Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (4): 1036-1046.doi: 10.12307/2025.969
Previous Articles Next Articles
Relationship between Alzheimer’s disease and sarcopenia and body mass index: analysis of GWAS datasets for European populations
He Qiwang1, 2, 3, Chen Bo4, Liang Fuchao4, Kang Zewei4, Zhou Yuan5, Ji Anxu1, Tang Xialin2, 3
- 1Hubei University of Chinese Medicine, Wuhan 430061, Hubei Province, China; 2Hubei Provincial Hospital of Traditional Chinese Medicine, Affiliated Hospital of Hubei University of Chinese Medicine, Wuhan 430061, Hubei Province, China; 3Hubei Shizhen Laboratory, Wuhan 430061, Hubei Province, China; 4Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang 441000, Hubei Province, China; 5Traditional Chinese Medical Hospital of Changxing, Huzhou 313100, Zhejiang Province, China
-
Received:2024-11-18Accepted:2024-12-25Online:2026-02-08Published:2025-05-23 -
Contact:Tang Xialin, MD, Attending physician, Hubei Provincial Hospital of Traditional Chinese Medicine, Affiliated Hospital of Hubei University of Chinese Medicine, Wuhan 430061, Hubei Province, China; Hubei Shizhen Laboratory, Wuhan 430061, Hubei Province, China -
About author:He Qiwang, Master candidate, Hubei University of Chinese Medicine, Wuhan 430061, Hubei Province, China; Hubei Provincial Hospital of Traditional Chinese Medicine, Affiliated Hospital of Hubei University of Chinese Medicine, Wuhan 430061, Hubei Province, China; Hubei Shizhen Laboratory, Wuhan 430061, Hubei Province, China -
Supported by:Hubei Provincial Natural Science Foundation for Youth Program, No. 2022CFB923 (to TXL); Young Talent Program of Hubei Provincial Administration of Traditional Chinese Medicine, No. ZY2023Q001 (to TXL)
CLC Number:
Cite this article
He Qiwang, , , Chen Bo, Liang Fuchao, Kang Zewei, Zhou Yuan, Ji Anxu, Tang Xialin, . Relationship between Alzheimer’s disease and sarcopenia and body mass index: analysis of GWAS datasets for European populations[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1036-1046.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
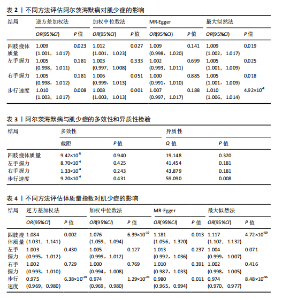
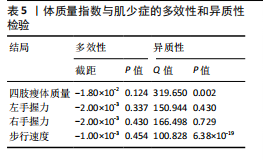
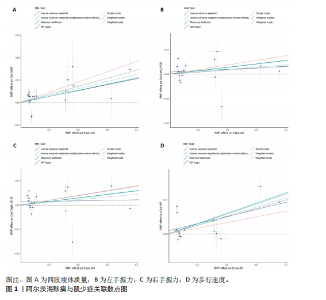
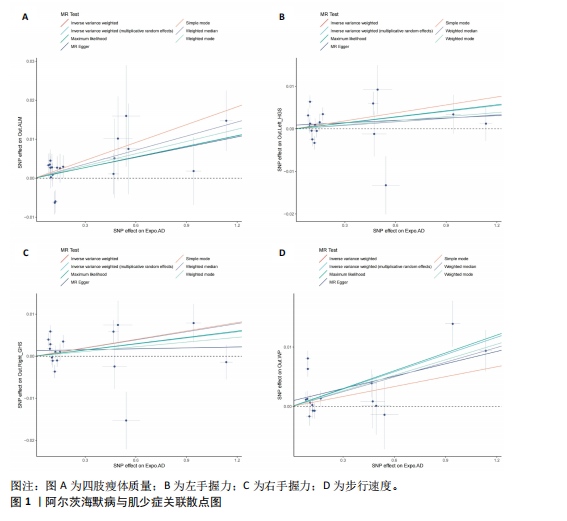
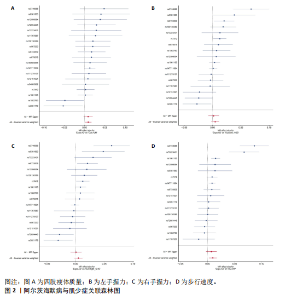
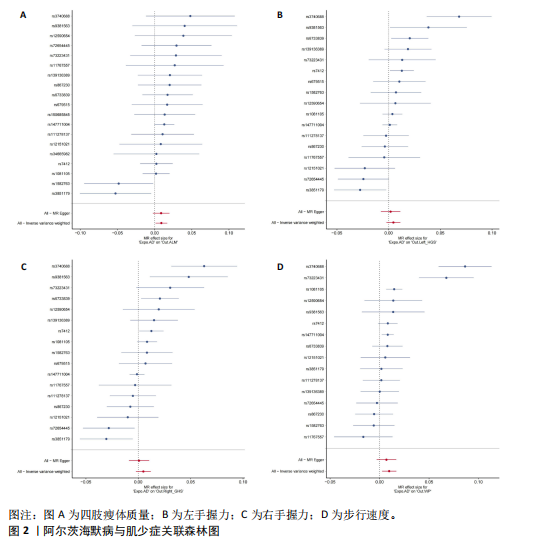
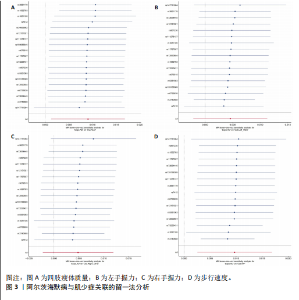
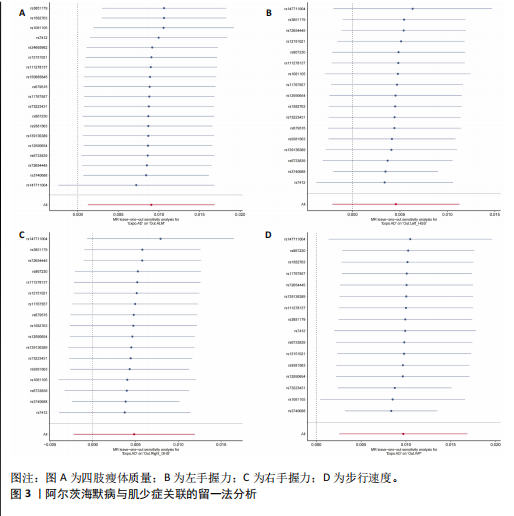
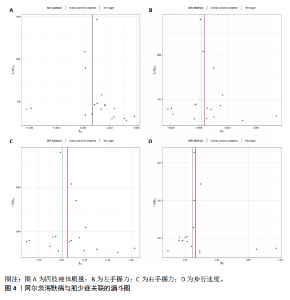
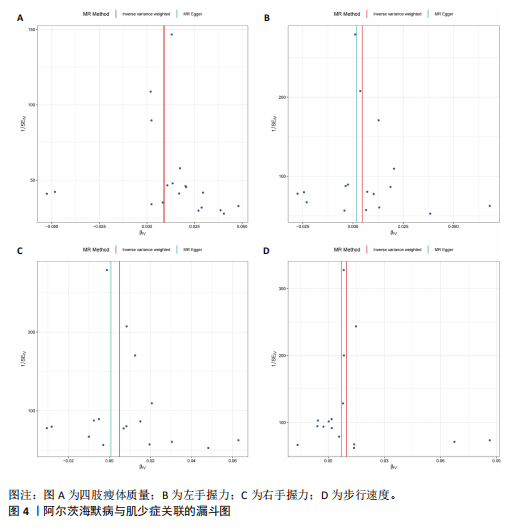
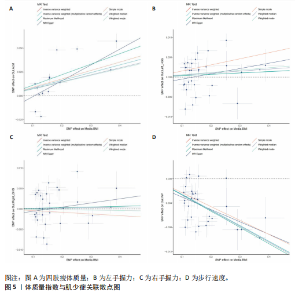
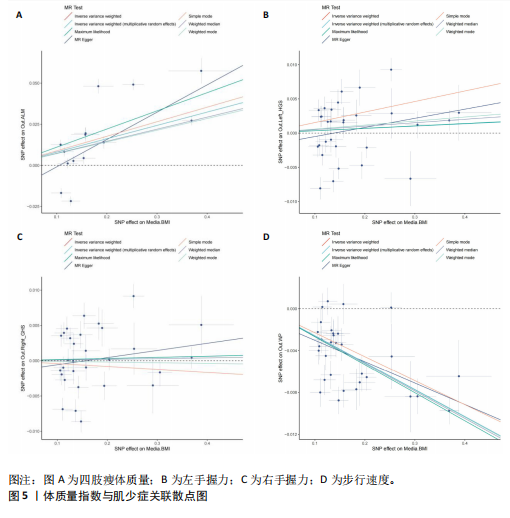
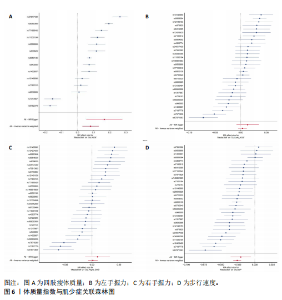
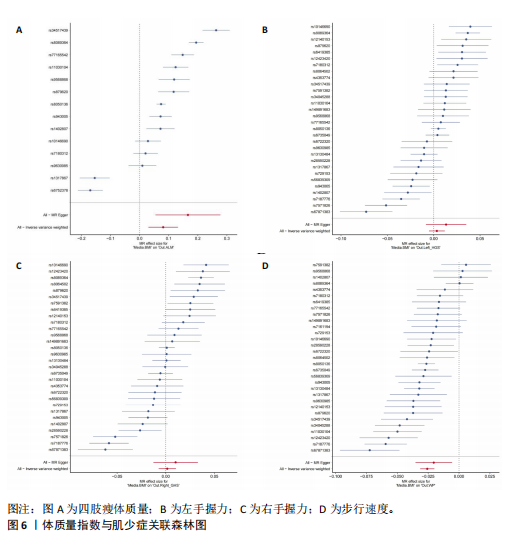
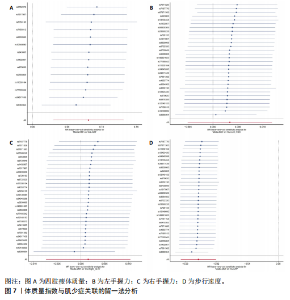
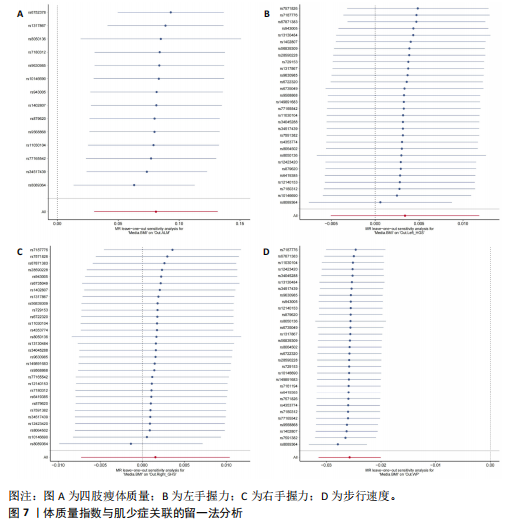
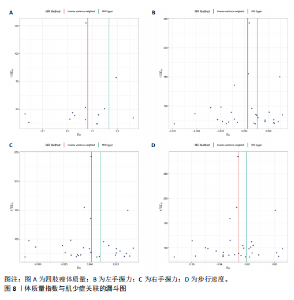
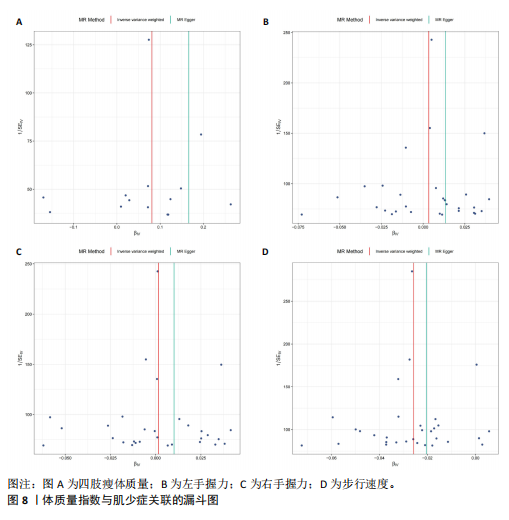
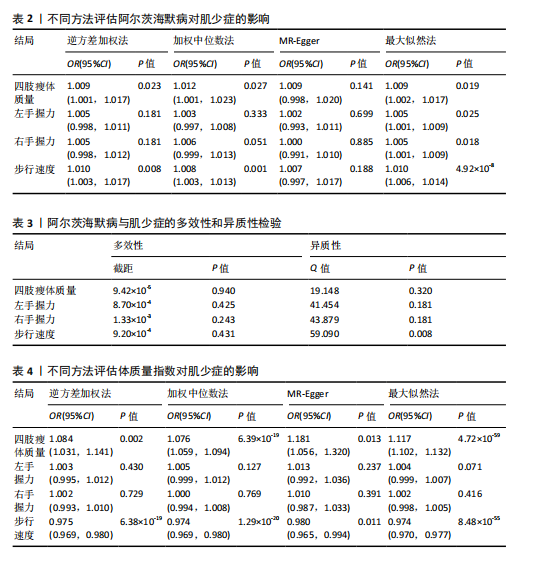
2.1 SNP的选择与验证 根据上述筛选条件,将暴露与结局相匹配,当F统计量> 10时,SNP被认为足够强大以减轻潜在偏倚的影响,结果如下:阿尔茨海默病-四肢瘦体质量共纳入19个SNP(30.77 < F < 962.36),其中经过P值过滤与去除连锁不平衡后剩余21个,将暴露与结局的效应等位基因对齐过程去除2个;阿尔茨海默病-左手握力共纳入17个SNP(30.77 < F < 962.36),其中经过P值过滤与去除连锁不平衡后剩余20个,将暴露与结局的效应等位基因对齐过程去除3个;阿尔茨海默病-右手握力共纳入17个SNP(30.77 < F < 962.36),其中经过P值过滤与去除连锁不平衡后剩余20个,将暴露与结局的效应等位基因对齐过程去除3个;阿尔茨海默病-步行速度共纳入16个SNP(30.77 < F < 962.36),其中经过P值过滤与去除连锁不平衡后剩余20个,将暴露与结局的效应等位基因对齐过程去除4个;阿尔茨海默病-体质量指数共纳入16个SNP(30.77 < F < 466.80),其中经过P值过滤与去除连锁不平衡后剩余19个,将暴露与结局的效应等位基因对齐过程去除3个;体质量指数-四肢瘦体质量共纳入14个SNP(30.45 < F < 372.83),其中经过P值过滤与去除连锁不平衡后剩余42个,将暴露与结局的效应等位基因对齐过程去除28个;体质量指数-左手握力共纳入30个SNP(30.00 < F < 372.83);体质量指数-右手握力共纳入30个SNP(30.00 < F < 372.83),体质量指数-步行速度共纳入31个SNP(30.00 < F < 372.83),其中经过P值过滤与去除连锁不平衡后剩余42个,将暴露与结局的效应等位基因对齐过程去除11个。 2.2 阿尔茨海默病对肌少症的影响 逆方差加权分析显示,阿尔茨海默病与四肢瘦体质量(OR=1.009,95%CI:1.001-1.017,P=0.023)及步行速度(OR=1.010,95%CI:1.003-1.017,P=0.008)呈正相关。相比之下,未观察到阿尔茨海默病与握力正相关的证据。加权中位数、MR-Egger与最大似然法分析显示了一致的估计值,但精度较低(表2)。未发现定向多效性的证据。某些参数的异质性较高,采用随机效应模型下的逆方差加权荟萃分析来减轻异质性的影响(表3)。 阿尔茨海默病与肌少症之间关系的散点图和森林图分别见图1,2,其中可以观察到类似的结果。如图3所示,留一法敏感性分析显示,总体估计值未受到任何单个SNP不成比例的影响。图4中的漏斗图也表明没有水平多效性的证据。 2.3 体质量指数对肌少症的影响 逆方差加权分析显示,体质量指数与四肢瘦体质量(OR=1.084,95%CI:1.031-1.141,P=0.002)呈正相关,与步行速度(OR=0.975,95%CI:0.969-0.980,P=6.38×10-19)呈负相关。相比之下,未观察到阿尔茨海默病与握力存在相关性的证据。加权中位数、MR-Egger与最大似然法分析显示了一致的估计值,但精度较低(表4);未发现定向多效性的证据;某些参数的异质性较高,采用随机效应模型下的逆方差加权荟萃分析来减轻异质性的影响(表5)。 体质量指数与肌少症之间关系的散点图和森林图分别见图5,6,其中可以观察到类似的结果。如图7所示,留一法敏感性分析显示,总体估计值未受到任何单个SNP不成比例的影响。图8中的漏斗图也表明没有水平多效性的证据。 2.4 体质量指数在阿尔茨海默病与肌少症中的中介作用 由于体质量指数对握力没有因果关系,因此不认为它们是阿尔茨海默病作为肌少症风险因素的潜在介质。于是仅对四肢瘦体质"
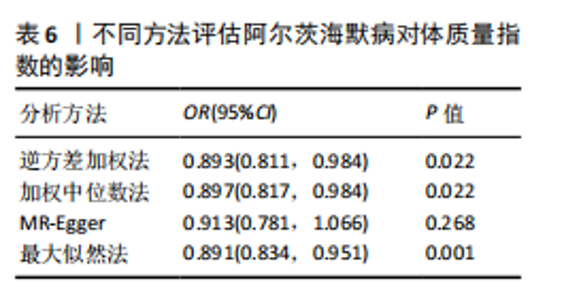
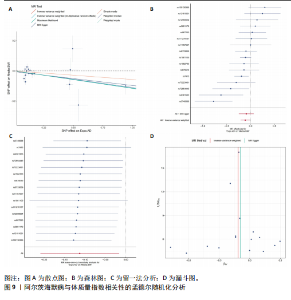
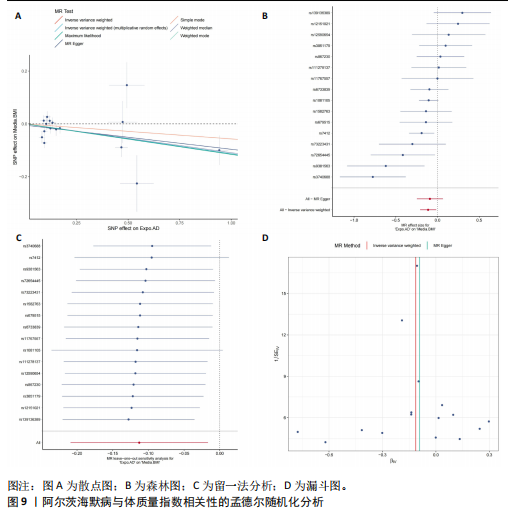
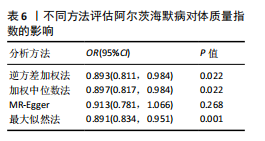
量与步行速度进行了中介分析。结果显示阿尔茨海默病与体质量指数呈负相关(OR=0.893,95%CI:0.811-0.984,P=0.022),多效性检验截距为-0.005,P > 0.05,未发现定向多效性的证据;异质性检验Q=33.293,P=0.022,采用随机效应模型下的逆方差加权荟萃分析来减轻异质性的影响,更多详情见表6及图9。在阿尔茨海默病对四肢瘦体质量的因果作用中,体质量指数的遮掩作用为-0.009 1(体质量指数遮掩效应比为50.25%),阿尔茨海默病对四肢瘦体质量的直接效应为0.018。在阿尔茨海默病对步行速度的因果作用中,体质量指数的中介作用为0.002 9(体质量指数中介率为32.11%),阿尔茨海默病对步行速度的直接效应为0.006 1。"
| [1] SCHELTENS P, DE STROOPER B, KIVIPELTO M, et al. Alzheimer’s disease. Lancet. 2021; 397(10284):1577-1590. [2] WU Y, ZHANG X, LI C, et al. Ecosystem service trade-offs and synergies under influence of climate and land cover change in an afforested semiarid basin, China. Ecol Eng. 2021;159:106083. [3] ALDRICH L, ISPOGLOU T, PROKOPIDIS K, et al. Acute Sarcopenia: Systematic Review and Meta-Analysis on Its Incidence and Muscle Parameter Shifts During Hospitalisation. J Cachexia Sarcopenia Muscle. 2024. doi: 10.1002/jcsm.13662. [4] AMINI N, IBN HACH M, LAPAUW L, et al. Meta-analysis on the interrelationship between sarcopenia and mild cognitive impairment, Alzheimer’s disease and other forms of dementia. J Cachexia Sarcopenia Muscle. 2024;15(4):1240-1253. [5] 何启旺, 夏宇阳, 卢博雯, 等. 基于生物信息学探讨肌少症与阿尔茨海默病的关系[J]. 中华骨质疏松和骨矿盐疾病杂志, 2024,17(5):430-445. [6] WENG X, LIU S, LI M, et al. White matter hyperintensities: a possible link between sarcopenia and cognitive impairment in patients with mild to moderate Alzheimer’s disease. Eur Geriatr Med. 2023;14(5): 1037-1047. [7] BARONE R, BRAMATO G, GNONI V, et al. Sarcopenia in subjects with Alzheimer’s disease: prevalence and comparison of agreement between EGWSOP1, EGWSOP2, and FNIH criteria. BMC Geriatr. 2024;24(1): 278. [8] CHOI S, CHON J, LEE SA, et al. Central obesity is associated with lower prevalence of sarcopenia in older women, but not in men: a cross-sectional study. BMC Geriatr. 2022;22(1):406. [9] KANJANAVAIKOON N, SAISIRIVECHAKUN P, CHAIAMNUAY S. Age, body mass index, and function as the independent predictors of sarcopenia in axial spondyloarthritis: a cross-sectional analysis. Clin Rheumatol. 2023;42(12):3257-3265. [10] CHALERMSRI C, AEKPLAKORN W, SRINONPRASERT V. Body Mass Index Combined With Possible Sarcopenia Status Is Better Than BMI or Possible Sarcopenia Status Alone for Predicting All-Cause Mortality Among Asian Community-Dwelling Older Adults. Front Nutr. 2022;9:881121. [11] GARCíA-MARíN LM, CAMPOS AI, MARTIN NG, et al. Phenome-wide analysis highlights putative causal relationships between self-reported migraine and other complex traits. J Headache Pain. 2021;22(1):66. [12] CRUZ-JENTOFT AJ, BAEYENS JP, BAUER JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412-423. [13] HU MJ, TAN JS, GAO XJ, et al. Effect of Cheese Intake on Cardiovascular Diseases and Cardiovascular Biomarkers. Nutrients. 2022;14(14):2936. [14] LIU C, LIU N, XIA Y, et al. Osteoporosis and sarcopenia-related traits: A bi-directional Mendelian randomization study. Front Endocrinol (Lausanne). 2022;13:975647. [15] HE J, HUANG M, LI N, et al. Genetic Association and Potential Mediators between Sarcopenia and Coronary Heart Disease: A Bidirectional Two-Sample, Two-Step Mendelian Randomization Study. Nutrients. 2023;15(13):3013. [16] DU Y, XIE B, WANG M, et al. Roles of sex hormones in mediating the causal effect of vitamin D on osteoporosis: A two-step Mendelian randomization study. Front Endocrinol (Lausanne). 2023;14:1159241. [17] SU Q, JIN C, YANG Y, et al. Association Between Autoimmune Diseases and Sarcopenia: A Two-Sample Mendelian Randomization Study. Clin Epidemiol. 2023; 15:901-910. [18] WANG J, YANG M, TIAN Y, et al. Causal associations between common musculoskeletal disorders and dementia: a Mendelian randomization study. Front Aging Neurosci. 2023;15:1253791. [19] BOEHM FJ, ZHOU X. Statistical methods for Mendelian randomization in genome-wide association studies: A review. Comput Struct Biotechnol J. 2022;20:2338-2351. [20] BURGESS S, BOWDEN J, FALL T, et al. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology. 2017;28(1):30-42. [21] BOWDEN J, DAVEY SMITH G, BURGESS S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512-525. [22] ZENG Y, GUO R, CAO S, et al. CSF N-acylethanolamine acid amidase level and Parkinson’s disease risk: A mendelian randomization study. Parkinsonism Relat Disord. 2024;123:106953. [23] ZHAO W, ZHANG X, LI F, et al. Mendelian Randomization Estimates the Effects of Plasma and Cerebrospinal Fluid Proteins on Intelligence, Fluid Intelligence Score, and Cognitive Performance. 2024. doi: 10.1007/s12035-024-04542-5. [24] LONGO S, MESSI ML, WANG ZM, et al. Accelerated sarcopenia precedes learning and memory impairments in the P301S mouse model of tauopathies and Alzheimer’s disease. J Cachexia Sarcopenia Muscle. 2024;15(4):1358-1375. [25] XU H, BHASKARAN S, PIEKARZ KM, et al. Age Related Changes in Muscle Mass and Force Generation in the Triple Transgenic (3xTgAD) Mouse Model of Alzheimer’s Disease. Front Aging Neurosci. 2022;14:876816. [26] WU MY, ZOU WJ, LEE D, et al. APP in the Neuromuscular Junction for the Development of Sarcopenia and Alzheimer’s Disease. Int J Mol Sci. 2023;24(9):7809. [27] KARIM A, IQBAL MS, MUHAMMAD T, et al. Elevated plasma zonulin and CAF22 are correlated with sarcopenia and functional dependency at various stages of Alzheimer’s diseases. Neurosci Res. 2022;184:47-53. [28] AUDOUARD E, VAN HEES L, SUAIN V, et al. Motor deficit in a tauopathy model is induced by disturbances of axonal transport leading to dying-back degeneration and denervation of neuromuscular junctions. Am J Pathol. 2015;185(10):2685-2697. [29] TORCINARO A, RICCI V, STRIMPAKOS G, et al. Peripheral Nerve Impairment in a Mouse Model of Alzheimer’s Disease. Brain Sci. 2021;11(9):1245. [30] WEN Y, ZHENG Y, HUA S, et al. Mechanisms of Bone Morphogenetic Protein 2 in Respiratory Diseases. Curr Allergy Asthma Rep. 2024;25(1):1. [31] SARTORI R, MILAN G, PATRON M, et al. Smad2 and 3 transcription factors control muscle mass in adulthood. Am J Physiol Cell Physiol. 2009;296(6):C1248-1257. [32] WANG S, GUSTAFSON S, DECKELMAN C, et al. Dysphagia Profiles Among Inpatients with Dementia Referred for Swallow Evaluation. J Alzheimers Dis. 2022;89(1):351-358. [33] SAARI T, HALLIKAINEN I, HINTSA T, et al. Neuropsychiatric symptoms and activities of daily living in Alzheimer’s disease: ALSOVA 5-year follow-up study. Int Psychogeriatr. 2020;32(6):741-751. [34] DZIEWAS R, ALLESCHER HD, AROYO I, et al. Diagnosis and treatment of neurogenic dysphagia - S1 guideline of the German Society of Neurology. Neurol Res Pract. 2021;3(1):23. [35] JUN L, ROBINSON M, GEETHA T, et al. Prevalence and Mechanisms of Skeletal Muscle Atrophy in Metabolic Conditions. Int J Mol Sci. 2023;24(3):2973. [36] LIGUORI C, CHIARAVALLOTI A, NUCCETELLI M, et al. Hypothalamic dysfunction is related to sleep impairment and CSF biomarkers in Alzheimer’s disease. J Neurol. 2017;264(11):2215-2223. [37] STEINBACH S, HUNDT W, VAITL A, et al. Taste in mild cognitive impairment and Alzheimer’s disease. J Neurol. 2010;257(2): 238-246. [38] KOPP KO, GLOTFELTY EJ, LI Y, et al. Glucagon-like peptide-1 (GLP-1) receptor agonists and neuroinflammation: Implications for neurodegenerative disease treatment. Pharmacol Res. 2022;186:106550. [39] YU X, CHEN S, FUNCKE JB, et al. The GIP receptor activates futile calcium cycling in white adipose tissue to increase energy expenditure and drive weight loss in mice. Cell Metab. 2024:S1550-4131(24)00449-2. [40] DODDS RM, GRANIC A, DAVIES K, et al. Prevalence and incidence of sarcopenia in the very old: findings from the Newcastle 85+ Study. J Cachexia Sarcopenia Muscle. 2017;8(2):229-237. [41] NASIMI N, DABBAGHMANESH MH, SOHRABI Z. Nutritional status and body fat mass: Determinants of sarcopenia in community-dwelling older adults. Exp Gerontol. 2019; 122:67-73. [42] FAN Y, ZHANG B, HUANG G, et al. Sarcopenia: Body Composition and Gait Analysis. Front Aging Neurosci. 2022;14: 909551. [43] MESINOVIC J, MCMILLAN LB, SHORE-LORENTI C, et al. Metabolic Syndrome and Its Associations with Components of Sarcopenia in Overweight and Obese Older Adults. J Clin Med. 2019;8(2):145. [44] YOO MC, WON CW, SOH Y. Association of high body mass index, waist circumference, and body fat percentage with sarcopenia in older women. BMC Geriatr. 2022;22(1): 937. [45] TOMLINSON DJ, ERSKINE RM, MORSE CI, et al. The impact of obesity on skeletal muscle strength and structure through adolescence to old age. Biogerontology. 2016;17(3): 467-483. [46] MORGAN PT, SMEUNINX B, BREEN L. Exploring the Impact of Obesity on Skeletal Muscle Function in Older Age. Front Nutr. 2020;7:569904. [47] BAHNIWAL RK, SADR N, SCHINDERLE C, et al. Obesity Paradox and the Effect of NT‐proBNP on All‐Cause and Cause‐Specific Mortality. Clin Cardiol. 2024;47(11):e70044. [48] SWAN L, WARTERS A, O’SULLIVAN M. Socioeconomic Inequality and Risk of Sarcopenia in Community-Dwelling Older Adults. Clin Interv Aging. 2021;16:1119-1129. [49] STUDENSKI S, PERERA S, PATEL K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50-58. [50] GONG Z, FAULKNER ME, AKHONDA M, et al. White matter integrity and motor function: a link between cerebral myelination and longitudinal changes in gait speed in aging. Geroscience. 2024. doi: 10.1007/s11357-024-01392-w. [51] NEWMAN AB, VISSER M, KRITCHEVSKY SB, et al. The Health, Aging, and Body Composition (Health ABC) Study-Ground-Breaking Science for 25 Years and Counting. J Gerontol A Biol Sci Med Sci. 2023;78(11): 2024-2034. [52] LIU H, JIN M, HAO H, et al. Association between relative fat mass and kidney stones in American adults. Sci Rep. 2024;14(1): 27045. [53] PRIETO J, WILSON J, TINGLE A, et al. Strategies for older people living in care homes to prevent urinary tract infection: the StOP UTI realist synthesis. Health Technol Assess. 2024;28(68):1-139. [54] CAO H, ZHANG D, WANG P, et al. Gut microbiome: a novel preventive and therapeutic target for prostatic disease. Front Cell Infect Microbiol. 2024;14: 1431088. [55] HENTZEN C, TURMEL N, CHESNEL C, et al. Effect of a strong desire to void on walking speed in individuals with multiple sclerosis and urinary disorders. Ann Phys Rehabil Med. 2020;63(2):106-110. [56] BAUER SR, PARKER-AUTRY C, LU K, et al. Skeletal Muscle Health, Physical Performance, and Lower Urinary Tract Symptoms in Older Adults: The Study of Muscle, Mobility, and Aging. J Gerontol A Biol Sci Med Sci. 2024;79(6):glad218. [57] HAN P, ZHAO J, GUO Q, et al. Incidence, Risk Factors, and the Protective Effect of High Body Mass Index against Sarcopenia in Suburb-Dwelling Elderly Chinese Populations. J Nutr Health Aging, 2016;20(10):1056-1060. [58] LI C, KANG B, ZHANG T, et al. High Visceral Fat Area Attenuated the Negative Association between High Body Mass Index and Sarcopenia in Community-Dwelling Older Chinese People. Healthcare (Basel). 2020;8(4):479. [59] ZHAO X, LIU D, ZHANG H, et al. Associations of physical activity intensity, frequency, duration, and volume with the incidence of sarcopenia in middle-aged and older adults: a 4-year longitudinal study in China. BMC Geriatr. 2024;24(1):258. [60] SENIOR HE, HENWOOD TR, BELLER EM, et al. Prevalence and risk factors of sarcopenia among adults living in nursing homes. Maturitas. 2015;82(4):418-423. [61] LUO S, CHEN X, HOU L, et al. The accuracy of body mass index and calf circumference values when assessing sarcopenia in a multi-ethnic cohort of middle-aged and older adults: West China health and aging trend study results. Heliyon. 2023;9(4):e15027. [62] BARDON LA, STREICHER M, CORISH CA, et al. Predictors of Incident Malnutrition in Older Irish Adults from the Irish Longitudinal Study on Ageing Cohort-A MaNuEL study. J Gerontol A Biol Sci Med Sci. 2020;75(2): 249-256. [63] CURTIS M, SWAN L, FOX R, et al. Associations between Body Mass Index and Probable Sarcopenia in Community-Dwelling Older Adults. Nutrients. 2023;15(6):1505. [64] DíAZ-AMAYA MJ, ROSALES-ARREOLA LF, HERNáNDEZ-LICONA J, et al. Postoperative complications in the pediatric population. Malnutrition or phase angle? Which one do we use? Front Nutr. 2024;11:1474616. [65] SCHNEIDER SM, CORREIA M. Epidemiology of weight loss, malnutrition and sarcopenia: A transatlantic view. Nutrition. 2020;69: 110581. [66] AI D, DING N, WU H. The impact of sarcopenia on nutritional status in elderly patients with gastrointestinal tumors. Sci Rep. 2023;13(1):10308. [67] PRADO CM, BATSIS JA, DONINI LM, et al. Sarcopenic obesity in older adults: a clinical overview. Nat Rev Endocrinol. 2024;20(5): 261-277. [68] CEDERHOLM T, JENSEN GL, CORREIA M, et al. GLIM criteria for the diagnosis of malnutrition - A consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle. 2019;10(1): 207-217. [69] NAHMIAS BLANK D, HERMANO E, SONNENBLICK A, et al. Macrophages Upregulate Estrogen Receptor Expression in the Model of Obesity-Associated Breast Carcinoma. Cells. 2022;11(18):2844. [70] MORYA VK, SHAHID H, LANG J, et al. Advancements in Therapeutic Approaches for Degenerative Tendinopathy: Evaluating Efficacy and Challenges. Int J Mol Sci. 2024; 25(21):11846. [71] HO PY, KOH YC, LU TJ, et al. Purple Napiergrass (Pennisetum purpureum Schumach) Hot Water Extracts Ameliorate High-Fat Diet-Induced Obesity and Metabolic Disorders in Mice. J Agric Food Chem. 2023;71(51):20701-20712. |
| [1] | Zhou Jian, Zhang Tao, Zhou Weili, Zhao Xingcheng, Wang Jun, Shen Jie, Qian Li, Lu Ming. Effects of resistance training on quadriceps mass and knee joint function in patients with osteoporosis and sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1081-1088. |
| [2] | Li Guangzheng, Li Wei, Zhang Bochun, Ding Haoqin, Zhou Zhongqi, Li Gang, Liang Xuezhen. A prediction model for sarcopenia in postmenopausal women: information analysis based on the China Health and Retirement Longitudinal Study database [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 849-857. |
| [3] | Sun Jiahe, Shi Jipeng, Zhu Tianrui, Quan Helong, Xu Hongqi. Effect of exercise intervention in elderly individuals with sarcopenia and its comorbidities: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 997-1007. |
| [4] | Rong Xiangbin, , Zheng Haibo, Mo Xueshen, Hou Kun, Zeng Ping, . Plasma metabolites, immune cells, and hip osteoarthritis: causal inference based on GWAS data from European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1028-1035. |
| [5] | Yuan Weiyuan, Lei Qinhui, Li Xiuqi, Lu Tiezhu, Fu Ziwen, Liang Zhili, Ji Shaoyang, Li Yijia, Ren Yu . Therapeutic effects of adipose-derived mesenchymal stem cells and their exosomes on dexamethasone-induced sarcopenia in mice [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 58-67. |
| [6] | Chen Jiayong, Tang Meiling, Lu Jianqi, Pang Yan, Yang Shangbing, Mao Meiling, Luo Wenkuan, Lu Wei, Zhou Jiatan. Based on Mendelian randomization, the causal relationship between 1400 metabolites and sarcopenia and the correlation analysis of cardiovascular disease were investigated [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-11. |
| [7] |
Li Tian, Ren Yuhua, Gao Yanping, Su Qiang.
Mechanism of agomelatine alleviating anxiety- and depression-like behaviors in APP/PS1 transgenic mice #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1176-1182.
|
| [8] | Li Jiatong, Jin Yue, Liu Runjia, Song Bowen, Zhu Xiaoqian, Li Nianhu . Association between thyroid function levels and phenotypes associated with sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1312-1320. |
| [9] | Jiang Siqi, Huang Huanhuan, Yu Xinyu, Peng Ying, Zhou Wei, Zhao Qinghua. Meta-analysis of dose-effect of exercise on improving muscle health in community-dwelling older adults with sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6295-6304. |
| [10] | Pang Jiahui, Wang Bo, Hu Yingxuan, Hu Ziwei, Wu Wen. Association between dietary preferences and the risk of osteoarthritis in Europeans: analysis of human genome-wide association study data [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6333-6342. |
| [11] | Chen Jiayong, Tang Meiling, Lu Jianqi, Pang Yan, Yang Shangbing, Mao Meiling, Luo Wenkuan, Lu Wei. Causal association between metabolites and sarcopenia: a big data analysis of genome-wide association studies in the European population [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6369-6380. |
| [12] | Sun Yahui, Wang Yufeng, Guo Chao, Yao Junjie, Ji Yuanyuan, Li Zhongxu, Lou Huijuan, Jiang Jinglei, Sun Yiping, Xu Jing, Cong Deyu. Effect of massage on extracellular matrix collagen deposition in skeletal muscle of type 2 diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5549-5555. |
| [13] | Zhang Xingyu, Wu Dou, Zhao Enzhe, Song Xubin, Zhang Xiaolun. Treatment and repair of musculoskeletal degenerative diseases and injuries from the perspective of muscle-bone crosstalk mechanism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(24): 5179-5186. |
| [14] | Mou Limin, Li Chao, Zhang Wenhao, Shi Zhengyu, Deng Yingjie, Fang Rui. Correlation between body mass index and efficacy after medial unicompartmental knee arthroplasty in postmenopausal women [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(21): 4537-4544. |
| [15] | Huo Jiang, Ding Yu, Yuan Jie. Immune cells mediate the association between different lipids and knee osteoarthritis: a genome-wide association analysis of European individuals [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(18): 3934-3940. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||