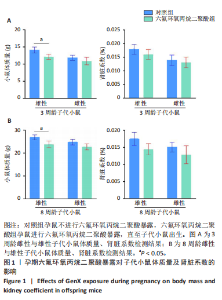
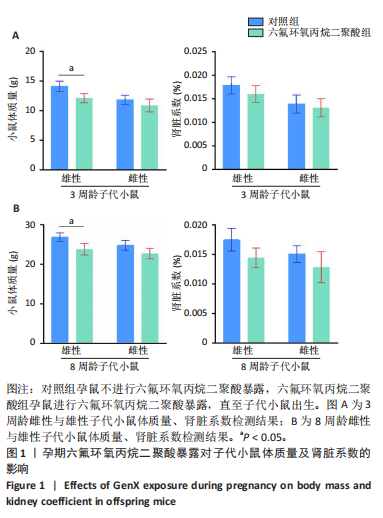
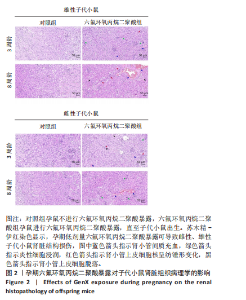
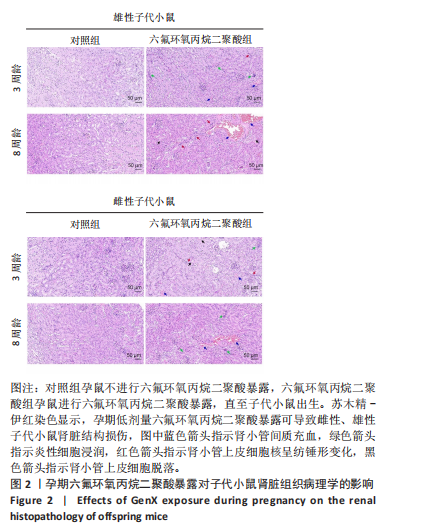
Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (11): 2752-2763.doi: 10.12307/2026.099
Previous Articles Next Articles
Impact and mechanism of low-dose hexafluoropropylene oxide dimer acid exposure during pregnancy on renal toxicity in offspring mice
Hong Runyang1, Zhou Qiyue1, Fan Zhencheng1, Shi Yujie1, Chen Hao1, 2, Pan Chun1, 2
- 1Institute of Translational Medicine, Medical College, Yangzhou University, Yangzhou 225000, Jiangsu Province, China; 2Jiangsu Key Lab of Non-Coding RNA Basic and Clinical Translational Research, Yangzhou 225000, Jiangsu Province, China
-
Received:2025-05-06Accepted:2025-06-05Online:2026-04-18Published:2025-09-05 -
Contact:Pan Chun, PhD, Lecturer, Institute of Translational Medicine, Medical College, Yangzhou University, Yangzhou 225000, Jiangsu Province, China; Jiangsu Key Lab of Non-Coding RNA Basic and Clinical Translational Research, Yangzhou 225000, Jiangsu Province, China Co-corresponding author: Chen Hao, PhD, Professor, Doctoral supervisor, Institute of Translational Medicine, Medical College, Yangzhou University, Yangzhou 225000, Jiangsu Province, China; Jiangsu Key Lab of Non-Coding RNA Basic and Clinical Translational Research, Yangzhou 225000, Jiangsu Province, China -
About author:Hong Runyang, Institute of Translational Medicine, Medical College, Yangzhou University, Yangzhou 225000, Jiangsu Province, China -
Supported by:National Natural Science Foundation of China, Nos. 32301416 (to PC), 82172468 and 82372436 (both to CH); the Yangzhou Lyu Yang Jin Feng Program, No. YZLVJFJH2022YXBS154 (to PC); the 2024 Innovation and Entrepreneurship Training Program for College Students of Innovation and Entrepreneurship School of Yangzhou University, No. C202411117002Y (to ZQY)
CLC Number:
Cite this article
Hong Runyang, Zhou Qiyue, Fan Zhencheng, Shi Yujie, Chen Hao, Pan Chun. Impact and mechanism of low-dose hexafluoropropylene oxide dimer acid exposure during pregnancy on renal toxicity in offspring mice[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(11): 2752-2763.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
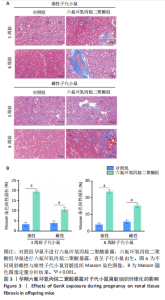
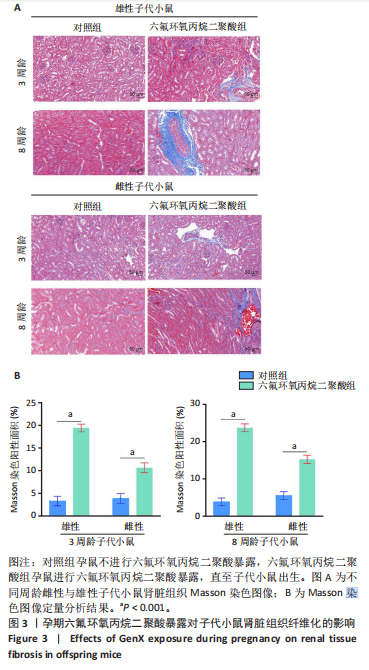
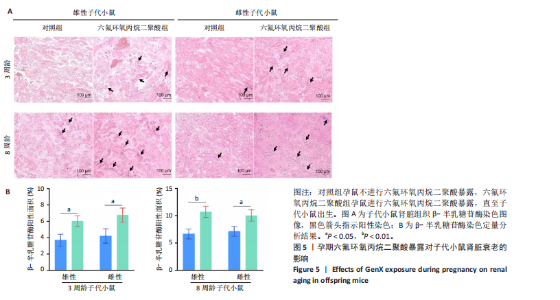
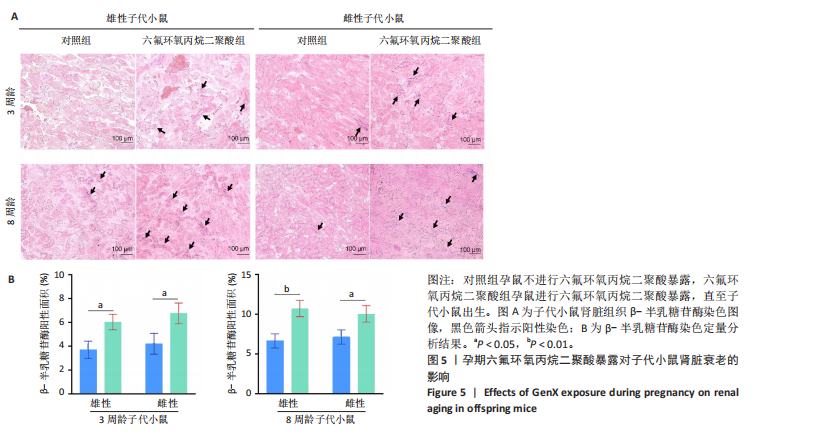
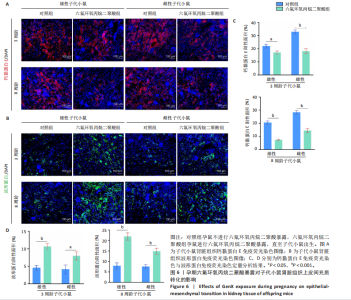
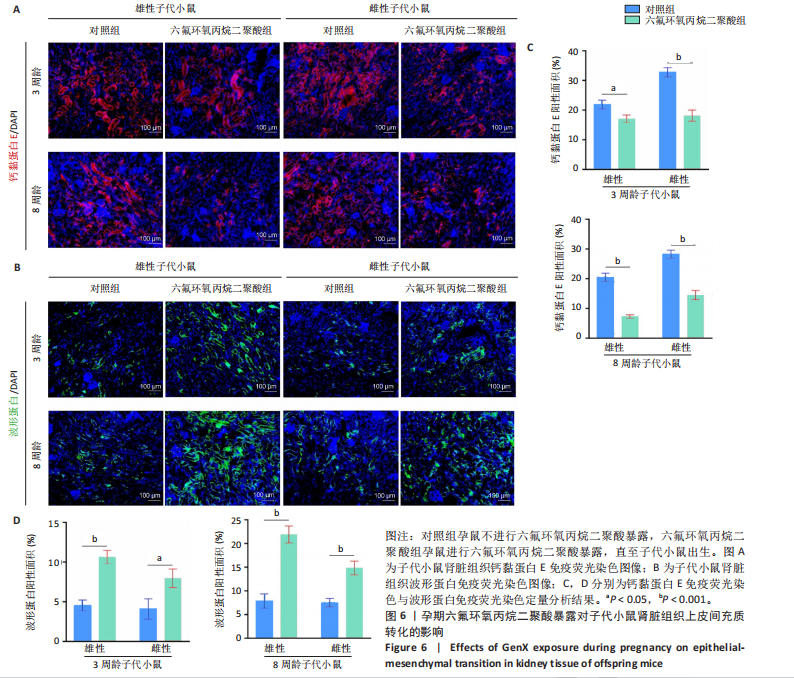
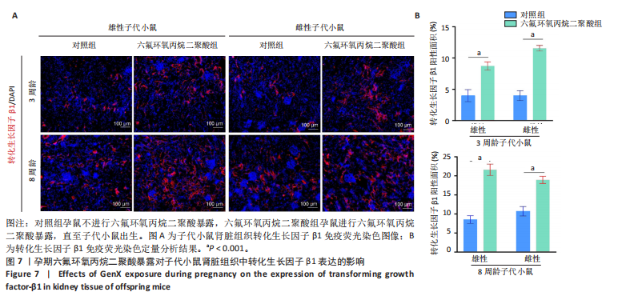
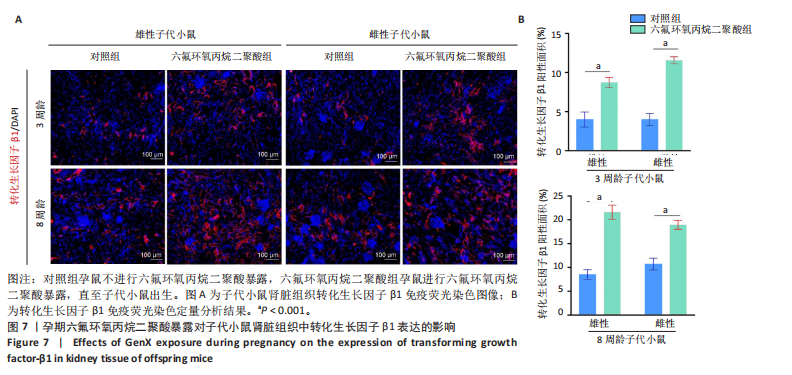
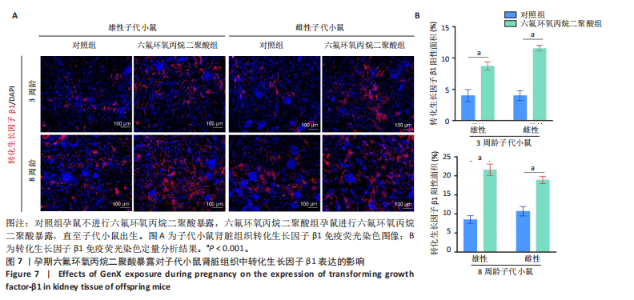
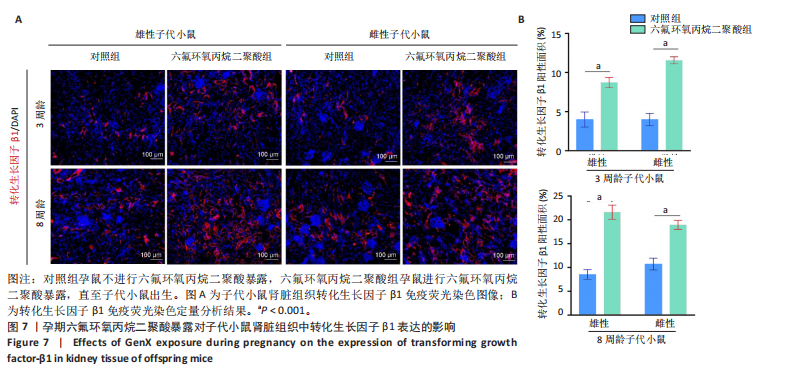
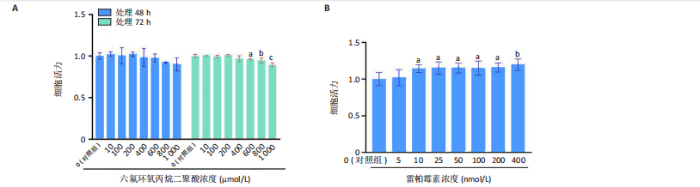
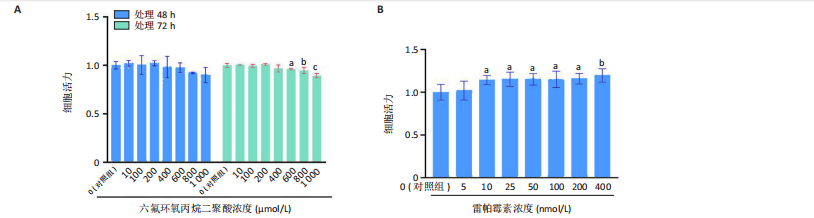
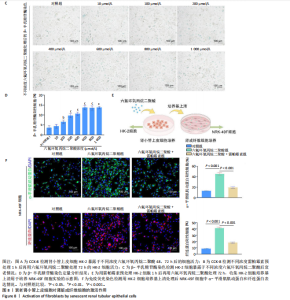
2.1.5 孕期六氟环氧丙烷二聚酸暴露对子代小鼠肾脏组织衰老的影响 细胞衰老与肾脏疾病的进展密切相关[26]。通过β-半乳糖苷酶染色评估孕期六氟环氧丙烷二聚酸暴露对子代小鼠肾脏衰老的影响,结果显示,与对照组同周龄、同性别子代小鼠比较,六氟环氧丙烷二聚酸组子代小鼠肾脏组织中β-半乳糖苷酶阳性染色面积显著增加,见图5。提示孕期六氟环氧丙烷二聚酸暴露可以导致子代小鼠肾脏衰老的发生。 2.1.6 孕期六氟环氧丙烷二聚酸暴露对子代小鼠肾脏组织上皮间充质转化的影响 上皮间充质转化是导致肾间质纤维化的重要机制[27],因此,采用免疫荧光染色评估上皮标志蛋白钙黏蛋白E和间质标志蛋白波形蛋白的表达。免疫荧光染色结果显示,与对照组同周龄、同性别子代小鼠比较,六氟环氧丙烷二聚酸组子代小鼠肾脏组织中钙黏蛋白E表达降低,波形蛋白表达升高,并且以8周龄子代小鼠肾脏组织中两指标变化更明显,见图6。结果表明,孕期六氟环氧丙烷二聚酸暴露诱导子代小鼠肾脏组织上皮间充质转化的发生。 2.1.7 孕期六氟环氧丙烷二聚酸暴露对子代小鼠肾脏组织中转化生长因子β1表达的影响 免疫荧光染色结果显示,与对照组同周龄、同性别子代小鼠比较,六氟环氧丙烷二聚酸组子代小鼠肾脏组织中促纤维化因子转化生长因子β1表达升高,见图7。 2.2 衰老肾小管上皮细胞对肾脏成纤维细胞的激活作用 CCK-8检测结果显示,培养72 h后,600-1 000 μmol/L六氟环氧丙烷二聚酸可降低HK-2细胞活力,见图8A。 雷帕霉素通过减少衰老相关分泌表型的产生可以抑制细胞衰老[28],并且已被广泛应用于抗衰老研究[29]。为了验证六氟环氧丙烷二聚酸暴露可能加速HK-2细胞衰老的假设,实验检测不同浓度雷帕霉素预处理HK-2细胞后暴露于600 μmol/L 六氟环氧丙烷二聚酸72 h的细胞活力变化,CCK-8检测结果显示10-400 nmol/L雷帕霉素可提升HK-2细胞活力,见图8B。β-半乳糖苷酶染色显示,在10-600 μmol/L浓度范围内,随着六氟环氧丙烷二聚酸浓度的升高,HK-2细胞衰老加速,此后再增加六氟环氧丙烷二聚酸浓度,细胞衰老不再发生明显变化,见图8C,D,提示六氟环氧丙烷二聚酸暴露可能显著诱导HK-2细胞加速衰老。衰老肾小管上皮细胞对肾脏成纤维细胞的激活作用的示意图,见图8E。 综合上述实验结果,选择10 nmol/L雷帕霉素与600 μmol/L六氟环氧丙烷二聚酸处理HK-2细胞,提取细胞上清干预NRK-49F细胞。免疫荧光染色结果显示,与对照组细胞上清处理的NRK-49 F细胞相比,六氟环氧丙烷二聚酸组细胞上清处理的NRK-49F细胞α-平滑肌肌动蛋白和纤连蛋白表达均升高;与六氟环氧丙烷二聚酸组细胞上清处理的NRK-49F细胞相比,六氟环氧丙烷二聚酸+雷帕霉素组细胞上清处理的NRK-49F细胞α-平滑肌肌动蛋白和纤连蛋白表达均降低,见图8F。结果表明,六氟环氧丙烷二聚酸暴露下HK-2细胞会加速衰老,同时释放促纤维化因子转化生长因子β1,激活肾脏成纤维细胞并促进肾纤维化。 "
| [1] COPERCHINI F, CROCE L, RICCI G, et al. Thyroid Disrupting Effects of Old and New Generation PFAS. Front Endocrinol (Lausanne). 2020; 11:612320. [2] DIXIT F, DUTTA R, BARBEAU B, et al. PFAS removal by ion exchange resins: A review. Chemosphere. 2021;272:129777. [3] PELCH KE, READE A, WOLFFE TAM, et al. PFAS health effects database: Protocol for a systematic evidence map. Environ Int. 2019;130:104851. [4] KOSKELA A, FINNILÄ MA, KORKALAINEN M, et al. Effects of developmental exposure to perfluorooctanoic acid (PFOA) on long bone morphology and bone cell differentiation. Toxicol Appl Pharmacol. 2016;301:14-21. [5] SHRESTHA S, BLOOM MS, YUCEL R, et al. Perfluoroalkyl substances, thyroid hormones, and neuropsychological status in older adults. Int J Hyg Environ Health. 2017;220(4):679-685. [6] RUMSBY PC, MCLAUGHLIN CL, HALL T. Perfluorooctane sulphonate and perfluorooctanoic acid in drinking and environmental waters. Philos Trans A Math Phys Eng Sci. 2009;367(1904):4119-4136. [7] WANG Z, DEWITT JC, HIGGINS CP, et al. A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environ Sci Technol. 2017;51(5):2508-2518. [8] BLAKE BE, COPE HA, HALL SM, et al. Evaluation of Maternal, Embryo, and Placental Effects in CD-1 Mice following Gestational Exposure to Perfluorooctanoic Acid (PFOA) or Hexafluoropropylene Oxide Dimer Acid (HFPO-DA or GenX). Environ Health Perspect. 2020;128(2):27006. [9] GALLOWAY JE, MORENO AVP, LINDSTROM AB, et al. Evidence of Air Dispersion: HFPO-DA and PFOA in Ohio and West Virginia Surface Water and Soil near a Fluoropolymer Production Facility. Environ Sci Technol. 2020;54(12):7175-7184. [10] WU S, XIE J, ZHAO H, et al. Pre-differentiation GenX exposure induced neurotoxicity in human dopaminergic-like neurons. Chemosphere. 2023;332:138900. [11] PAN Y, ZHANG H, CUI Q, et al. Worldwide Distribution of Novel Perfluoroether Carboxylic and Sulfonic Acids in Surface Water. Environ Sci Technol. 2018;52(14):7621-7629. [12] HEIDARI H, ABBAS T, OK YS, et al. GenX is not always a better fluorinated organic compound than PFOA: A critical review on aqueous phase treatability by adsorption and its associated cost. Water Res. 2021;205:117683. [13] REN W, WANG Z, GUO H, et al. GenX analogs exposure induced greater hepatotoxicity than GenX mainly via activation of PPARα pathway while caused hepatomegaly in the absence of PPARα in female mice. Environ Pollut. 2024;344:123314. [14] PENG BX, LI F, MORTIMER M, et al. Perfluorooctanoic acid alternatives hexafluoropropylene oxides exert male reproductive toxicity by disrupting blood-testis barrier. Sci Total Environ. 2022;846:157313. [15] LV D, LIU H, AN Q, et al. Association of adverse fetal outcomes with placental inflammation after oral gestational exposure to hexafluoropropylene oxide dimer acid (GenX) in Sprague-Dawley rats. J Hazard Mater. 2024;461:132536. [16] XIE X, ZHOU J, HU L, et al. Exposure to hexafluoropropylene oxide dimer acid (HFPO-DA) disturbs the gut barrier function and gut microbiota in mice. Environ Pollut. 2021;290:117934. [17] WANG X, WANG K, MAO W, et al. Emerging perfluoroalkyl substances retard skeletal growth by accelerating osteoblasts senescence via ferroptosis. Environ Res. 2024;258:119483. [18] YAO D, SHAO J, JIA D, et al. Immunotoxicity of legacy and alternative per- and polyfluoroalkyl substances on zebrafish larvae. Environ Pollut. 2024;358:124511. [19] PETRIELLO MC, MOTTALEB MA, SERIO TC, et al. Serum concentrations of legacy and emerging per- and polyfluoroalkyl substances in the Anniston Community Health Surveys (ACHS I and ACHS II). Environ Int. 2022;158:106907. [20] CALAFAT AM, KATO K, HUBBARD K, et al. Legacy and alternative per- and polyfluoroalkyl substances in the U.S. general population: Paired serum-urine data from the 2013-2014 National Health and Nutrition Examination Survey. Environ Int. 2019;131:105048. [21] LESLIE HA, VAN VELZEN MJM, BRANDSMA SH, et al. Discovery and quantification of plastic particle pollution in human blood. Environ Int. 2022;163:107199. [22] DO NASCIMENTO LCP, NETO J, DE ANDRADE BV, et al. Maternal exposure to high-fat and high-cholesterol diet induces arterial hypertension and oxidative stress along the gut-kidney axis in rat offspring. Life Sci. 2020;261:118367. [23] NIELSEN F, FISCHER FC, LETH PM, et al. Occurrence of Major Perfluorinated Alkylate Substances in Human Blood and Target Organs. Environ Sci Technol. 2024;58(1):143-149. [24] RHEE J, CHANG VC, CHENG I, et al. Serum concentrations of per- and polyfluoroalkyl substances and risk of renal cell carcinoma in the Multiethnic Cohort Study. Environ Int. 2023;180:108197. [25] GUO WQ, HAO WD, XIAO WS. Emerging Perfluorinated Chemical GenX: Environmental and Biological Fates and Risks. Environment & Health. 2024; doi: 10.1021/envhealth.4c00164. [26] HUANG W, HICKSON LJ, EIRIN A, et al. Cellular senescence: the good, the bad and the unknown. Nat Rev Nephrol. 2022;18(10):611-627. [27] SHENG L, ZHUANG S. New Insights Into the Role and Mechanism of Partial Epithelial-Mesenchymal Transition in Kidney Fibrosis. Front Physiol. 2020;11:569322. [28] SELVARANI R, MOHAMMED S, RICHARDSON A. Effect of rapamycin on aging and age-related diseases-past and future. Geroscience. 2021;43(3):1135-1158. [29] MANNICK JB, LAMMING DW. Targeting the biology of aging with mTOR inhibitors. Nat Aging. 2023;3(6):642-660. [30] LAU C, THIBODEAUX JR, HANSON RG, et al. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol Sci. 2006;90(2): 510-518. [31] PAN C, WANG X, FAN Z, et al. Polystyrene microplastics facilitate renal fibrosis through accelerating tubular epithelial cell senescence. Food Chem Toxicol. 2024;191:114888. [32] CONLEY JM, LAMBRIGHT CS, EVANS N, et al. Dose additive maternal and offspring effects of oral maternal exposure to a mixture of three PFAS (HFPO-DA, NBP2, PFOS) during pregnancy in the Sprague-Dawley rat. Sci Total Environ. 2023;892:164609. [33] KATO K, KALATHIL AA, PATEL AM, et al. Per- and polyfluoroalkyl substances and fluorinated alternatives in urine and serum by on-line solid phase extraction-liquid chromatography-tandem mass spectrometry. Chemosphere. 2018;209:338-345. [34] FENG L, LANG Y, FENG Y, et al. Maternal F-53B exposure during pregnancy and lactation affects bone growth and development in male offspring. Ecotoxicol Environ Saf. 2024;279:116501. [35] IMAM MU, ISMAIL M, OOI DJ, et al. Increased risk of insulin resistance in rat offsprings exposed prenatally to white rice. Mol Nutr Food Res. 2015;59(1):180-184. [36] MA T, ZHOU Y, XIA Y, et al. Maternal Exposure to Di-n-butyl Phthalate Promotes the Formation of Testicular Tight Junctions through Downregulation of NF-κB/COX-2/PGE(2)/MMP-2 in Mouse Offspring. Environ Sci Technol. 2020;54(13):8245-8258. [37] LUO C, ZHOU S, ZHOU Z, et al. Wnt9a Promotes Renal Fibrosis by Accelerating Cellular Senescence in Tubular Epithelial Cells. J Am Soc Nephrol. 2018;29(4):1238-1256. [38] BAKER DJ, CHILDS BG, DURIK M, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530(7589):184-189. [39] CHOU X, LI X, MIN Z, et al. Sirtuin-1 attenuates cadmium-induced renal cell senescence through p53 deacetylation. Ecotoxicol Environ Saf. 2022;245:114098. [40] KALLURI R, NEILSON EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112(12):1776-1784. [41] BURNS WC, KANTHARIDIS P, THOMAS MC. The role of tubular epithelial-mesenchymal transition in progressive kidney disease. Cells Tissues Organs. 2007;185(1-3):222-231. [42] PAN C, WU Y, HU S, et al. Polystyrene microplastics arrest skeletal growth in puberty through accelerating osteoblast senescence. Environ Pollut. 2023;322:121217. [43] WANG S, CHEN L, SHI X, et al. Polystyrene microplastics-induced macrophage extracellular traps contributes to liver fibrotic injury by activating ROS/TGF-β/Smad2/3 signaling axis. Environ Pollut. 2023; 324:121388. [44] ISHII T, WARABI E. Mechanism of Rapid Nuclear Factor-E2-Related Factor 2 (Nrf2) Activation via Membrane-Associated Estrogen Receptors: Roles of NADPH Oxidase 1, Neutral Sphingomyelinase 2 and Epidermal Growth Factor Receptor (EGFR). Antioxidants (Basel). 2019;8(3):69. [45] CHU C, GAO X, LI X, et al. Involvement of Estrogen Receptor-α in the Activation of Nrf2-Antioxidative Signaling Pathways by Silibinin in Pancreatic β-Cells. Biomol Ther (Seoul). 2020;28(2):163-171. [46] TAWARAYAMA H, UCHIDA K, HASEGAWA H, et al. Estrogen, via ESR2 receptor, prevents oxidative stress-induced Müller cell death and stimulates FGF2 production independently of NRF2, attenuating retinal degeneration. Exp Eye Res. 2024;248:110103. [47] VILLA A, RIZZI N, VEGETO E, et al. Estrogen accelerates the resolution of inflammation in macrophagic cells. Sci Rep. 2015;5:15224. [48] SVEDLUND EE, LANTERO RM, HALVORSEN B, et al. Testosterone exacerbates neutrophilia and cardiac injury in myocardial infarction via actions in bone marrow. Nat Commun. 2025;16(1):1142. [49] KANG DH, YU ES, YOON KI, et al. The impact of gender on progression of renal disease: potential role of estrogen-mediated vascular endothelial growth factor regulation and vascular protection. Am J Pathol. 2004;164(2):679-688. [50] DISTECHE CM. Dosage compensation of the sex chromosomes and autosomes. Semin Cell Dev Biol. 2016;56:9-18. [51] LI X, HOU M, ZHANG F, et al. Per- and Polyfluoroalkyl Substances and Female Health Concern: Gender-based Accumulation Differences, Adverse Outcomes, and Mechanisms. Environ Sci Technol. 2025;59(3): 1469-1486. |
| [1] | Li Sa, Sun Ning, Sun Zhaozhong, Feng Zhimeng, Li Xuedong. Evaluation parameters and specific region of C6 nerve oppression by uncinate process degeneration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2294-2302. |
| [2] | Yang Lixia, Diao Liqin, Li Hua, Feng Yachan, Liu Xin, Yu Yuexin, Dou Xixi, Gu Huifeng, Xu Lanju. Regulatory mechanism of recombinant type III humanized collagen protein improving photoaging skin in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1988-2000. |
| [3] | Ye Qianqian, Pan Hang, Tian Chuan, Zhu Xiangqing, Ye Li, Zhao Xiaojuan, Shu Liping, Pan Xinghua. Effects of highly active umbilical cord mesenchymal stem cells on structure and function of thymus in elderly tree shrews [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1720-1729. |
| [4] | Pan Dong, Yang Jialing, Tian Wei, Wang Dongji, Zhu Zheng, Ma Wenchao, Liu Na, Fu Changxi. Resistance exercise activates skeletal muscle satellite cells in aged rats: role of adiponectin receptor 1 pathway [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1736-1746. |
| [5] | Liu Huan, Zeng Shaopeng, Chen Jun, He Linqian, Yang Ying, Zhang Jing. Aging-related dysregulation of glucose metabolism: crossroads of cancer and neurodegenerative diseases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1527-1538. |
| [6] | Hou Chaowen, Li Zhaojin, Kong Jianda, Zhang Shuli. Main physiological changes in skeletal muscle aging and the multimechanism regulatory role of exercise [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1464-1475. |
| [7] | Peng Tuanhui, Song Hongming, Yang Ling, Ding Xiaoge, Meng Pengjun. Effects of long-term endurance exercise on kl/FGF23 axis and calcium-phosphorus metabolism in naturally aging mice [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1089-1095. |
| [8] | Zhou Feng, Fu Pengfei, Qian Yufan, Xu Pingcheng, Guo Jiongjiong, Zhang Lei. Correlation between spinal sagittal imbalance and knee joint parameters detected by whole-body EOS imaging [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 596-603. |
| [9] | Zhang Qian, Wang Fuxia, Wang Wen, Zhang Kun. Characteristic analysis of nanogel composite system and its application strategies in visualization of diagnostic imaging and therapy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 480-488. |
| [10] | Tan Fengyi, Xie Jiamin, Pan Zhenfeng, Zhang Xinxu, Zheng Zetai, Zeng Zhiying, Zhou Yanfang. Effect and mechanism of collagen combined with microneedles in treatment of skin photoaging [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 451-458. |
| [11] | Wang Yaping, Gao Tianyun, Wang Bin. Senescence of human bone marrow mesenchymal stromal cells with increasing age is not dependent on the mediation of endogenous retroviruses [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 10-20. |
| [12] | Yuan Weiyuan, Lei Qinhui, Li Xiuqi, Lu Tiezhu, Fu Ziwen, Liang Zhili, Ji Shaoyang, Li Yijia, Ren Yu . Therapeutic effects of adipose-derived mesenchymal stem cells and their exosomes on dexamethasone-induced sarcopenia in mice [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 58-67. |
| [13] | Miao Jiahang, Ma Sheng, Li Qupeng, Yu Huilin, Hu Tianyu, Gao Xiao, Feng Hu. Cervical lordosis ratio can be used as a decision-making indicator for selection of posterior surgical approach for multi-level cervical spondylotic myelopathy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1796-1802. |
| [14] | Lyu Liting, Yu Xia, Zhang Jinmei, Gao Qiaojing, Liu Renfan, Li Meng, Wang Lu. Bibliometric analysis of research process and current situation of brain aging and exosomes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1457-1465. |
| [15] | Chen Yilin, Jiang Xiaobo, Qu Honglin, Liu Ruilian. General pattern of GSK3/Nrf2-regulated biological rhythms in organismal aging [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1257-1264. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||