Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (16): 4278-4288.doi: 10.12307/2026.730
Bibliometric analysis of ferroptosis and Alzheimer’s disease
Liu Annan1, Li Jianhui1, Gao Wei1, Li Xue1, Song Jing1, Xing Liping1, Li Honglin2
- 1Graduate School of Heilongjiang University of Chinese Medicine, Harbin 150006, Heilongjiang Province, China; 2Heilongjiang University of Chinese Medicine Affiliated Second Hospital, Harbin 150001, Heilongjiang Province, China
-
Received:2025-07-11Accepted:2025-08-24Online:2026-06-08Published:2025-11-29 -
Contact:Li Honglin, Chief physician, Doctoral supervisor, Heilongjiang University of Chinese Medicine Affiliated Second Hospital, Harbin 150001, Heilongjiang Province, China -
About author:Liu Annan, PhD candidate, Graduate School of Heilongjiang University of Chinese Medicine, Harbin 150006, Heilongjiang Province, China -
Supported by:National Natural Science Foundation of China, No. 82105035 (to LHL); Heilongjiang Provincial Natural Science Foundation, No. LH2023H064 (to LHL)
CLC Number:
Cite this article
Liu Annan, Li Jianhui, Gao Wei, Li Xue, Song Jing, Xing Liping, Li Honglin. Bibliometric analysis of ferroptosis and Alzheimer’s disease[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(16): 4278-4288.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
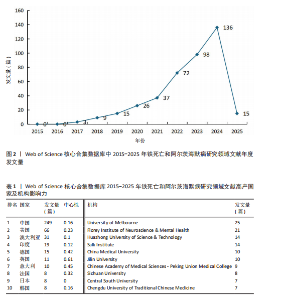
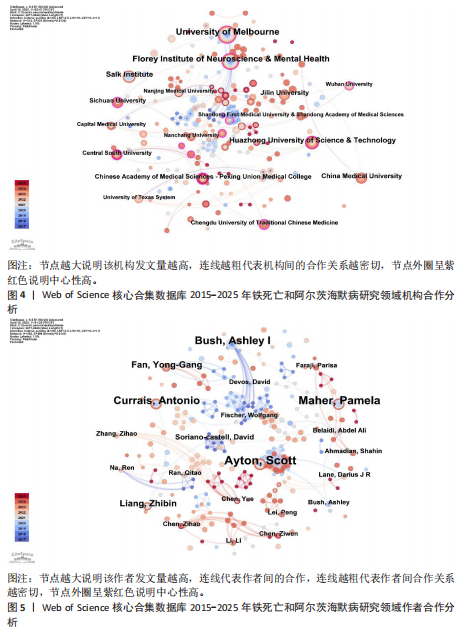
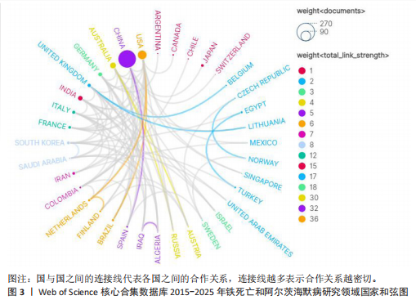
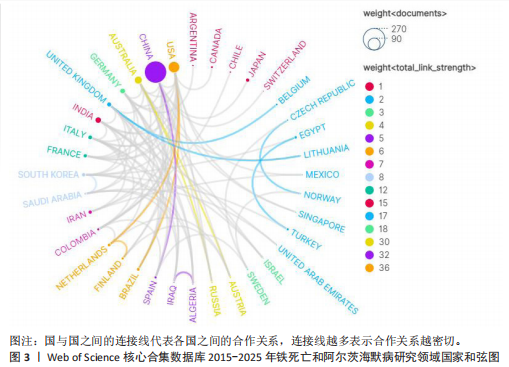
2.1 铁死亡与阿尔茨海默病研究领域年度发文量分析 2015-2025年,共发表了411篇关于铁死亡与阿尔茨海默病关系的研究,在此期间发文量总体上呈显著上升趋势(图2),特别是在2021年大幅增加。年度发文量可分为2个阶段,第一阶段为2015-2020 年,第二阶段 为2021-2024 年。2015-2020 年,此阶段属于“机制探索时期”,该阶段以细胞模型研究与转基因动物实验为主导,由于铁死亡这一概念在2012年被首次提出,所以这一阶段内发文量呈缓慢增长趋势,每年发文量低于40篇,相关领域逐渐引起学界的重视。自 2021 年以来,此阶段属于“临床转化期”,该阶段铁螯合剂临床试验激增,年发文量呈现高速增长态势,仅2025年1月份相关领域就发表15篇研究,这与2019年全年发文量相当。此结果显示目前铁死亡与阿尔茨海默病关系的研究受到国内外的广泛关注。 2.2 铁死亡与阿尔茨海默病研究领域国家分析 对国家进行可视化分析,结果显示共有43个国家参与铁死亡和阿尔茨海默病的研究。其中,中国发文量最多,共249篇,其次是美国(66篇)和澳大利亚(31篇),见表1。图3显示了国家和国家之间的合作,结果显示国家之间展开了较为密切的合作,其中美国的连接线最多,表明美国在该领域发挥了重要作用;中国虽然发布了最多的研究文章,但合作有限,论文的国际影响力还有待提高。 2.3 铁死亡与阿尔茨海默病研究领域机构分析 对研究机构进行可视化分析,结果显示共生成 213个节点和301 条连线,图谱密度为 0.013 3(图4)。数据分析显示,复旦大学的中心性最高,在该研究领域属于核心机构,由此可知高校是该领域研究的主力军。其中,墨尔本大学(中心性"
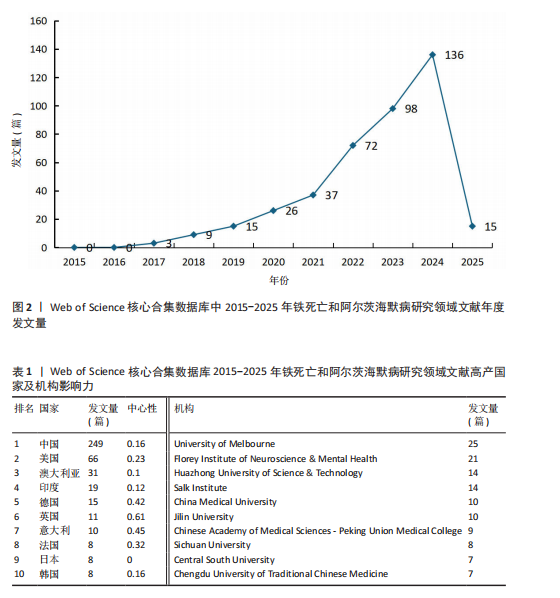
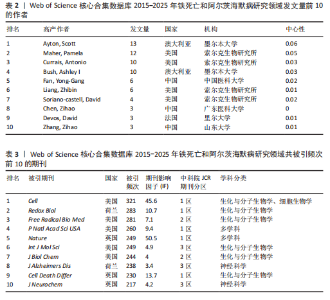
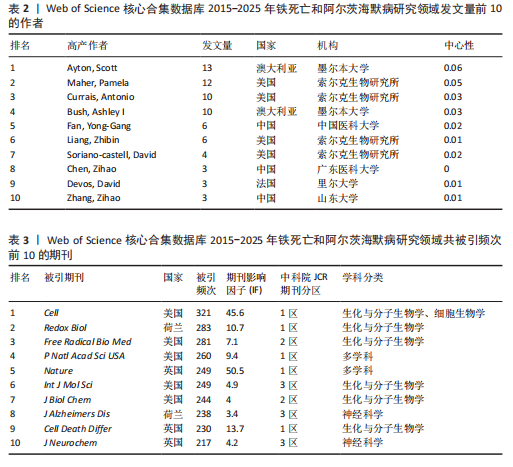
0.11)、弗洛里神经科学与心理健康研究所(中心性0.12)、华中科技大学(中心性0.16)、索尔克生物研究所(中心性0.14)和中国医科大学(中心性0.08) 的发文量排在前 5 位,分别为25,21,14,14,10篇,这些机构来自不同的国家,包括澳大利亚、中国和美国,它们代表了不同类型的机构,涵盖综合性大学和专业科研机构,并且这 5 所机构与其他机构建立了较为广泛的合作关系。 2.4 铁死亡与阿尔茨海默病研究领域作者分析 对411篇文献的作者进行分析,显示作者单位分散但彼此联系较多,说明目前全球的作者在铁死亡和阿尔茨海默病领域开展了广泛的合作(图5)。在总发文量中,Ayton Scott发文量最多,为13篇,其次"
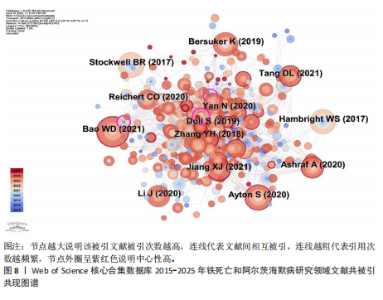
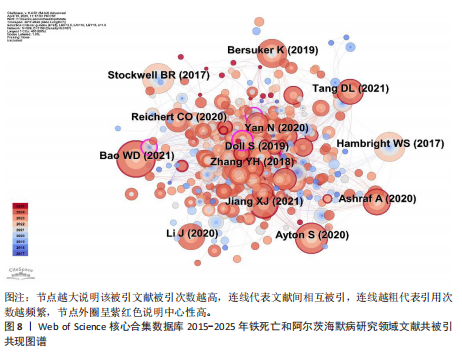
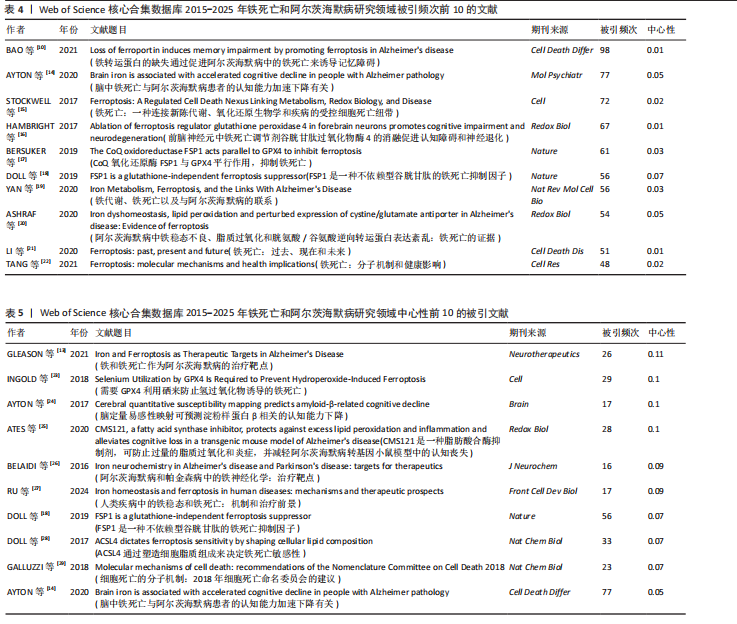
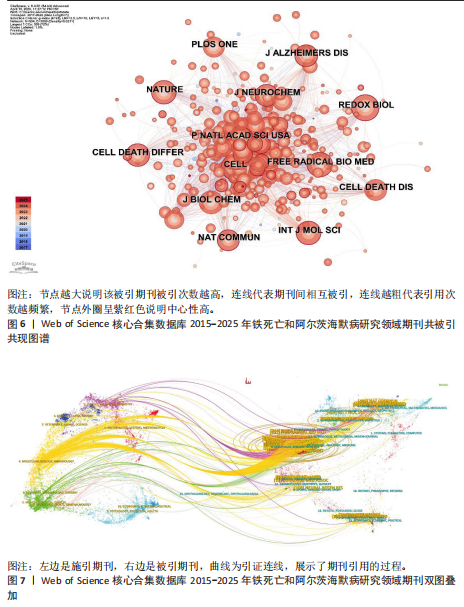
为Maher Pamela,发文量为12篇,Currais Antonio发文量为10篇,见表2 。依据Price Law核心作者发文量最小值(M)=0.749× nmax (nmax为最高产作者发文量)[12],M=0.749×3.6≈2.70,取整数3,即核心作者发文量≥3篇,筛选出22位核心作者,共发106篇,发文量占总发文量的25.79% (106/411),未达到总文献量的50%,表明尚未形成显著的核心作者群。 2.5 铁死亡与阿尔茨海默病研究领域期刊共被引分析 共73种期刊被铁死亡和阿尔茨海默病相关研究引用,最常被引用的10种期刊如表3所示。按学科门类进行分析,以生化与分子生物学和神经科学为主,结果表明该领域已在不同期刊和平台上获得广泛关注,可作为研究人员的主要参考。以被引期刊为节点进行期刊共被引分析(图 6),《Cell》《Redox Biol》和《Free Radical Biology And Medicine》是最常被引用的期刊,这些期刊的出版商主要位于美国、英国和荷兰。被引期刊影响因子普遍较高,所录用文章主要从大脑结构、基因和细胞分子等角度研究铁死亡和阿尔茨海默病的现状和发病机制等热点问题。 期刊的双图叠加显示了研究主题相对于主要研究学科的位置,通过期刊层面的信息流动直观地反映学科的研究动态(图7)。期刊双图叠加中主要的引用路径有 2 条,黄色路径表明分子/生物/免疫学领域的期刊通常受到分子/生物/遗传学和心理学/教育学/社会学领域期刊的影响,绿色路径表明神经科学/运动学/眼科学领域的期刊受分子/生物/遗传学和心理学/教育学/社会领域期刊的影响。 2.6 铁死亡和阿尔茨海默病研究领域文献共被引分析 以被引文献为节点,对文献数据进行文献共被引分析(图8)。被引用频次和中心性排名前10文献的信息分别见表 4,5[10,13-29]。高被引文献和中心性较高的文献揭示了铁死亡和阿尔茨海默病研究中关注较多的研究内容和进展。被引次数最多的文献为BAO 等[10]发表的《铁转运蛋"
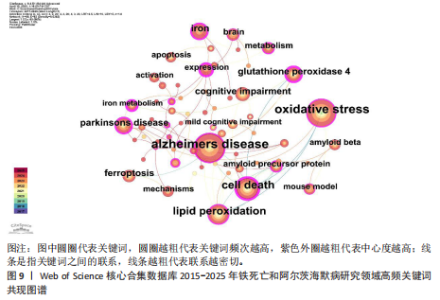
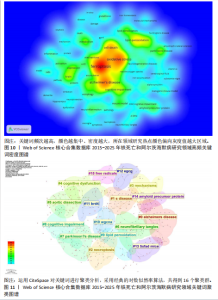
白的缺失通过促进阿尔茨海默病中的铁死亡来诱导记忆障碍》,被引次数为 98 次,该研究表明铁转运蛋白和铁死亡在阿尔茨海默病进展中的关键作用,从而为这种疾病提供了有前途的治疗方法。被引文献中中心性最高的为GLEASON等[13]发表的《铁和铁死亡作为阿尔茨海默病的治疗靶点》,中心性为 0.11,该研究表明铁与铁死亡抑制剂在治疗阿尔茨海默病方面具有巨大的前景,并可能据此生产出目前市面上急需的减缓阿尔茨海默病神经变性的药物。 2.7 铁死亡和阿尔茨海默病研究领域研究热点分析 2.7.1 关键词共现和频次分析 关键词是文章内容的核心要素,通过分析文章中的关键词可以洞察文章的主旨,深入研究关键词的使用有助于揭示特定学术领域的研究趋势和热点问题[30]。使用 CiteSpace 和VOSviewer软件绘制铁死亡和阿尔茨海默病的研究热点图谱,其中关键词共现图谱生成 66 个节点,82条连线,图谱密度为0.038 2(图9)。关键词密度图谱显示ferroptosis(铁死亡)、Alzheimers disease和oxidative stress(氧化应激)等词汇颜色较深,提示在铁死亡和阿尔茨海默病研究领域扮演着重要角色(图10)。分析发现Alzheimers disease出现的频次最高,其次是 oxidative stress(氧化应激)、cell death (细胞死亡)、lipid peroxidation (脂质过氧化)和iron(铁)等,见表6。这反映了氧化应激是铁死亡和阿尔茨海默病研究中的一个热门话题。 2.7.2 关键词聚类分析 关键词聚类揭示了研究领域内围绕共同主题的关键词所构成的相互关联的网络群体,每个群体的特征由其组成文章中频繁出现的标题词汇来定义。采用经典的对数似然率算法得到16个聚类组,聚类从 0 开始编号(图11)。聚类模块化值(Q)=0.762 > 0.3,平均轮廓值(S)=0.903 2 > 0.5,说明聚类结果均显著且可信任,见表7。分析聚类图谱可以发现,聚类#0主要涉及β-淀粉样蛋白、铁代谢和羟吲哚等主题;聚"
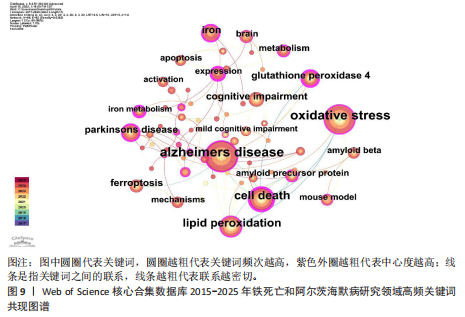
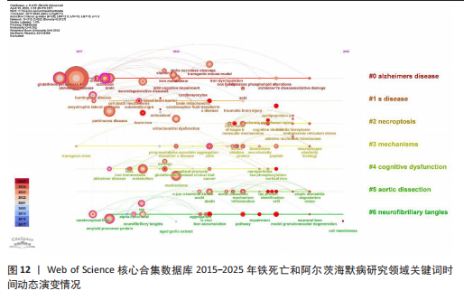
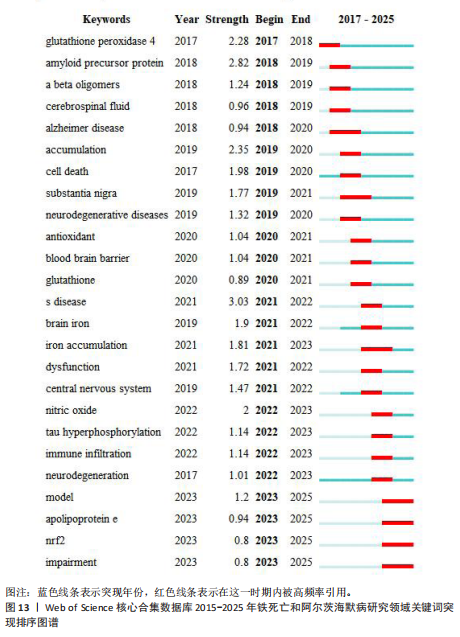
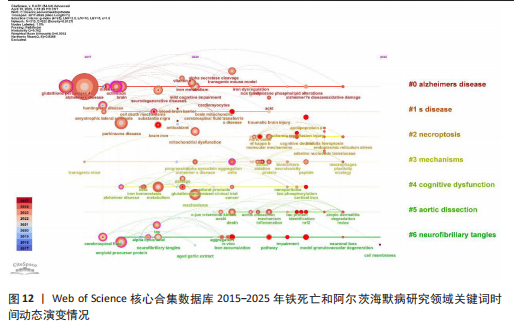
类#1围绕帕金森病、亨廷顿病和肌萎缩侧索硬化症等主题;聚类#2围绕坏死性凋亡、细胞焦亡和细胞程序坏死等主题,主要探讨阿尔茨海默病的发病机制;聚类#3围绕人参皂苷、多奈哌齐和谷胱甘肽等主题,集中研究阿尔茨海默病的治疗药物研发。从这些聚类的结果可以看出,目前的研究主要集中在发病机制、疾病预防和治疗3个方面。 以 Timeline view显示聚类关键词的时间动态变化(图12)。从2017年阿尔茨海默病、谷胱甘肽过氧化物酶4、脑脊髓液等关键词开始得到广泛关注,铁代谢、血脑屏障、线粒体功能障碍、转基因小鼠模型等关键词的热度2020年开始出现,2020-2025年,氧化应激、神经通路、生物标志物和铁积累等成为新的热门词汇。国际学术界对铁死亡和阿尔茨海默病研究热点的关注,反映了对阿尔茨海默病发病机制、临床治疗和诊断模型的极大关注。预测未来铁死亡在阿尔茨海默病病因学中发挥的作用及在临床早期诊断中的运用逐渐成为关注的焦点。 2.7.3 关键词突现分析 关键词突现是指频率急剧增加的关键词,关键词突现是在一段时间内受到相关研究领域学者们特别关注的关键词的一种有用算法,可反映一段时间内该领域的研究前沿及变化趋势,在分析研究前沿、研究趋势和热点以及预测未来研究方向具有重要的价值[31]。突现强度 (strength) 是对关键词的一种量化表达方式,表示该关键词在短时间内被大量引用[32]。分析突现图谱(图13)发现,淀粉样前体蛋白突现强度(2.82)较高,爆发起始年份为2018年,说明淀粉样前体蛋白作为阿尔茨海默病发病过程中一个关键的病理机制,在 2018-2019 年期间被重点研究。同时,“铁积累”“脑中铁浓度”“中枢神经系统”等关键词的关注度近几年也明显上升,表明学者们逐渐将研究重点转向铁代谢研究。"
| [1] SCHELTENS P, DE STROOPER B, KIVIPELTO M, et al. Alzheimer’s disease. The Lancet. 2021; 397:1577-1590. [2] GUSTAVSSON A, NORTON N, FAST T, et al. Global estimates on the number of persons across the Alzheimer’s disease continuum. Alzheimers Demen. 2023;19(2):658-670. [3] ZHANG XX, TIAN Y, WANG ZT, et al. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J Prev Alzheimers Dis. 2021;8(3):313-321. [4] GOETZL EJ. Current Developments in Alzheimer’s Disease. Am J Med. 2025; 138(1):15-20. [5] THE LANCET N. Treatment for Alzheimer’s disease: time to get ready. Lancet Neurol. 2023;22(6):455. [6] LIU E, ZHANG Y, WANG JZ. Updates in Alzheimer’s disease: from basic research to diagnosis and therapies. Transl Neurodegener. 2024;13(1):45. [7] JIANG X, STOCKWELL BR, CONRAD M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021; 22(4):266-282. [8] FENG L, SUN J, XIA L, et al. Ferroptosis mechanism and Alzheimer’s disease. Neural Regen Res. 2024;19(8):1741-1750. [9] LEI P, AYTON S, BUSH AI. The essential elements of Alzheimer’s disease. J Biol Chem. 2021;296:100105. [10] BAO WD, PANG P, ZHOU XT, et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ. 2021;28(5):1548-1562. [11] LI D, YU D, LI Y, et al. A bibliometric analysis of PROTAC from 2001 to 2021. Eur J Med Chem. 2022;244:114838. [12] REDONDO M, LEON L, POVEDANO FJ, et al. A bibliometric study of the scientific publications on patient-reported outcomes in rheumatology. Semin Arthritis Rheum. 2017;46(6):828-833. [13] GLEASON A, BUSH AI. Iron and Ferroptosis as Therapeutic Targets in Alzheimer’s Disease. Neurotherapeutics. 2021;18(1):252-264. [14] AYTON S, WANG Y, DIOUF I, et al. Brain iron is associated with accelerated cognitive decline in people with Alzheimer pathology. Mol Psychiatry. 2020;25(11):2932-2941. [15] STOCKWELL BR, FRIEDMANN ANGELI JP, BAYIR H, et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171(2): 273-285. [16] HAMBRIGHT WS, FONSECA RS, CHEN L, et al. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017; 12:8-17. [17] BERSUKER K, HENDRICKS JM, LI Z, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019; 575(7784):688-692. [18] DOLL S, FREITAS FP, SHAH R, et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575(7784):693-698. [19] YAN N, ZHANG J. Iron Metabolism, Ferroptosis, and the Links With Alzheimer’s Disease. Front Neurosci. 2020;13:1443. [20] ASHRAF A, JEANDRIENS J, PARKES HG, et al. Iron dyshomeostasis, lipid peroxidation and perturbed expression of cystine/glutamate antiporter in Alzheimer’s disease: Evidence of ferroptosis. Redox Biol. 2020;32:101494. [21] LI J, CAO F, YIN HL, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020; 11(2):88. [22] TANG D, CHEN X, KANG R, et al. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31(2):107-125. [23] INGOLD I, BERNDT C, SCHMITT S, et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell. 2018;172(3):409-422.e21. [24] AYTON S, FAZLOLLAHI A, BOURGEAT P, et al. Cerebral quantitative susceptibility mapping predicts amyloid-β-related cognitive decline. Brain. 2017;140(8):2112-2119. [25] ATES G, GOLDBERG J, CURRAIS A, et al. CMS121, a fatty acid synthase inhibitor, protects against excess lipid peroxidation and inflammation and alleviates cognitive loss in a transgenic mouse model of Alzheimer’s disease. Redox Biol. 2020;36: 101648. [26] BELAIDI AA, BUSH AI. Iron neurochemistry in Alzheimer’s disease and Parkinson’s disease: targets for therapeutics. J Neurochem. 2016;139 Suppl 1:179-197. [27] RU Q, LI Y, CHEN L, et al. Iron homeostasis and ferroptosis in human diseases: mechanisms and therapeutic prospects. Signal Transduct Target Ther. 2024;9(1):271. [28] DOLL S, PRONETH B, TYURINA YY, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13(1):91-98. [29] GALLUZZI L, VITALE I, AARONSON SA, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25(3):486-541. [30] AI Y, XING Y, YAN L, et al. Atrial Fibrillation and Depression: A Bibliometric Analysis From 2001 to 2021. Front Cardiovasc Med. 2022;9:775329. [31] ZHOU X, KANG C, HU Y, et al. Study on insulin resistance and ischemic cerebrovascular disease: A bibliometric analysis via CiteSpace. Front Public Health. 2023;11:1021378. [32] LIU S, SUN YP, GAO XL, et al. Knowledge domain and emerging trends in Alzheimer’s disease: a scientometric review based on CiteSpace analysis. Neural Regen Res. 2019;14(9):1643-1650. [33] 《中国应对阿尔茨海默病战略行动计划》建议书[J].科技导报,2021,39(20):149. [34] BERNDT C, ALBORZINIA H, AMEN VS, et al. Ferroptosis in health and disease. Redox Biol. 2024;75:103211. [35] NIKSERESHT S, BUSH AI, AYTON S. Treating Alzheimer’s disease by targeting iron. Br J Pharmacol. 2019;176(18):3622-3635. [36] AYTON S, BARTON D, BREW B, et al. Deferiprone in Alzheimer Disease: A Randomized Clinical Trial. JAMA Neurol. 2025;82(1):11-18. [37] SCHUBERT D, CURRAIS A, GOLDBERG J, et al. Geroneuroprotectors: Effective Geroprotectors for the Brain. Trends Pharmacol Sci. 2018;39(12):1004-1007. [38] MAHER P, CURRAIS A, SCHUBERT D. Using the Oxytosis/Ferroptosis Pathway to Understand and Treat Age-Associated Neurodegenerative Diseases. Cell Chem Biol. 2020;27(12):1456-1471. [39] KEPCHIA D, CURRAIS A, DARGUSCH R, et al.Geroprotective effects of Alzheimer’s disease drug candidates. Aging (Albany NY). 2021;13(3):3269-3289. [40] XU D, WANG YL, WANG KT, et al. A Scientometrics Analysis and Visualization of Depressive Disorder. Curr Neuropharmacol. 2021;19(6):766-786. [41] HIRSCHHORN T, STOCKWELL BR. The development of the concept of ferroptosis. Free Radic Biol Med. 2019;133:130-143. [42] STOCKWELL BR. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185(14):2401-2421. [43] CHEN Z, TAO S, LI X, et al. Anagliptin protects neuronal cells against endogenous amyloid β (Aβ)-induced cytotoxicity and apoptosis. Artif Cells Nanomed Biotechnol. 2019;47(1):2213-2220. [44] MOU Y, WANG J, WU J, et al. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol. 2019;12(1):34. [45] LIU JL, FAN YG, YANG ZS, et al. Iron and Alzheimer’s Disease: From Pathogenesis to Therapeutic Implications. Front Neurosci. 2018;12:632. [46] BENSENY-CASES N, KLEMENTIEVA O, COTTE M, et al. Microspectroscopy (μFTIR) reveals co-localization of lipid oxidation and amyloid plaques in human Alzheimer disease brains. Anal Chem. 2014;86(24):12047-12054. [47] PEÑA-BAUTISTA C, BAQUERO M, VENTO M, et al. Free radicals in Alzheimer’s disease: Lipid peroxidation biomarkers. Clin Chim Acta. 2019;491:85-90. [48] LLORET A, ESTEVE D, MONLLOR P, et al. The Effectiveness of Vitamin E Treatment in Alzheimer’s Disease. Int J Mol Sci. 2019; 20(4):879. [49] JIMÉNEZ-JIMÉNEZ FJ, ALONSO-NAVARRO H, GARCÍA-MARTÍN E, et al. Coenzyme Q10 and Dementia: A Systematic Review. Antioxidants (Basel). 2023;12(2):533. [50] WANG F, WANG J, SHEN Y, et al. Iron Dyshomeostasis and Ferroptosis: A New Alzheimer’s Disease Hypothesis? Front Aging Neurosci. 2022;14:830569. [51] JIANG S, LIU Y, ZHENG H, et al. Evolutionary patterns and research frontiers in neoadjuvant immunotherapy: a bibliometric analysis. Int J Surg. 2023;109(9):2774-2783. [52] SELKOE DJ, HARDY J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8(6):595-608. [53] OROBETS KS, KARAMYSHEV AL. Amyloid Precursor Protein and Alzheimer’s Disease. Annu Rev Neurosci. 2011;34:185-204. [54] CHO Y, BAE HG, OKUN E, et al. Physiology and pharmacology of amyloid precursor protein. Pharmacol Ther. 2022;235:108122. [55] ZHENG J, CONRAD M. The Metabolic Underpinnings of Ferroptosis. Cell Metab. 2020;32(6):920-937. [56] PENG Y, CHANG X, LANG M. Iron Homeostasis Disorder and Alzheimer’s Disease. Int J Mol Sci. 2021;22(22):12442. [57] WU L, XIAN X, TAN Z, et al. The Role of Iron Metabolism, Lipid Metabolism, and Redox Homeostasis in Alzheimer’s Disease: from the Perspective of Ferroptosis. Mol Neurobiol. 2023;60(5):2832-2850. |
| [1] | Xu Canli, He Wenxing, Wang Yuping, Ba Yinying, Chi Li, Wang Wenjuan, Wang Jiajia. Research context and trend of TBK1 in autoimmunity, signaling pathways, gene expression, tumor prevention and treatment [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(在线): 1-11. |
| [2] | Zhu Xiaolong, Zhang Wei, Yang Yang. Visualization analysis of research hotspots and cutting-edge information in the field of intervertebral disc regeneration and repair [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2391-2402. |
| [3] | Wen Fayan, Li Yan, Qiang Tianming, Yang Chen, Shen Linming, Li Yadong, Liu Yongming. Unilateral biportal endoscopic technology for treatment of lumbar degenerative diseases: global research status and changing trends [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2380-2390. |
| [4] | Sun Yaotian, Xu Kai, Wang Peiyun. Potential mechanisms by which exercise regulates iron metabolism in immune inflammatory diseases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1486-1498. |
| [5] | Huang Jie, Zeng Hao, Wang Wenchi, Lyu Zhucheng, Cui Wei. Visualization analysis of literature on the effect of lipid metabolism on osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1558-1568. |
| [6] | Lyu Guoqing, Aizimaitijiang·Rouzi, Xiong Daohai. Irisin inhibits ferroptosis in human articular chondrocytes: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1359-1367. |
| [7] | Wen Xiaolong, Weng Xiquan, Feng Yao, Cao Wenyan, Liu Yuqian, Wang Haitao. Effects of inflammation on serum hepcidin and iron metabolism related parameters in patients with type 2 diabetes mellitus: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1294-1301. |
| [8] | Zou Rongji, Yu Fangfang, Wang Maolin, Jia Zhuopeng. Triptolide inhibits ferroptosis and improves cerebral ischemia-reperfusion injury in a rat model of cerebral artery occlusion/reperfusion [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 873-881. |
| [9] | Yang Xiao, Bai Yuehui, Zhao Tiantian, Wang Donghao, Zhao Chen, Yuan Shuo. Cartilage degeneration in temporomandibular joint osteoarthritis: mechanisms and regenerative challenges [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 926-935. |
| [10] | Jiang Kan, Alimujiang·Abudourousuli, Shalayiding·Aierxiding, Aikebaierjiang·Aisaiti, Kutiluke·Shoukeer, Aikeremujiang·Muheremu. Biomaterials and bone regeneration: research hotspots and analysis of 500 influential papers [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 528-536. |
| [11] | Yu Le, Nan Songhua, Shi Zijian, He Qiqi, Li Zhenjia, Cui Yinglin. Mechanisms underlying mitophagy, ferroptosis, cuproptosis, and disulfidptosis in Parkinson’s disease [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(17): 4446-4456. |
| [12] | Li Ruiying, Xia Hong. Visual analysis of cuproptosis research: global landscape of hotspots and frontiers [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(17): 4529-4541. |
| [13] | Wei Jingyi, Wang Xiaojing, Liu Xihua. Application trends of eye-tracking technology in rehabilitation: a visualization analysis based on CiteSpace and VOSviewer [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(16): 4265-4277. |
| [14] | Chen Xinlong, Meng Tao, Wang Yaomin, Zhang Kefan, Li Jian, Shi Hui, Zhang Chenchen. Ferroptosis inhibitors in the treatment of osteoarthritis: diversity and multitarget characteristics [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(16): 4166-4179. |
| [15] | Zou Shunyi, Yi Jin, Zeng Hao, Li Jianqi, Wu Zhongping. Postmenopausal osteoporosis: visualization analysis of related signaling pathways [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(16): 4229-4239. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||