功能的影响、隐花色素基因在无光情况下参与昼夜节律的具体机制目前仍不清楚。未来的研究重点可能会关注永恒蛋白基因/周期蛋白基因在运动、饮食及衰老方面的具体作用机制。
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||