Chinese Journal of Tissue Engineering Research ›› 2013, Vol. 17 ›› Issue (8): 1473-1480.doi: 10.3969/j.issn.2095-4344.2013.08.024
Previous Articles Next Articles
Characteristics and experimental application of polylactic acid copolymer composite sustained-release drug materials
Wang Dan1, Jiang Hang-hang2
- 1 Seventh People’s Hospital of Shenyang, Shenyang 110003, Liaoning Province, China
2 China Medical University, Shenyang 110000, Liaoning Province, China
-
Received:2012-10-18Revised:2012-12-27Online:2013-02-19Published:2013-03-23 -
Contact:Jiang Hang-hang, Studying for master’s degree, China Medical University, Shenyang 110000, Liaoning Province, China Jwb20082000@yahoo.cn -
About author:Wan Dan, Associate chief pharmacist, Seventh People’s Hospital of Shenyang, Shenyang 110003, Liaoning Province, China qywd123@yahoo.cn
CLC Number:
Cite this article
Wang Dan, Jiang Hang-hang. Characteristics and experimental application of polylactic acid copolymer composite sustained-release drug materials[J]. Chinese Journal of Tissue Engineering Research, 2013, 17(8): 1473-1480.
share this article
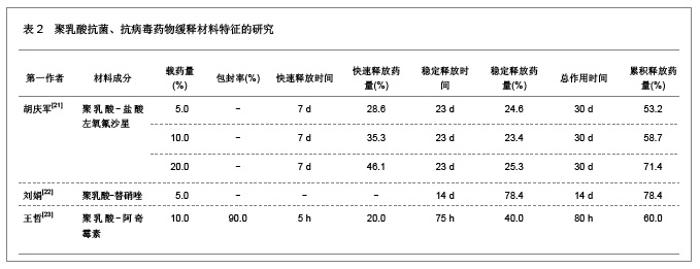
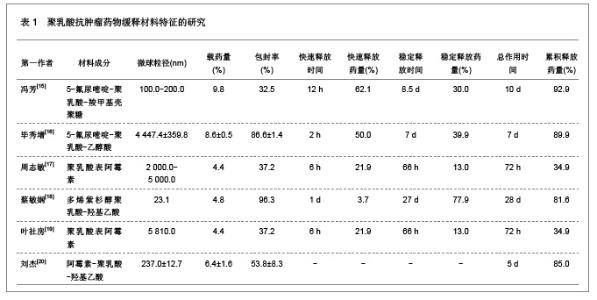
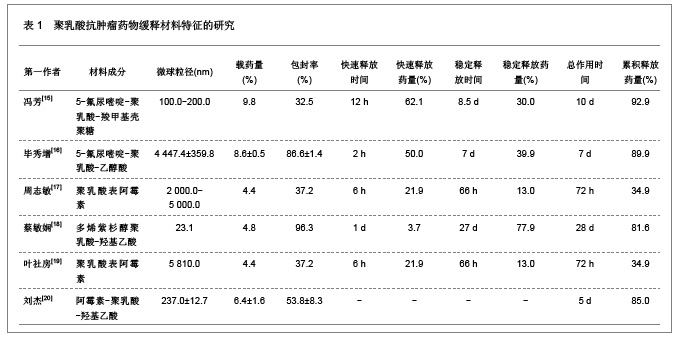
肿瘤是一种常见病与多发病,严重威胁着人类的健康,其死亡率仅次于心脑血管疾病的死亡率,并且正在逐年增加。恶性肿瘤的治疗主要以化疗为主,各种化疗药物如5-氟尿嘧啶、阿霉素、表阿霉素、环磷酰胺、长春新碱、喜树碱和紫杉醇等在恶性肿瘤的治疗中发挥重要的作用,但是绝大部分药物具有较强的毒性以及非靶向性,而微粒系统在肿瘤细胞表面的吸附能力较强,能够增强肿瘤细胞的摄粒活性,从而提高药物的靶向性,明显降低药物的毒副作用,增强治疗效果。诸多研究对5-氟尿嘧啶、阿霉素、表阿霉素以及紫杉醇的聚乳酸共聚物的缓释药物特征进行了分析。 2.1.2 聚乳酸抗菌、抗病毒药物缓释材料的特征 应用抗菌、抗病毒聚乳酸聚合物缓释材料可以降低药物的毒性,提高治疗效果,原因是细菌和病毒感染的主要部位肝、脾等器官也是药物缓释微粒的靶向器官,此外,微粒可以进入细胞内释放药物,并且可以持续较长时间的药物作用,从而消除细菌和病毒的耐药性。文章对盐酸左氧氟沙星、替硝唑以及阿奇霉素聚乳酸共聚物的缓释药物特征进行了研究分析,见表2。"
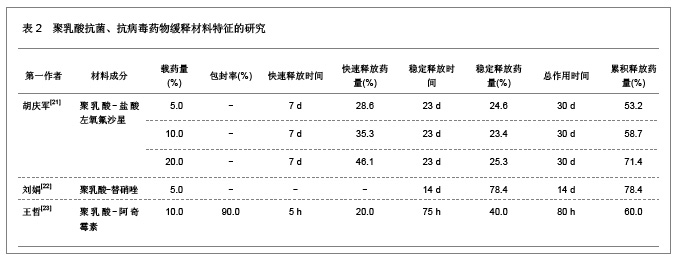
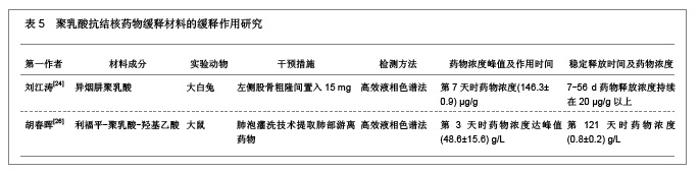
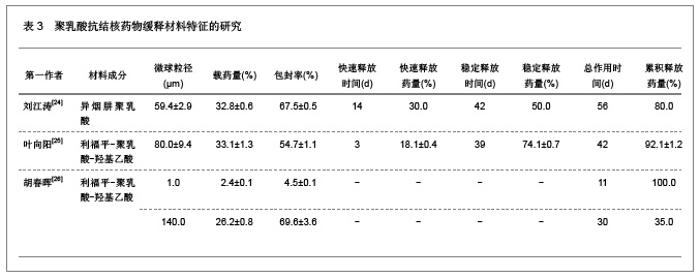
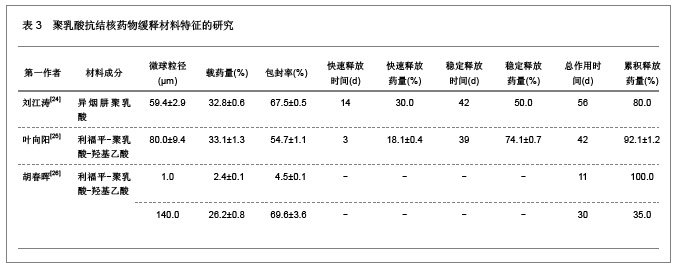
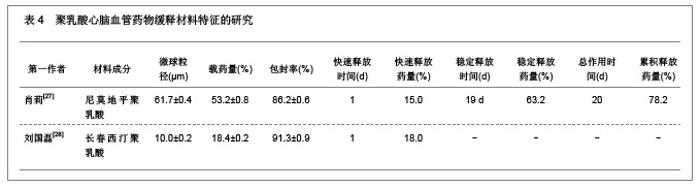
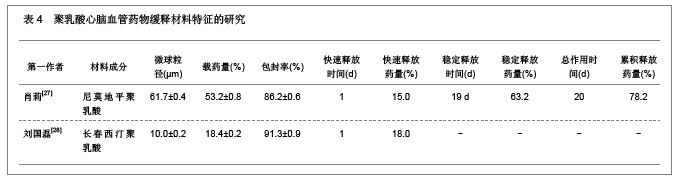
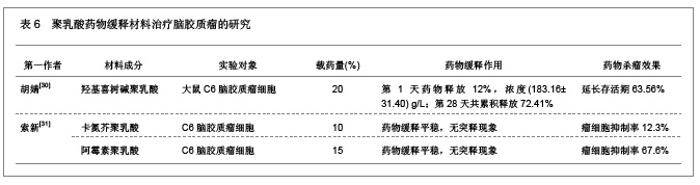
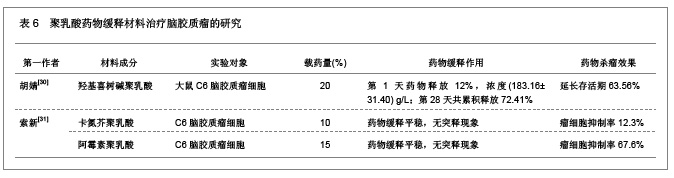
2.1.5 聚乳酸降胆固醇药物缓释材料的特征 洛伐他汀是降低胆固醇药物,半衰期较短,口服药物后血药浓度变化较大,加入聚乳酸载体后能够长时间维持药物的血药浓度,延长药物作用时间。张敏[29]对洛伐他汀聚乳酸微球的药物缓释性能进行了研究分析,实验结果发现洛伐他汀聚乳酸微球的形态圆整,粒径分布均匀,最佳微球粒径65.8 μm,载药量32.2%,包封率81.8%,可持续释放药物达10 d,累积释放药量34.8%。同时研究还发现载体材料的亲水性越高,微球的包封率和载药量越大,药物释放速率也越快。 2.2 聚乳酸共聚物复合药物缓释材料的动物实验应用 2.2.1 聚乳酸抗结核药物缓释材料治疗结核的应用实验 刘江涛等[24]和胡春晖[26]分别对异烟肼聚乳酸共聚物和利福平聚乳酸共聚物进行了药物缓释作用的动物实验研究,同样显示出聚乳酸抗结核药物良好的药物缓释特征,具体实验方法及结果见表5。"

| [1] Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303(5665):1818-1822. [2] Deitzel JM, Kleinmeyer JD, Hirvonen JK, et al. Controlled deposition of electrospun poly(ethylene oxide) fibers. Polymer. 2000;42:8163-8170.[3] Zussman E, Burman M, Yarin AL, et al. Tensile deformation of electrospun nylon-6,6 nanofibers. Journal of Polymer Science B: Polymer Physics. 2006;44:1482-1489.[4] Auras R, Harte B, Selke S. An overview of polylactides as packaging materials. Macromol Biosci. 2004;4(9):835-864. [5] 石淑先,夏宇正,郭祖鹏,等.聚D,L-乳酸的合成及表征[J].弹性体,2002,12(6):10-13. [6] Drumright RE, Gruber PR, Henton DE. Polylactic acid technology. Advanced Materials. 2000;12:1841-1846.[7] Pelouze J. Memoire sure l'acide lactique. Annual Chimie. 1845;3(13):257-268.[8] Carothers WH, Dorough GL, Van Natta FJ. Studies of polymerization and ring formation. X. The reversible polymerization of six-membered cyclic ester. Journal of the American Chemical Society. 1932;54:761-772. [9] Lowe CE. Preparation of high molecular weight polyhydroxyacetic ester. US Patent, 2668162,1954.[10] Yolles S, Leaffe T, Ward L, et al. Controlled release of biologically active drugs. Bull Parenter Drug Assoc. 1976; 30(6):306-312. [11] Uchida T, Yoshida K, Ninomiya A, et al. Optimization of preparative conditions for polylactide (PLA) microspheres containing ovalbumin. Chem Pharm Bull (Tokyo). 1995;43(9): 1569-1573. [12] Cao X, Schoichet MS. Delivering neuroactive molecules from biodegradable microspheres for application in central nervous system disorders. Biomaterials. 1999;20(4):329-339. [13] 郭圣荣.医药用生物降解性高分子材料[M].北京:化学工业出版社,2003.42-43.[14] 中国知网.中国学术期刊总库[DB/OL].2012-11-28. https://www.cnki.net [15] 冯芳.5-FU载药纳米微粒的制备、表征及体外实验[D].甘肃:兰州大学,2009:1-46.[16] 毕秀增,潘伟华,南开辉,等.5-氟尿嘧啶缓释剂制备及体外释药性能比较[J].中国组织工程研究,2012,16(8):1430-1434.[17] 周志敏.抗肿瘤药物表阿霉素缓释制剂及聚乳酸自组装行为的研究[D].福建:厦门大学,2006:1-82.[18] 蔡敏娴,陈志奎,林礼务,等.载多烯紫杉醇聚乳酸-羟基乙酸微球的制备、表征及其药物稳定性[J].中国组织工程研究与临床康复,2010,14(21):3856-3860.[19] 叶社房,侯振清,周志敏,等.植入型表阿霉素缓释药膜的制备及体内抑瘤活性[J].中国生物医学工程学报,2008,27(4): 586-590.[20] 刘杰,徐伟华,金成,等.载阿霉素PLGA纳米微球的制备及性质研究[J].现代生物医学进展,2010,10(24):4661-4663.[21] 胡庆军.聚DL-乳酸在HA复合材料与药物缓释中的应用[D].湖北:华中科技大学,2006:1-59.[22] 刘娟,肖丽英,李伟,等.可吸收聚乳酸-替硝唑抗菌缓释剂体外药物释放效果的测定[J].华西口腔医学杂志,2005,23(3):237-239.[23] 王哲,张秀梅,倪宏哲,等.聚乳酸载阿奇霉素微球包裹和体外释放行为[J].中国生物医学工程学报,2009,28(2):314-316.[24] 刘江涛,王永清,夏侃,等.异烟肼聚乳酸缓释体的制备及体内外释药特性[J].中国脊柱脊髓杂志,2008,18(4):290-293.[25] 叶向阳,孙湘,贾会文,等.利福平/聚乳酸-聚羟基乙酸缓释微球的制备及特性[J].中国组织工程研究与临床康复,2011,15(51): 9608-9612.[26] 胡春晖.利福平微球-原位凝胶复合缓释载体的研究[D].北京:北京协和医学院,2011:1-109.[27] 肖莉,张韵慧,崔颖,等.尼莫地平聚乳酸缓释微球的制备及其药剂学性质[J].中国医院药学杂志,2008,28(3):194-198.[28] 刘国磊,王帅,王静,等.长春西汀聚乳酸-聚乙醇酸缓释微球的研制[J].中国药房,2012,23(13):1203-1206.[29] 张敏.洛伐他汀聚乳酸微球的制备及释药特性[D].北京:北京化工大学,2011:1-89.[30] 胡婧.羟基喜树碱缓释片脑内植入治疗脑胶质瘤的研究[D].重庆:第三军医大学,2008:1-68.[31] 索新,刘伟,郭永川,等.可生物降解高分子超细纤维PLA药物缓释体作用于脑胶质瘤的体外研究[J].中国微侵袭神经外科杂志, 2006, 11(12):558-561.[32] Asmus LR, Tille JC, Kaufmann B, et al. In vivo biocompatibility, sustained-release and stability of triptorelin formulations based on a liquid, degradable polymer. J Control Release. 2012;165(3):199-206. [33] Ranjan AP, Mukerjee A, Helson L, et al. Scale up, optimization and stability analysis of Curcumin C3 complex-loaded nanoparticles for cancer therapy. J Nanobiotechnology. 2012; 10:38. [34] Asmus LR, Kaufmann B, Melander L, et al. Single processing step toward injectable sustained-release formulations of Triptorelin based on a novel degradable semi-solid polymer. Eur J Pharm Biopharm. 2012;81(3):591-599. [35] Agrawal R, Shanavas A, Yadav S, et al. Polyelectrolyte coated polymeric nanoparticles for controlled release of docetaxel. J Biomed Nanotechnol. 2012;8(1):19-28. [36] Parker T, Davé V, Falotico R, et al. Control of cilostazol release kinetics and direction from a stent using a reservoir-based design. J Biomed Mater Res B Appl Biomater. 2012;100(3):603-610. [37] Asmus LR, Gurny R, Möller M. Solutions as solutions--synthesis and use of a liquid polyester excipient to dissolve lipophilic drugs and formulatesustained-release parenterals. Eur J Pharm Biopharm. 2011;79(3):584-591. [38] Zhan W, Zhang X. Preparation of sustained release microspheres containing extracts from Caulis sinomenii with polylactic acid and their release property in vitro. Zhongguo Zhong Yao Za Zhi. 2010;35(16):2142-2145. [39] Wang TW, Wu Y, Li MJ, et al. Preparation and drug release property of paclitaxel nanoparticles. Zhong Yao Cai. 2009; 32(9): 1447-1449. [40] Kang C, Yuan X, Zhong Y, et al. Growth inhibition against intracranial C6 glioma cells by stereotactic delivery of BCNU by controlled release from poly(D,L-lactic acid) nanoparticles. Technol Cancer Res Treat. 2009;8(1):61-70. [41] Gao H, Ping Q, Gu Y. Fabrication, characterization and drug release characteristics of drug loaded poly (L-lactic acid) fiber. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2008;25(4): 870-873. [42] Rothen-Weinhold A, Besseghir K, Vuaridel E, et al. Injection-molding versus extrusion as manufacturing technique for the preparation of biodegradable implants. Eur J Pharm Biopharm. 1999;48(2):113-121. [43] Pardridge WM. Drug targeting to the brain. Pharm Res. 2007; 24(9):1733-1744. [44] Mariette B, Coudane J, Vert M. Interactions of GRF(1-29)NH2 with plasma proteins and their effects on the release of the peptide from a PLAGA matrix. J Control Release. 2005;106(3): 253-262. [45] Yamagata T, Morishita M, Kavimandan NJ, et al. Characterization of insulin protection properties of complexation hydrogels in gastric and intestinal enzyme fluids. J Control Release. 2006;112(3):343-349. [46] Edelman ER, Langer R. Optimization of release from magnetically controlled polymeric drug release devices. Biomaterials. 1993;14(8):621-626. |
| [1] | Min Changqin, Huang Ying. Construction of pH/near-infrared laser stimuli-responsive drug delivery system and its application in treatment of oral squamous cell carcinoma [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1940-1951. |
| [2] | Wang Songpeng, Liu Yusan, Yu Huanying, Gao Xiaoli, Xu Yingjiang, Zhang Xiaoming, Liu Min. Bidirectional regulation of reactive oxygen species based on zeolitic imidazolate framework-8 nanomaterials: from tumor therapy and antibacterial activity to cytoprotection [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2033-2013. |
| [3] | Li Qingbin, Lin Jianhui, Huang Wenjie, Wang Mingshuang, Du Jiankai, Lao Yongqiang. Bone cement filling after enlarged curettage of giant cell tumor around the knee joint: a comparison of subchondral bone grafting and non-grafting [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1896-1902. |
| [4] | Chen Ju, Zheng Jinchang, Liang Zhen, Huang Chengshuo, Lin Hao, Zeng Li. Effect and mechanism of beta-caryophyllene in mice with osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1341-1347. |
| [5] | Ma Hong, Ding Xueling, Wang Qi, Lyu Hui, Asya Albusm, Cheng Xinyi, Ma Xiang. Expression and significance of tumor necrosis factor alpha, nuclear factor kappaB and ionized calcium binding adaptor molecule-1 in the hippocampus of mice with aortic dissection [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 858-863. |
| [6] | Guo Jiachen, Gao Jun, Dai Wenhao, Liao Huayuan, Jiang You, Zhang Xi . Effect of compressive stress microenvironment on cytokines during fracture healing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 908-916. |
| [7] | Yu Shiyu, Yu Sutong, Xu Yang, Zhen Xiangyan, Han Fengxuan. Advances in research and application of tissue engineering therapeutic strategies in oral submucous fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 936-948. |
| [8] | Yang Hu, Zheng Yu, Jia Chengming, Wang Tong, Zhang Guangfei, Ji Yaoyao. Immune microenvironment regulates bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 701-710. |
| [9] | Yang Fengli, Zhou Chao, Xiong Wei, Zhou Yuxiang, Li Dengshun, Wang Xin, Li Zhanzhen. 3D printed poly-L-lactic acid bone scaffolds in repair of bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 507-515. |
| [10] | Xu Wenhe, Li Xiaobing, Liu Fang. Functionalized biomimetic mineralized collagen modified orthopedic implants [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 516-527. |
| [11] | Chen Yaodong, Ren Jiayi, Cao Jingwei, Fan Wenwen, Chen Wu. Near-infrared photoresponsive h-PCuNF nanoparticles mediate multimodal therapeutics against malignant tumors [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 780-788. |
| [12] | Li Mingzhe, Ye Xiangling, Wang Bing, Yu Xiang. Preparation and osteogenic properties of liquid crystal display light-cured polylactic acid scaffold loaded with nano-tantalum [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 670-677. |
| [13] | Yu Shuangqi, Ding Fan, Wan Song, Chen Wei, Zhang Xuejun, Chen Dong, Li Qiang, Lin Zuoli. Effects of polylactic acid-glycolic acid copolymer/lysine-grafted graphene oxide nanoparticle composite scaffolds on osteogenic differentiation of MC3T3 cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 707-712. |
| [14] | Dai Yueyou, Guo Dandan, Wang Qianqian, Wang Baiyan, Feng Shuying. Anti-tumor effects of engineered exosomes for targeted drug delivery [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6753-6764. |
| [15] | Zhou Ying, Tian Yong, Zhong Zhimei, Gu Yongxiang, Fang Hao. Inhibition of tumor necrosis factor receptor associated factor 6 regulates mTORC1/ULK1 signaling and promotes autophagy to improve myocardial injury in sepsis mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6434-6440. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||