Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (35): 7490-7498.doi: 10.12307/2025.568
Previous Articles Next Articles
Differential effects of kartogenin on chondrogenic and osteogenic differentiation of rat and rabbit bone marrow mesenchymal stem cells
Liu Chengyuan1, 2, Guo Qianping1, 2
- 1Department of Orthopedic Surgery, First Affiliated Hospital, Soochow University, Suzhou 215000, Jiangsu Province, China; 2Orthopedic Institute, Suzhou Medical College, Soochow University, Suzhou 215000, Jiangsu Province, China
-
Received:2024-09-10Accepted:2024-11-22Online:2025-12-18Published:2025-04-30 -
Contact:Guo Qianping, MS, Associate researcher, Department of Orthopedic Surgery, First Affiliated Hospital, Soochow University, Suzhou 215000, Jiangsu Province, China; Orthopedic Institute, Suzhou Medical College, Soochow University, Suzhou 215000, Jiangsu Province, China -
About author:Liu Chengyuan, MS, Department of Orthopedic Surgery, First Affiliated Hospital, Soochow University, Suzhou 215000, Jiangsu Province, China; Orthopedic Institute, Suzhou Medical College, Soochow University, Suzhou 215000, Jiangsu Province, China -
Supported by:National Natural Science Foundation of China, No. 82072424 (to GQP)
CLC Number:
Cite this article
Liu Chengyuan, Guo Qianping. Differential effects of kartogenin on chondrogenic and osteogenic differentiation of rat and rabbit bone marrow mesenchymal stem cells[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7490-7498.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
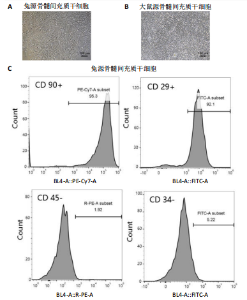
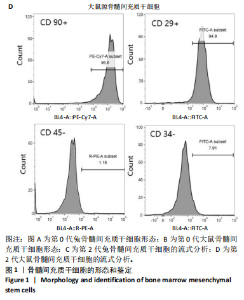
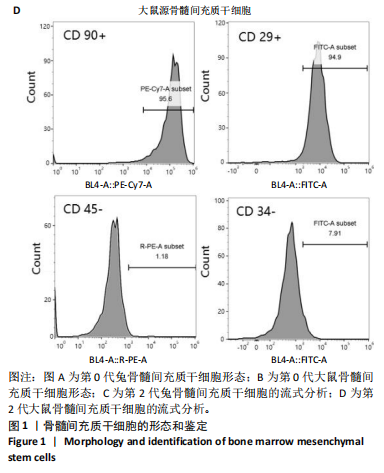
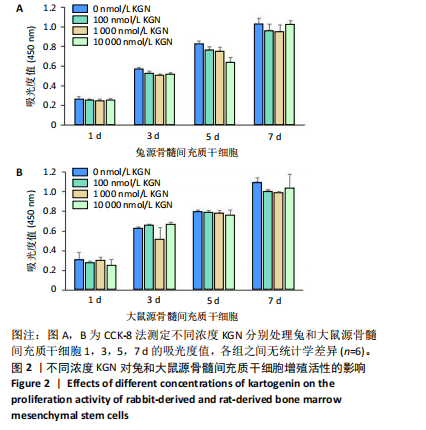
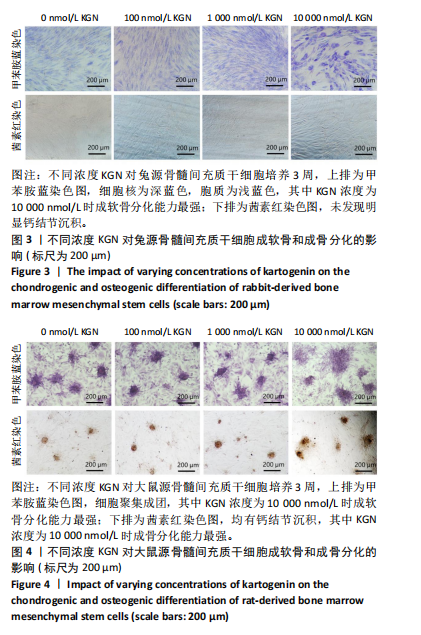
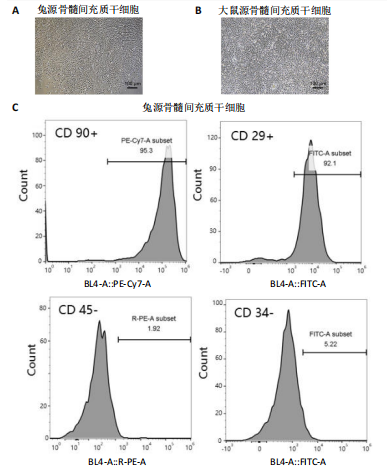
2.1 骨髓间充质干细胞的形态和流式分析结果 采用全骨髓分离贴壁法分离并培养兔和大鼠源骨髓间充质干细胞,培养5 d后,可以观察到细胞形态非常相似,兔源骨髓间充质干细胞为长梭形,大鼠源骨髓间充质干细胞为梭形,两者都表现出强烈的贴壁生长特性。此外,细胞核较大,呈椭圆形或圆形,核仁清晰可见,显示出明显的干细胞特征,见图1A,B。为了验证培养的骨髓间充质干细胞的纯度,流式细胞仪检测结果显示,第2代兔源骨髓间充质干细胞高表达特异性表面抗原CD90和CD29,阳性率分别达到95.3%和92.1%;同时,这些细胞低表达或不表达骨髓成纤维细胞、造血干细胞表面抗原CD45和CD34,阳性率分别为1.92%和5.22%。同样,鼠源骨髓间充质干细胞也高表达CD90和CD29,阳性率分别为95.6%和94.9%,而CD45和CD34低表达或不表达,阳性率分别为1.18%和7.91%,见图1C,D。 2.2 KGN对细胞增殖的影响 采用CCK-8法评估不同浓度KGN(0,100,1 000,10 000 nmol/L)对兔和大鼠源骨髓间充质干细胞增殖的影响。培养1,3,5,7 d后,各组之间的细胞增殖数量无显著统计学差异,这表明在0-10 000 nmol/L的浓度范围内,KGN对两种骨髓间充质干细胞的增殖均无明显影响,见图2。 2.3 不同浓度KGN对兔源骨髓间充质干细胞成骨和成软骨分化的影响 通过甲苯胺蓝染色和茜素红染色来表征细胞的成软骨和成骨分化情况,见图3。在甲苯胺蓝染色图像中,细胞形态为长梭形,无明显聚集现象,KGN处理组的细胞核呈深蓝色,胞质为浅蓝色。其中,10 000 nmol/L KGN组染色极为明显,表现出最显著的促成软骨分化效果。在茜素红染色图像中,各组细胞均未着色,未观察到钙结节的沉积,表明在这些条件下未出现明显的成骨分化。 2.4 不同浓度KGN对大鼠源骨髓间充质干细胞成骨和成软骨分化的影响 由图4可见,在甲苯胺蓝染色图像中,大鼠源骨髓间充质干细胞形态为梭形,具有明显的聚集现象,形成软骨小节。低浓度KGN对细胞聚集没有明显影响,但"

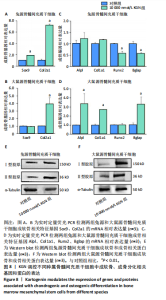
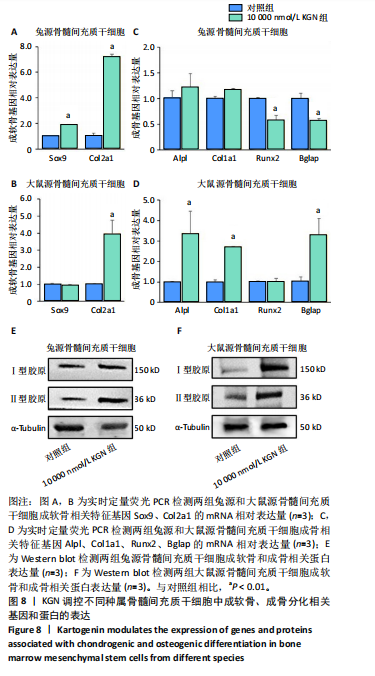
2.5 KGN在早期对兔和大鼠源骨髓间充质干细胞成骨的影响 因10 000 nmol/L KGN对兔源和大鼠源骨髓间充质干细胞的成骨分化具有不同的调控效果,进一步使用含10 000 nmol/L KGN的培养基对兔源和大鼠源骨髓间充质干细胞培养1周,通过碱性磷酸酶染色和定量分析KGN对不同种属骨髓间充质干细胞成骨早期的影响。兔源骨髓间充质干细胞对照组中碱性磷酸酶染色较弱,经KGN处理后碱性磷酸酶阳性染色进一步减弱,定量分析结果也显示出相同的趋势,见图5A,B。然而,在大鼠源骨髓间充质干细胞中,对照组表现出较强的碱性磷酸酶阳性染色,而经KGN处理后碱性磷酸酶阳性染色进一步增强,定量分析结果同样支持这一现象,见图5A,C。 2.6 KGN在晚期对兔和大鼠源骨髓间充质干细胞成骨的影响 使用含10 000 nmol/L KGN的培养基对兔源和大鼠源骨髓间充质干细胞培养3周,通过碱性磷酸酶染色和定量分析KGN对不同种属骨髓间充质干细胞成骨晚期的影响。兔源骨髓间充质干细胞的对照组和KGN组均表达碱性磷酸酶,但阳性率较低,且定量结果显示两组之间无显著性差异,见图6A,B。相反,在大鼠源骨髓间充质干细胞中,无论是对照组还是KGN组,碱性磷酸酶表达均较高,其中KGN组的阳性染色面积更大,定量结果显示碱性磷酸酶含量更高,见图6A,C。这些结果表明,与兔源骨髓间充质干细胞相比,大鼠源骨髓间充质干细胞本身表达更高的碱性磷酸酶,而在KGN作用下大鼠源骨髓间充质干细胞表现出更强的成骨潜能。 2.7 KGN对兔和大鼠源骨髓间充质干细胞的软骨诱导作用 高密度微团(Micromass)法是一种高密度细胞培养系统,能够模拟软骨组织的细胞环境,促进细胞的自然聚集和分化,从而展示软骨细胞的特征。采用Micromass培养法进一步证实KGN的软骨诱导作用,将兔源和大鼠源骨髓间充质干细胞置于含KGN的软骨诱导培养基中进行培养。经过1周的诱导处理后,番红染色结果显示,细胞中出现大量软骨细胞外基质的沉积,并伴随明显的细胞团聚现象,见图7。这些结果表明,KGN能够有效诱导兔源和大鼠源骨髓间充质干细胞分化为软骨细胞。 2.8 KGN对兔和大鼠源骨髓间充质干细胞成软骨/成骨分化基因和蛋白表达的影响 由图8可见,qRT-PCR结果显示,在KGN处理的兔源骨髓间充质干细胞中,成软骨相关基因Col2a1和Sox9 的表达显著上调;同时成骨相关基因Alpl、Col1a1、Runx2和Bglap的表达水平降低或无显著变化。Western blot结果显示,KGN可以促进兔源骨髓间充质干细胞成软骨蛋白Ⅱ型胶原的表达上调,同时成骨相关蛋白Ⅰ型胶原的表达无明显变化。这些结果表明KGN在兔源骨髓间充质干细胞中主要促进软骨分化并抑制成骨分化。相反,在大鼠源骨髓间充质干细胞中,KGN上调了成软骨相关基因 Col2a1 和成软骨相关蛋白Ⅱ型胶原的表达,同时还显著上调了成骨相关基因 Alpl、Col1a1、Bglap和成骨相关蛋白Ⅰ型胶原的表达。这些结果表明,KGN在鼠源骨髓间充质干细胞中能同时促进成软骨和成骨分化。"

| [1] HOU M, TIAN B, BAI B, et al. Dominant role of in situ native cartilage niche for determining the cartilage type regenerated by BMSCs. Bioact Mater. 2021;13:149-160. [2] WANG Y, KONG B, CHEN X, et al. BMSC exosome-enriched acellular fish scale scaffolds promote bone regeneration. J Nanobiotechnology. 2022;20(1):444. [3] JIA R, SUN T, ZHAO X, et al. DEX-Induced SREBF1 Promotes BMSCs Differentiation into Adipocytes to Attract and Protect Residual T-Cell Acute Lymphoblastic Leukemia Cells After Chemotherapy. Adv Sci (Weinh). 2023;10(19):e2205854. [4] ZHONG G, YAO J, HUANG X, et al. Injectable ECM hydrogel for delivery of BMSCs enabled full-thickness meniscus repair in an orthotopic rat model. Bioact Mater. 2020;5(4):871-879. [5] ZHANG Q, LI J, WANG C, et al. N6-Methyladenosine in Cell-Fate Determination of BMSCs: From Mechanism to Applications. Research (Wash D C). 2024;7:0340. [6] BIANCO P, ROBEY PG, SIMMONS PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2(4): 313-319. [7] LI J, MA J, FENG Q, et al. Building Osteogenic Microenvironments with a Double-Network Composite Hydrogel for Bone Repair. Research (Wash D C). 2023;6:0021. [8] LIU J, TANG C, HUANG J, et al. Nanofiber Composite Microchannel-Containing Injectable Hydrogels for Cartilage Tissue Regeneration. Adv Healthc Mater. 2023;12(31):e2302293. [9] WANG L, GUAN C, ZHANG T, et al. Comparative effect of skeletal stem cells versus bone marrow mesenchymal stem cells on rotator cuff tendon-bone healing. J Orthop Translat. 2024;47:87-96. [10] LIU F, WU M, WU X, et al. TGM2 accelerates migration and differentiation of BMSCs by activating Wnt/β-catenin signaling. J Orthop Surg Res. 2023;18(1):168. [11] XU X, LIANG Y, LI X, et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021;269:120539. [12] SHIMOYAMA M, SMITH JR, BRYDA E, et al. Rat Genome and Model Resources. ILAR J. 2017;58(1):42-58. [13] DENG S, ZHU F, DAI K, et al. Harvest of functional mesenchymal stem cells derived from in vivo osteo-organoids. Biomater Transl. 2023;4(4):270-279. [14] WANG X, MA Y, CHEN J, et al. A novel decellularized matrix of Wnt signaling-activated osteocytes accelerates the repair of critical-sized parietal bone defects with osteoclastogenesis, angiogenesis, and neurogenesis. Bioact Mater. 2022;21:110-128. [15] QIN W, GAO J, YAN J, et al. Microarray analysis of signalling interactions between inflammation and angiogenesis in subchondral bone in temporomandibular joint osteoarthritis. Biomater Transl. 2024;5(2):175-184. [16] GUO J, WANG F, HU Y, et al. Exosome-based bone-targeting drug delivery alleviates impaired osteoblastic bone formation and bone loss in inflammatory bowel diseases. Cell Rep Med. 2023;4(1):100881. [17] KANG N, LIU X, GUAN Y, et al. Effects of co-culturing BMSCs and auricular chondrocytes on the elastic modulus and hypertrophy of tissue engineered cartilage. Biomaterials. 2012;33(18):4535-4544. [18] CAI JY, ZHANG L, CHEN J, et al. Kartogenin and Its Application in Regenerative Medicine. Curr Med Sci. 2019;39(1):16-20. [19] TIAN X, ZHANG Y, SHEN L, et al. Kartogenin-enhanced dynamic hydrogel ameliorates intervertebral disc degeneration via restoration of local redox homeostasis. J Orthop Translat. 2023;42:15-30. [20] LIU G, GUO Q, LIU C, et al. Cytomodulin-10 modified GelMA hydrogel with kartogenin for in-situ osteochondral regeneration. Acta Biomater. 2023;169:317-333. [21] ZHANG J, WANG JH. Kartogenin induces cartilage-like tissue formation in tendon-bone junction. Bone Res. 2014;2:14008. [22] WANG Y, CHEN G, YAN J, et al. Upregulation of SIRT1 by Kartogenin Enhances Antioxidant Functions and Promotes Osteogenesis in Human Mesenchymal Stem Cells. Oxid Med Cell Longev. 2018; 2018:1368142. [23] JOHNSON K, ZHU S, TREMBLAY MS, et al. A stem cell-based approach to cartilage repair. Science. 2012;336(6082):717-721. [24] ZHANG S, HU P, LIU T, et al. Kartogenin hydrolysis product 4-aminobiphenyl distributes to cartilage and mediates cartilage regeneration. Theranostics. 2019;9(24):7108-7121. [25] WANG H, PENG T, WU H, et al. In situ biomimetic lyotropic liquid crystal gel for full-thickness cartilage defect regeneration. J Control Release. 2021;338:623-632. [26] WEI J, RAN P, LI Q, et al. Hierarchically structured injectable hydrogels with loaded cell spheroids for cartilage repairing and osteoarthritis treatment. Chem Eng J. 2022;430:132211. [27] CHEN YR, YAN X, YUAN FZ, et al. Kartogenin-Conjugated Double-Network Hydrogel Combined with Stem Cell Transplantation and Tracing for Cartilage Repair. Adv Sci (Weinh). 2022;9(35):e2105571. [28] JANG Y, JUNG H, NAM Y, et al. Centrifugal gravity-induced BMP4 induces chondrogenic differentiation of adipose-derived stem cells via SOX9 upregulation. Stem Cell Res Ther. 2016;7(1):184. [29] ZHANG W, XUE W, JIA Z, et al. Photo-driven dynamic hydrogel modulates bone marrow mesenchymal stem cells behavior for enhanced cartilage regeneration. Chem Eng J. 2024;484:149689. [30] XUE JX, GONG YY, ZHOU GD, et al. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells induced by acellular cartilage sheets. Biomaterials. 2012;33(24):5832-5840. [31] CAO L, YANG F, LIU G, et al. The promotion of cartilage defect repair using adenovirus mediated Sox9 gene transfer of rabbit bone marrow mesenchymal stem cells. Biomaterials. 2011;32(16):3910-3920. [32] DUFFY DJ, KRSTIC A, HALASZ M, et al. Retinoic acid and TGF-β signalling cooperate to overcome MYCN-induced retinoid resistance. Genome Med. 2017;9(1):15. [33] LEFEBVRE V, BEHRINGER RR, DE CROMBRUGGHE B. L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. Osteoarthritis Cartilage. 2001;9 Suppl A:S69-75. [34] TUAN RS, BOLAND G, TULI R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5(1):32-45. [35] WEHRLI BM, HUANG W, DE CROMBRUGGHE B, et al. Sox9, a master regulator of chondrogenesis, distinguishes mesenchymal chondrosarcoma from other small blue round cell tumors. Hum Pathol. 2003;34(3):263-269. [36] VAN GASTEL N, STEGEN S, EELEN G, et al. Lipid availability determines fate of skeletal progenitor cells via SOX9. Nature. 2020;579(7797): 111-117. [37] SHI H, ZHOU K, WANG M, et al. Integrating physicomechanical and biological strategies for BTE: biomaterials-induced osteogenic differentiation of MSCs. Theranostics. 2023;13(10):3245-3275. [38] BAHARLOU HOUREH A, MASAELI E, NASR-ESFAHANI MH. Chitosan/polycaprolactone multilayer hydrogel: A sustained Kartogenin delivery model for cartilage regeneration. Int J Biol Macromol. 2021;177:589-600. [39] YAN H, YU T, LI J, et al. Kartogenin Improves Osteogenesis of Bone Marrow Mesenchymal Stem Cells via Autophagy. Stem Cells Int. 2022; 2022:1278921. [40] MENG X, LI L, HUANG C, et al. Anti-inflammatory and anabolic biphasic scaffold facilitates osteochondral tissue regeneration in osteoarthritic joints. J Mater Sci Mater Med. 2023;156:20-31. [41] 田鑫,刘滔,杨惠林,等.甲基丙烯化明胶微球缓释Kartogenin修复髓核退变的体外评估[J].中国组织工程研究,2024,28(5):724-730. |
| [1] | Chi Wenxin, Zhang Cunxin, Gao Kai, Lyu Chaoliang, Zhang Kefeng. Mechanism by which nobiletin inhibits inflammatory response of BV2 microglia [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1321-1327. |
| [2] | Yang Zhihang, Sun Zuyan, Huang Wenliang, Wan Yu, Chen Shida, Deng Jiang. Nerve growth factor promotes chondrogenic differentiation and inhibits hypertrophic differentiation of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1336-1342. |
| [3] | Hu Taotao, Liu Bing, Chen Cheng, Yin Zongyin, Kan Daohong, Ni Jie, Ye Lingxiao, Zheng Xiangbing, Yan Min, Zou Yong. Human amniotic mesenchymal stem cells overexpressing neuregulin-1 promote skin wound healing in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1343-1349. |
| [4] | Liu Qi, Li Linzhen, Li Yusheng, Jiao Hongzhuo, Yang Cheng, Zhang Juntao. Icariin-containing serum promotes chondrocyte proliferation and chondrogenic differentiation of stem cells in the co-culture system of three kinds of cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1371-1379. |
| [5] | Aikepaer · Aierken, Chen Xiaotao, Wufanbieke · Baheti. Osteogenesis-induced exosomes derived from human periodontal ligament stem cells promote osteogenic differentiation of human periodontal ligament stem cells in an inflammatory microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1388-1394. |
| [6] | Li Jialin, Zhang Yaodong, Lou Yanru, Yu Yang, Yang Rui. Molecular mechanisms underlying role of mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1512-1522. |
| [7] | Sun Yuting, Wu Jiayuan, Zhang Jian. Physical factors and action mechanisms affecting osteogenic/odontogenic differentiation of dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1531-1540. |
| [8] | Ge Xiao, Zhao Zhuangzhuang, Guo Shuyu, Xu Rongyao. HOXA10 gene-modified bone marrow mesenchymal stem cells promote bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7701-7708. |
| [9] | Ma Weibang, Xu Zhe, Yu Qiao, Ouyang Dong, Zhang Ruguo, Luo Wei, Xie Yangjiang, Liu Chen. Screening and cytological validation of cartilage degeneration-related genes in exosomes from osteoarthritis synovial fluid [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7783-7789. |
| [10] | Fang Yuan, Qian Zhiyong, He Yuanhada, Wang Haiyan, Sha Lirong, Li Xiaohe, Liu Jing, He Yachao, Zhang Kai, Temribagen. Mechanism of Mongolian medicine Echinops sphaerocephalus L. in proliferation and angiogenesis of vascular endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7519-7528. |
| [11] | Shi Tongtong, Deng Rongxia, Zhang Jianguang. Differences in physicochemical properties and collagen secretion stimulation of natural and synthetic hydroxyapatite particles [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7278-7285. |
| [12] | Yin Hang, Song Kui. Effect of crocin hydrogel on chondrocytes and MC3T3-E1 cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7293-7300. |
| [13] | Yi Xiaoding, Zhang Di, Guo Hong, Qing Liang, Zhao Tianyu. Decellularized tendon scaffold: a biomedical material for tendon injury repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7385-7392. |
| [14] | Tang Haoxu, Liang Yingjie, Li Ce, Ding Penglin, Qian Minlong, Yuan Lingli. Deferoxamine alleviates the inhibitory effect of glucocorticoids on osteogenic differentiation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6821-6827. |
| [15] | Wang Zhaoyan, Wang Qian, Liu Weipeng, Yang Hui, Luan Zuo, Qu Suqing. Effect of fibronectin on differentiation of human neural stem cells into oligodendrocyte precursor cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6661-6666. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||