Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (35): 7481-7489.doi: 10.12307/2025.991
Asperosaponin VI promotes osteogenic differentiation of MC3T3-E1 cells under hypoxia environment #br#
#br#
Li Yunzhe1, 2, Niu Zefan1, 2, Wang Zirou2, Ai Chongyi2, Chen Gang1, Wang Xinxing2
- 1College of Stomatology, Tianjin Medical University, Tianjin 300041, China; 2Institute of Military Medical Research, Academy of Military Medical Sciences, Beijing 100000, China
-
Received:2024-11-18Accepted:2024-12-31Online:2025-12-18Published:2025-04-30 -
Contact:Chen Gang, PhD, Professor, College of Stomatology, Tianjin Medical University, Tianjin 300041, China Co-corresponding author: Wang Xinxing, PhD, Professor, Institute of Military Medical Research, Academy of Military Medical Sciences, Beijing 100000, China -
About author:Li Yunzhe, Master candidate, College of Stomatology, Tianjin Medical University, Tianjin 300041, China; Institute of Military Medical Research, Academy of Military Medical Sciences, Beijing 100000, China
CLC Number:
Cite this article
Li Yunzhe, Niu Zefan, Wang Zirou, Ai Chongyi, Chen Gang, Wang Xinxing. Asperosaponin VI promotes osteogenic differentiation of MC3T3-E1 cells under hypoxia environment #br#
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

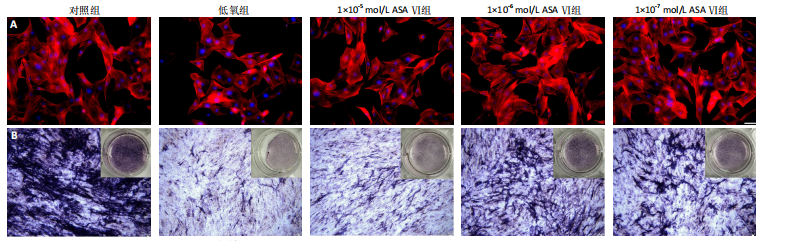
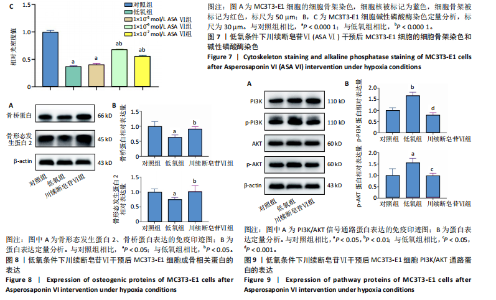
2.1 川续断皂苷Ⅵ对低氧条件下MC3T3-E1细胞增殖和凋亡的影响 如图1所示,与对照组相比,低氧处理24 h后低氧组细胞活力降低,加入1×10-5,1×10-6,1×10-7,1×10-8 mol/L川续断皂苷Ⅵ均可使低氧状态下细胞活力上升,促进细胞增殖,其中1×10-6 mol/L组效果最佳。EdU染色结果见图2,与对照组相比,低氧组细胞增殖受到抑制,EdU染色细胞阳性率明显下降;与低氧组相比,1×10-6mol/L川续断皂苷Ⅵ可以增强MC3T3-E1细胞增殖能力,EdU染色细胞阳性率最高。TUNEL原位荧光染色法检测MC3T3-E1细胞凋亡情况,如图3所示,倒置荧光显微镜下对照组几乎未见绿色荧光标记的凋亡细胞,与对照组相比,低氧组凋亡细胞明显增多;与低氧组相比,1×10-6 mol/L川续断皂苷Ⅵ组凋亡细胞明显减少。Western blot结果也显示,在低氧条件下Bcl-2蛋白表达下降;与低氧组相比,川续断皂苷Ⅵ组Bcl-2蛋白表达明显升高,见图4。 2.2 川续断皂苷Ⅵ对低氧条件下MC3T3-E1细胞内活性氧的影响 采用DCFH-DA 探针来检测低氧条件下各组MC3T3-E1细胞活性氧水平。DCFH-DA本身没有荧光,可以自由穿过细胞膜,进入细胞内后可以被细胞内的酯酶水解生成DCFH,细胞内的活性氧可以氧化无荧光的DCFH生成有荧光的DCF,通过流式细胞仪检测DCF的荧光,反映细胞内活性氧水平。由图5可见,与对照组相比,低氧组活性氧水平明显升高,说明低氧条件会导致细胞氧化应激;与低氧组相比,川续断皂苷Ⅵ浓度为1×10-6,1×10-7 mol/L组细胞内活性氧水平显著降低。 2.3 低氧条件下MC3T3-E1细胞在川续断皂苷Ⅵ处理后细胞周期的分布情况 使用流式细胞仪检测MC3T3-E1细胞的细胞周期分布。在G1期,低氧组和1×10-5 mol/L川续断皂苷Ⅵ组的细胞比例明显增多,低氧条件阻滞了细胞向S期的进展;在S期,低氧组细胞比例明显低于对照组,而经过不同浓度川续断皂苷Ⅵ干预后,S期细胞比例高于低氧组,其中川续断皂苷Ⅵ浓度为1×10-6 mol/L组细胞比例高于对照组,说明低氧条件下1×10-6 mol/L川续断皂苷Ⅵ显著促进MC3T3-E1细胞由G1期进入S期,见图6。 2.4 川续断皂苷Ⅵ对低氧条件下MC3T3-E1细胞成骨分化后细胞形态的影响 成骨诱导7 d后,倒置荧光显微镜下观察可见细胞骨架被红色荧光标记,细胞核被蓝色荧光标记,如图7A所示,对照组细胞分布密集且形态伸展,呈梭形,有较多树枝状突起;与对照组相比,低氧组细胞分布分散,伪足伸展不明显,细胞表面相对更平滑;与低氧组相比,1×10-6 mol/L川续断皂苷Ⅵ组细胞呈星状伸展,细胞突起明显,可以清晰地观察到细胞骨架呈丝状排列,形态更接近对照组。 2.5 川续断皂苷Ⅵ对低氧条件下MC3T3-E1细胞碱性磷酸酶染色的影响 成骨诱导7 d后,碱性磷酸酶染色结果显示,与对照组相比,低氧组碱性磷酸酶染色面积较小,颜色较浅;与低氧组相比,1×10-5 mol/L川续断皂苷Ⅵ组成骨效果不明显,1×10-6 mol/L川续断皂苷Ⅵ组染色面积较大,染色程度明显变深,成骨效果更好,见图7B,C。 2.6 川续断皂苷Ⅵ对低氧条件中MC3T3-E1细胞成骨分化的影响及潜在机制 Western blot检测结果显示,与对照组比较,低氧组骨形态发生蛋白2、骨桥蛋白表达降低;与低氧组相比,川续断皂苷Ⅵ组骨形态发生蛋白2、骨桥蛋白表达增加,见图8。PI3K/AKT信号通路是细胞生长与代谢的一个关键途径,参与增殖、分化、凋亡等多种细胞功能的调节,由图9可见,与对照组相比,低氧组p-PI3K、p-AKT蛋白表达明显上调;与低氧组相比,川续断皂苷Ⅵ组p-PI3K、p-AKT蛋白表达明显下调。各组PI3K、AKT总蛋白表达无明显差异。"

| [1] SARKAR SK, LEE BT. Hard tissue regeneration using bone substitutes: an update on innovations in materials. Korean J Intern Med. 2015; 30(3):279-293. [2] 熊伟,袁灵梅,钱国文,等. “补肾壮骨”中药应用于骨组织工程支架修复节段性骨缺损[J].中国组织工程研究,2023,27(21):3438-3444. [3] GORDILLO GM, SEN CK. Revisiting the essential role of oxygen in wound healing. Am J Surg. 2003;186(3):259-263. [4] FOLCO EJ, SUKHOVA GK, QUILLARD T, et al. Moderate hypoxia potentiates interleukin-1β production in activated human macrophages. Circ Res. 2014;115(10):875-883. [5] HU Z, WANG F, WU Z, et al. FOXO3a-dependent up-regulation of Mxi1-0 promotes hypoxia-induced apoptosis in endothelial cells. Cell Signal. 2018;51:233-242. [6] 武成,郑伟伟,杨民.氧环境对成骨细胞活性的影响[J].中华医学杂志,2017,97(3):217-222. [7] XUE JF, SHI ZM, ZOU J, et al. Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy of articular chondrocytes and attenuates inflammatory response in rats with osteoarthritis. Biomed Pharmacother. 2017;89:1252-1261. [8] MIRICESCU D, BALAN DG, TULIN A, et al. PI3K/AKT/mTOR signalling pathway involvement in renal cell carcinoma pathogenesis (Review). Exp Ther Med. 2021;21(5):540. [9] DING X, LI W, CHEN D, et al. Asperosaponin VI stimulates osteogenic differentiation of rat adipose-derived stem cells. Regen Ther. 2019; 11:17-24. [10] GU M, JIN J, REN C, et al. Akebia Saponin D suppresses inflammation in chondrocytes via the NRF2/HO-1/NF-κB axis and ameliorates osteoarthritis in mice. Food Funct. 2020;11(12):10852-10863. [11] VIOZZI CF. Maxillofacial and Mandibular Fractures in Sports. Clin Sports Med. 2017;36(2):355-368. [12] HO-SHUI-LING A, BOLANDER J, RUSTOM LE, et al. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials. 2018;180:143-162. [13] LIU J, RUAN J, WEIR MD, et al. Periodontal Bone-Ligament-Cementum Regeneration via Scaffolds and Stem Cells. Cells. 2019;8(6):537. [14] MOSIER KM. Lesions of the Jaw. Semin Ultrasound CT MR. 2015;36(5): 444-450. [15] ZHANG Q, WU W, QIAN C, et al. Advanced biomaterials for repairing and reconstruction of mandibular defects. Mater Sci Eng C Mater Biol Appl. 2019;103:109858. [16] 申琳,王杰,徐桂军,等.应用支架植入递送局部药物在治疗骨质疏松骨缺损的研究进展[J].中国中西医结合外科杂志,2023, 29(3):403-406. [17] SCARPA ES, ANTONELLI A, BALERCIA G, et al. Antioxidant, Anti-Inflammatory, Anti-Diabetic, and Pro-Osteogenic Activities of Polyphenols for the Treatment of Two Different Chronic Diseases: Type 2 Diabetes Mellitus and Osteoporosis. Biomolecules. 2024;14(7):836. [18] YU Y, FU D, ZHOU H, et al. Potential application of Atractylodes macrocephala Koidz. as a natural drug for bone mass regulation: A review. J Ethnopharmacol. 2023;315:116718. [19] GIORDANI C, MATACCHIONE G, GIULIANI A, et al. Pro-Osteogenic and Anti-Inflammatory Synergistic Effect of Orthosilicic Acid, Vitamin K2, Curcumin, Polydatin and Quercetin Combination in Young and Senescent Bone Marrow-Derived Mesenchymal Stromal Cells. Int J Mol Sci. 2023;24(10):8820. [20] GUPTA A, MEHTA SK, KUMAR A, et al. Advent of phytobiologics and nano-interventions for bone remodeling: a comprehensive review. Crit Rev Biotechnol. 2023;43(1):142-169. [21] WOJDASIEWICZ P, BRODACKI S, CIEŚLICKA E, et al. Salidroside: A Promising Agent in Bone Metabolism Modulation. Nutrients. 2024; 16(15):2387. [22] HUANG J, YUAN L, WANG X, et al. Icaritin and its glycosides enhance osteoblastic, but suppress osteoclastic, differentiation and activity in vitro. Life Sci. 2007;81(10):832-840. [23] GAO ZR, FENG YZ, ZHAO YQ, et al. Traditional Chinese medicine promotes bone regeneration in bone tissue engineering. Chin Med. 2022;17(1):86. [24] 杨斌,王楠,谭睿,等.中药及其单体对骨髓间充质干细胞诱导分化的研究进展[J].中草药,2022,53(24):7915-7924. [25] TAO Y, CHEN L, YAN J. Traditional uses, processing methods, phytochemistry, pharmacology and quality control of Dipsacus asper Wall. ex C.B. Clarke: A review. J Ethnopharmacol. 2020;258:112912. [26] 黄媛,徐艳,易学良,等.川续断皂苷Ⅵ通过JNK信号通路促进骨髓间充质干细胞成骨分化 [J].广州中医药大学学报, 2018, 35(5): 887-893. [27] NIU Y, LI Y, HUANG H, et al. Asperosaponin VI, a saponin component from Dipsacus asper wall, induces osteoblast differentiation through bone morphogenetic protein-2/p38 and extracellular signal-regulated kinase 1/2 pathway. Phytother Res. 2011;25(11):1700-1706. [28] WANG CG, LOU YT, TONG MJ, et al. Asperosaponin VI promotes angiogenesis and accelerates wound healing in rats via up-regulating HIF-1α/VEGF signaling. Acta Pharmacol Sin. 2018;39(3):393-404. [29] LIU J, PEI C, JIA N, et al. Preconditioning with Ginsenoside Rg3 mitigates cardiac injury induced by high-altitude hypobaric hypoxia exposure in mice by suppressing ferroptosis through inhibition of the RhoA/ROCK signaling pathway. J Ethnopharmacol. 2025;337(Pt 2):118861. [30] CHEN R, SONG C, QIU J, et al. Exploring the potential mechanism of Taohong Siwu decoction in the treatment of avascular necrosis of the femoral head based on network pharmacology and molecular docking. Medicine (Baltimore). 2023;102(50):e35312. [31] HAN J, CHAI Y, ZHANG XY, et al. Gujiansan Ameliorates Avascular Necrosis of the Femoral Head by Regulating Autophagy via the HIF-1α/BNIP3 Pathway. Evid Based Complement Alternat Med. 2021; 2021:6683007. [32] MA L, LIU X, ZHANG M, et al. Paeoniflorin alleviates ischemia/reperfusion induced acute kidney injury by inhibiting Slc7a11-mediated ferroptosis. Int Immunopharmacol. 2023;116:109754. [33] SEN CK. Wound healing essentials: let there be oxygen. Wound Repair Regen. 2009;17(1):1-18. [34] PRABHAKAR NR, KUMAR GK, NANDURI J, et al. ROS signaling in systemic and cellular responses to chronic intermittent hypoxia. Antioxid Redox Signal. 2007;9(9):1397-1403. [35] MALDA J, KLEIN TJ, UPTON Z. The roles of hypoxia in the in vitro engineering of tissues. Tissue Eng. 2007;13(9):2153-2162. [36] CAMACHO-CARDENOSA M, CAMACHO-CARDENOSA A, TIMÓN R, et al. Can Hypoxic Conditioning Improve Bone Metabolism? A Systematic Review. Int J Environ Res Public Health. 2019;16(10):1799. [37] YANG X, LIANG J, SHU Y, et al. Asperosaponin VI facilitates the regeneration of skeletal muscle injury by suppressing GSK-3β-mediated cell apoptosis. J Cell Biochem. 2024;125(1):115-126. [38] LIU K, LIU Y, XU Y, et al. Asperosaponin VI protects against bone destructions in collagen induced arthritis by inhibiting osteoclastogenesis. Phytomedicine. 2019;63:153006. [39] FRUMAN DA, CHIU H, HOPKINS BD, et al. The PI3K Pathway in Human Disease. Cell. 2017;170(4):605-635. [40] MANNING BD, TOKER A. AKT/PKB Signaling: Navigating the Network. Cell. 2017;169(3):381-405. [41] ZHANG Z, YAO L, YANG J, et al. PI3K/Akt and HIF‑1 signaling pathway in hypoxia‑ischemia (Review). Mol Med Rep. 2018;18(4):3547-3554. [42] 田启会,张亮,龙亚丽.黄芪影响缺氧微环境中骨髓间充质干细胞增殖活性的PI3K-AKT信号通路分析[J].畜牧兽医学报,2024,55(1): 346-354. [43] 宋晨阳,孟庆良.基于PI3K/AKT信号通路探讨补肾壮骨汤对骨关节炎防治作用的实验研究[J].陕西中医,2024,45(10):1311-1314+1319. [44] ZHANG LY, ZHANG K, ZHAO X, et al. Fetal hypoxia exposure induces Hif1a activation and autophagy in adult ovarian granulosa cells†. Biol Reprod. 2024;111(6):1220-1234. [45] KRATCHMAROVA I, BLAGOEV B, HAACK-SORENSEN M, et al. Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science. 2005;308(5727):1472-1477. |
| [1] | Yu Shuai, Liu Jiawei, Zhu Bin, Pan Tan, Li Xinglong, Sun Guangfeng, Yu Haiyang, Ding Ya, Wang Hongliang. Hot issues and application prospects of small molecule drugs in treatment of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1913-1922. |
| [2] | Yin Lu, Jiang Chuanfeng, Chen Junjie, Yi Ming, Wang Zihe, Shi Houyin, Wang Guoyou, Shen Huarui. Effect of Complanatoside A on the apoptosis of articular chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1541-1547. |
| [3] | Li Huayuan, Li Chun, Liu Junwei, Wang Ting, Li Long, Wu Yongli. Effect of warm acupuncture on PINK1/Parkin pathway in the skeletal muscle of rats with chronic fatigue syndrome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1618-1625. |
| [4] | Wang Qiuyue, Jin Pan, Pu Rui . Exercise intervention and the role of pyroptosis in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1667-1675. |
| [5] | Aikepaer · Aierken, Chen Xiaotao, Wufanbieke · Baheti. Osteogenesis-induced exosomes derived from human periodontal ligament stem cells promote osteogenic differentiation of human periodontal ligament stem cells in an inflammatory microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1388-1394. |
| [6] | Zhang Haojun, Li Hongyi, Zhang Hui, Chen Haoran, Zhang Lizhong, Geng Jie, Hou Chuandong, Yu Qi, He Peifeng, Jia Jinpeng, Lu Xuechun. Identification and drug sensitivity analysis of key molecular markers in mesenchymal cell-derived osteosarcoma [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1448-1456. |
| [7] | Sun Yuting, Wu Jiayuan, Zhang Jian. Physical factors and action mechanisms affecting osteogenic/odontogenic differentiation of dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1531-1540. |
| [8] | Yu Ting, Lyu Dongmei, Deng Hao, Sun Tao, Cheng Qian. Icariin pretreatment enhances effect of human periodontal stem cells on M1-type macrophages [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1328-1335. |
| [9] | Zhao Ruihua, Chen Sixian, Guo Yang, Shi Lei, Wu Chengjie, Wu Mao, Yang Guanglu, Zhang Haoheng, Ma Yong. Wen-Shen-Tong-Du Decoction promoting spinal cord injury repair in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1118-1126. |
| [10] | Zheng Lin, Jin Wenjun, Luo Shanshan, Huang Rui, Wang Jie, Cheng Yuting, An Zheqing, Xiong Yue, Gong Zipeng, Liao Jian. Eucommia ulmoides promotes alveolar bone formation in ovariectomized rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1159-1167. |
| [11] | Zhang Debao, Wang Peng, Li Kun, Zhang Shaojie, Li Zhijun, Li Shuwen, Wu Yimin. Epidural fibrous scar formation in rabbits following autologous ligamentum flavum intervention [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1168-1175. |
| [12] | Ji Huihui, Jiang Xu, Zhang Zhimin, Xing Yunhong, Wang Liangliang, Li Na, Song Yuting, Luo Xuguang, Cui Huilin, Cao Ximei. SR9009 combined with indolepropionic acid alleviates inflammation in C2C12 myoblasts through the nuclear factor-kappa B signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1220-1229. |
| [13] | He Bo, Chen Wen, Ma Suilu, He Zhijun, Song Yuan, Li Jinpeng, Liu Tao, Wei Xiaotao, Wang Weiwei, Xie Jing . Pathogenesis and treatment progress of flap ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1230-1238. |
| [14] | Zhang Wenhua, Li Xun, Zhang Weichao, Li Xinying, Ma Guoao, Wang Xiaoqiang . Promoting myogenesis based on the SphK1/S1P/S1PR2 signaling pathway: a new perspective on improving skeletal muscle health through exercise [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1265-1275. |
| [15] | Lan Shuangli, Xiang Feifan, Deng Guanghui, Xiao Yukun, Yang Yunkang, Liang Jie. Naringin inhibits iron deposition and cell apoptosis in bone tissue of osteoporotic rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 888-898. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||