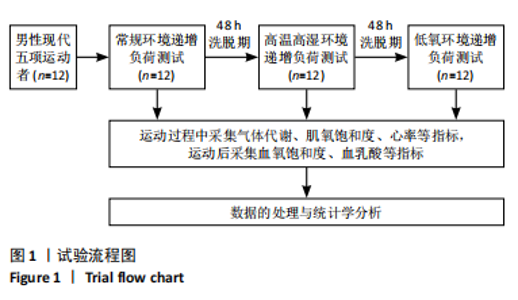
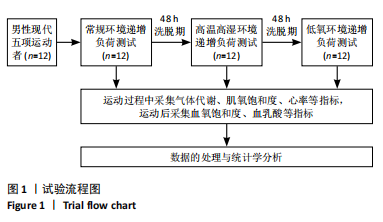
Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (32): 6866-6876.doi: 10.12307/2025.783
Previous Articles Next Articles
Difference of energy metabolism and skeletal muscle oxygenation in athletes under high temperature, high humidity and low oxygen environment
Geng Zhizhong1, Wang Jinhao2, Cao Guohuan2, Tan Chenhao2, Li Longji1, Qiu Jun2
- 1Shanghai Sports of University, Shanghai 200438, China; 2Shanghai Research Institute of Sports Science, Shanghai 200030, China
-
Received:2024-09-10Accepted:2024-10-31Online:2025-11-18Published:2025-04-25 -
Contact:Qiu Jun, PhD, Researcher, Shanghai Research Institute of Sports Science, Shanghai 200030, China -
About author:Geng Zhizhong, Doctoral candidate, Shanghai Sports of University, Shanghai 200438, China -
Supported by:Shanghai Science and Technology Commission Project, No. 22dz1204601 (to QJ)
CLC Number:
Cite this article
Geng Zhizhong, Wang Jinhao, Cao Guohuan, Tan Chenhao, Li Longji, Qiu Jun. Difference of energy metabolism and skeletal muscle oxygenation in athletes under high temperature, high humidity and low oxygen environment[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6866-6876.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
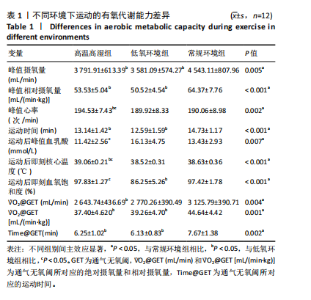
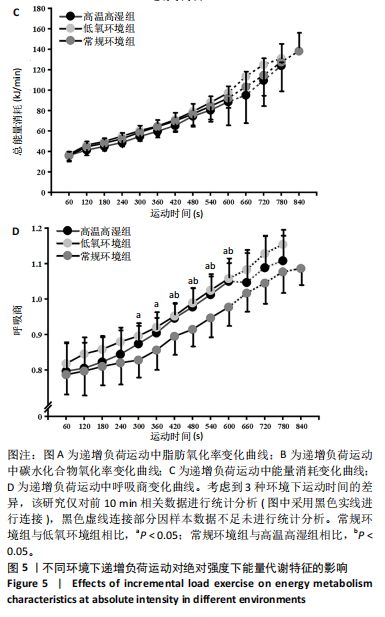
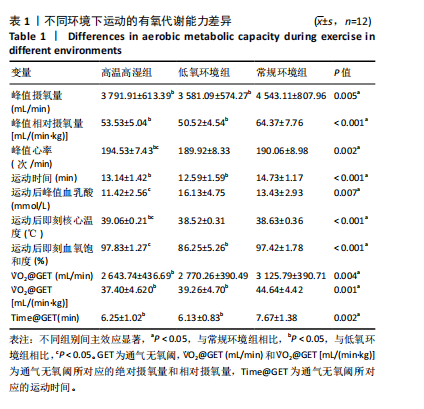
2.3 有氧代谢能力 运动者在不同环境中递增负荷运动时绝对V?O2peak存在主效应显著[F(2,22)=6.832,P=0.005,ηp2=0.383],与常规环境组相比,高温高湿组[P=0.038,95%CI(47.983,1 876.067)]、低氧环境组[P=0.012,95%CI (46.351,375.302)]的V?O2peak降低。相对V?O2peak存在主效应显著[F(2,22)=17.161,P < 0.001,ηp2=0.609],与常规环境组相比,高温高湿组[P=0.012,95%CI(1.231,20.459)]、低氧环境组[P=0.002,95%CI(4.282,23.423)]的V?O2peak降低。运动时间存在主效应显著[F(2,22)=10.971,P < 0.001,ηp2=0.499],与常规环境组相比,高温高湿组[P=0.040,95%CI(0.064,3.105)]与低氧环境组[P=0.009,95%CI (0.544,3.720)]的运动时间均出现降低。运动后血乳酸峰值浓度存在主效应[F(1.583,17.416)=7.383,P=0.007,ηp2=0.402],与高温高湿组相比,低氧环境组的血乳酸峰值浓度升高[P=0.025,95%CI (0.015,9.402 )]。运动后即刻核心温度存在主效应显著[F(1.842,20.259)=14.663,P < 0.001,ηp2=0.571],与高温高湿组相比,低氧环境组[P=0.012,95%CI(0.500,0.813)]、常规环境组[P < 0.001,95%CI(0.230,0.838)]的核心温度降低。峰值心率存在主效应显著[F(2,22)=8.044,P=0.002,ηp2=0.422],与高温高湿组相比,低氧环境组[P=0.039,95%CI(0.217,9.117)]、常规环境组[P=0.010,95%CI(1.099,7.958)]的峰值心率降低。运动后即刻血氧饱和度存在主效应显著[F(2,22)=49.023,P < 0.001,ηp2=0.817],与低氧环境组相比,高温高湿组[P < 0.001,95%CI (6.062,17.104)]与常规环境组[P < 0.001,95%CI(6.880,15.453)]运动后即刻血氧饱和度升高。GET时刻对应绝对V?O2存在主效应显著[F(1.766,19.421)=7.285,P=0.004,ηp2=0. 398],与常规环境组相比,高温高湿组的GET时刻对应绝对V?O2出现降低[P=0.004,95%CI(165.044,799.057)]。同时GET时刻对应相对V?O2存在主效应显著[F(1.743,19.170)=9.805,P=0.002,ηp2=0.471],与常规环境组相比,高温高湿组[P=0.001,95%CI(3.484,11.090)]与低氧环境组[P=0.049,95%CI(0.027,10.807)]的GET时刻对应相对V?O2均出现降低。GET时刻所对应的运动时间存在主效应显著[F(2,22)=8.278,P=0.002,ηp2=0. 429],与常规环境组相比,高温高湿组[P=0.017,95%CI(0.249,2.586)]与低氧环境组[P=0.026,95%CI(0.181,2.904)]的GET时刻所对应的运动时间出现降低。见表1。"
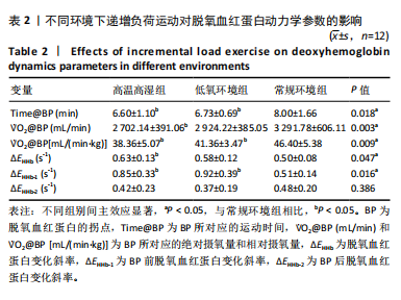
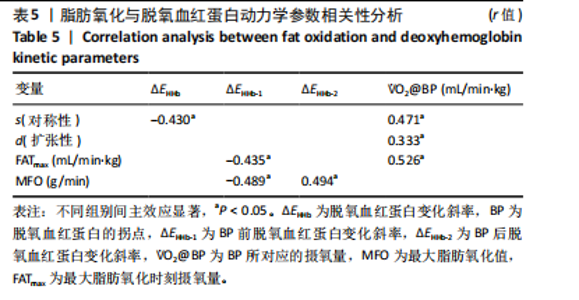
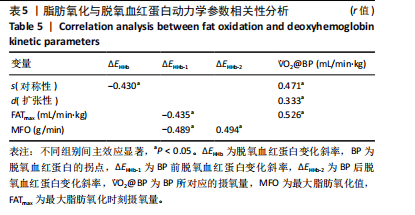
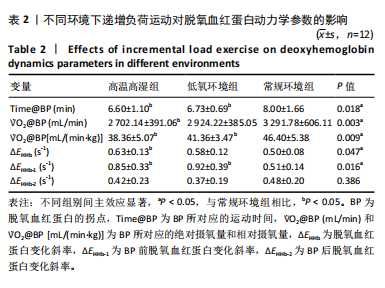
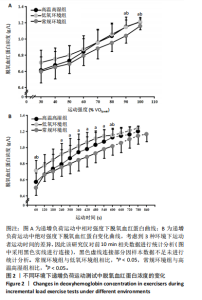
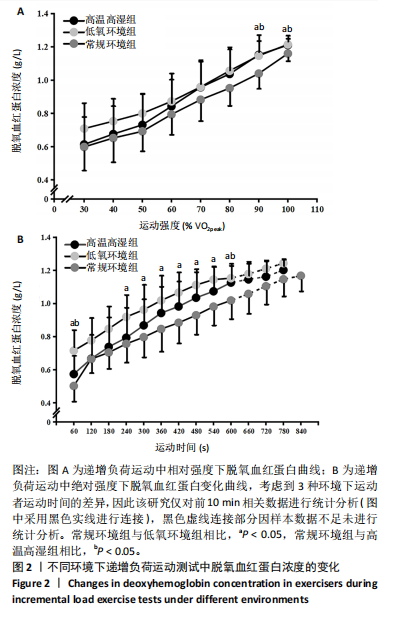
2.4 脱氧血红蛋白动力学参数 BP所对应的运动时间存在主效应显著[F(2,22)=4.860,P=0.018,ηp2=0.306],与常规环境组相比,高温高湿组[P=0.049,95%CI(0.004,2.857)]与低氧环境组BP所对应的运动时间[P=0.031,95%CI(0.144,2.420)]均出现降低。BP所对应的绝对V?O2存在主效应显著[F(2,22)=7.488, P=0.003,ηp2=0.405],与常规环境组相比,高温高湿组BP所对应的绝对V?O2降低[P=0.048,95%CI (5.893, 1 173.387)]。BP所对应的相对V?O2存在主效应显著[F(2,22)=8.177,P=0.009,ηp2= 0.426],与常规环境组相比,高温高湿组[P=0.035,95%CI(0.517,15.574)]与低氧环境组[P=0.046,95%CI(0.088,10.001)]BP所对应的相对V?O2均出现降低。ΔEHHb存在主效应显著[F(1.695,18.643)=3.796,P=0.047,ηp2=0.257],与常规环境组相比,高温高湿组的ΔEHHb升高[P=0.044,95%CI(0.003,0.248)]。ΔEHHb-1存在主效应显著[F(2,22)=4.984,P=0.016,ηp2=0.312],与常规环境组相比,高温高湿组[P=0.005,95%CI (0.109,0.566)]与低氧环境组[P=0.029,95%CI(0.039,0.782)]的ΔEHHb-1均出现升高。各组间的ΔEHHb-2差异无显著性意义[F(2, 22)=0.994,P=0.386,ηp2=0.083],见表2。在绝对强度下低氧环境组的HHb在4 min后显著大于常规环境组(P < 0.05),而高温高湿组在10 min时显著大于常规环境组(P < 0.05)。在相对强度下低氧环境组与高温高湿组在90%-100%V?O2peak时显著大于常规环境组(P < 0.05),如图2所示。"
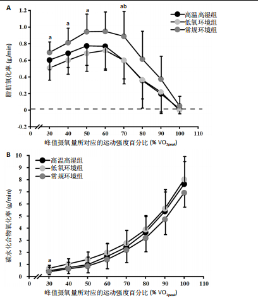
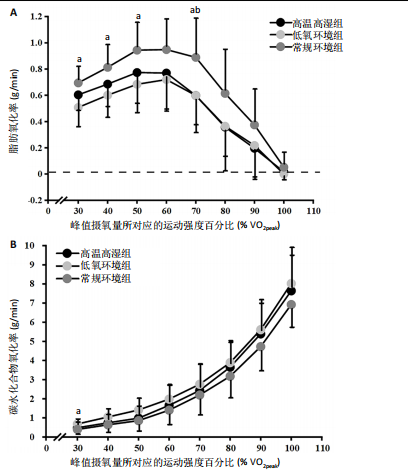
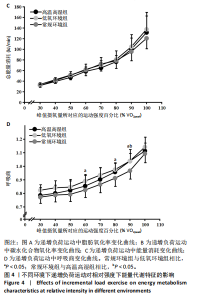
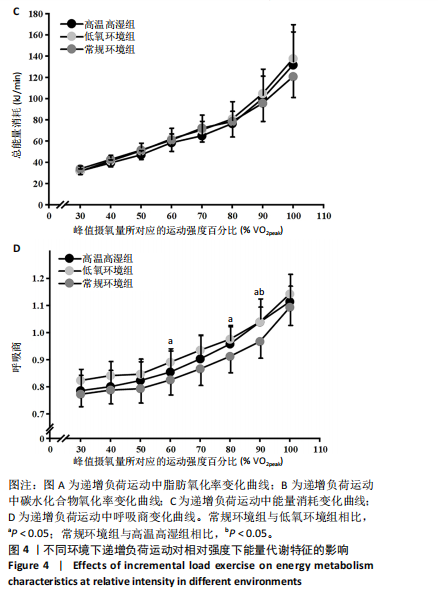
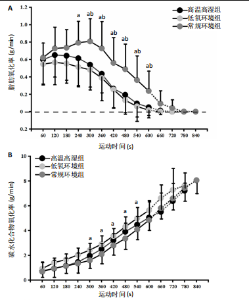
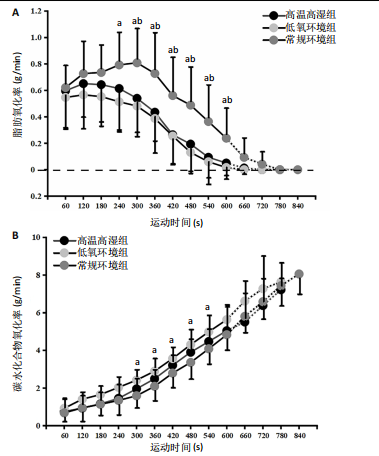
2.5 能量代谢 2.5.1 运动过程中能量代谢 不同环境下递增负荷运动各组间的MFO差异无显著性意义[F(1.206,13.269)=3.329,P=0.085,ηp2=0.232]。MFO所对应的运动时间存在主效应显著[F(2,22)=14.577,P < 0.001,ηp2=0.570],与常规环境组相比,高温高湿组[P=0.003,95%CI(39.535,179.702)]与低氧环境组[P=0.005,95%CI(30.715,164.296)]MFO所对应的运动时间均出现降低。%MFO存在主效应显著[F(2,22)=5.992,P=0.008,ηp2=0.353],与常规环境组相比,低氧环境组%MFO出现降低[P=0.010,95%CI(1.745,12.357)]。FATmax存在主效应显著[F(2,22)=18.429,P < 0.001,ηp2=0.626],与常规环境组相比,高温高湿组[P=0.001,95%CI(4.255,13.240)]与低氧环境组[P=0.001,95%CI(2.810,10.177)]的FATmax均出现降低。在脂肪氧化动力学参数方面,各组间对称性[F(2,22)=3.352,P=0.054,ηp2=0.234]与平移性[F(2,22)=0.724,P=0.496,ηp2=0.062]差异无显著性意义,而扩张性存在主效应显著[F(2,22)=5.652,P=0.010,ηp2=0.339],与常规环境组相比,高温高湿组[P=0.014,95%CI(0.031,0.266)]与低氧环境组[P=0.010,95%CI(0.014,0.322)]的扩张性出现降低。在供能系统占比方面,有氧供能占比存在主效应显著[F(2,22)=5.745,P=0.010,ηp2=0.343],与常规环境组"
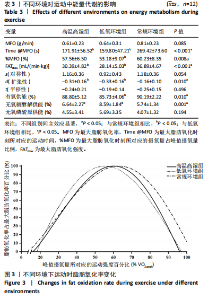
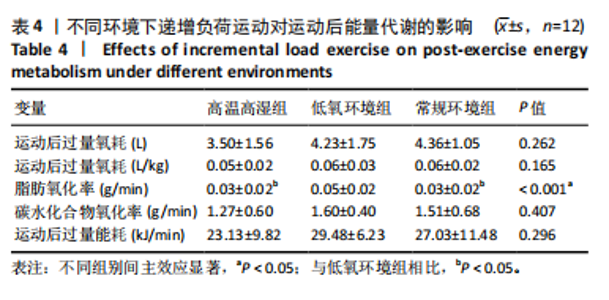
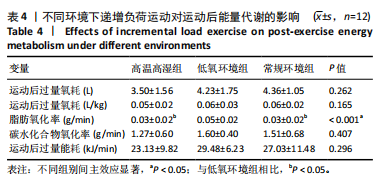
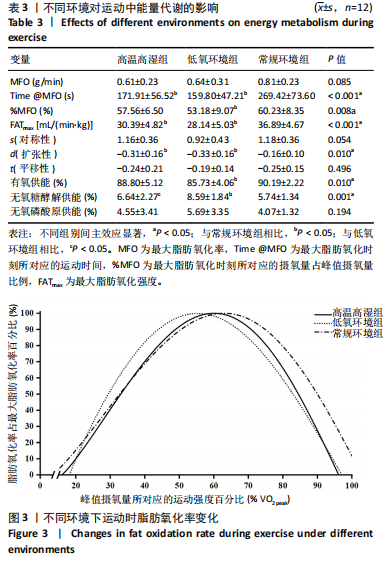
相比,低氧环境组的有氧供能占比降低[P=0.016,95%CI (0.842,8.086)]。无氧糖酵解供能占比存在主效应显著[F(2,22)=10.876,P=0.001,ηp2=0.497],与低氧环境组相比,高温高湿组[P=0.012,95%CI(0.434,3.453)]与常规环境组[P=0.005,95%CI(0.922,4.777)]的无氧糖酵解供能占比均出现升高。各组间无氧磷酸原供能占比差异无显著性意义[F(2,22)=1.767,P=0.194,ηp2=0.138]。如表3,图3所示。此外在相对运动负荷与绝对运动负荷下低氧环境组表现出较低的脂肪氧化率、较高的碳水化合物氧化率与较高的呼吸商,同时高温高湿组在较高的相对运动负荷与绝对运动负荷下表现出较低的脂肪氧化率与较高的呼吸商,如图4,5所示。 2.5.2 运动后能量代谢 各组间的运动后绝对过量氧耗[F(2,22)=1.423,P=0.262,ηp2=0.115]与相对过量氧耗[F(2,22)=1.956,P=0.165,ηp2=0.151]差异均无显著性意义。运动后的脂肪氧化率存在主效应显著[F(2,22)=11.031,P < 0.001,ηp2=0.501],与低氧环境组相比,高温高湿组[P=0.018, 95%CI(0.003,0.037)]与常规环境组[P=0.006,"
| [1] 赵杰修,张漓,路瑛丽,等.高原(低氧)和高温环境下运动训练生理生化监控研究进展[J].中国运动医学杂志,2016,35(12):1165-1171. [2] GENG Z, WANG J, CAO G, et al. Differential impact of heat and hypoxia on dynamic oxygen uptake and deoxyhemoglobin parameters during incremental exhaustive exercise. Front Physiol. 2024;14:1247659. [3] TWOMEY R, WRIGHTSON J, FLETCHER H, et al. Exercise-induced Fatigue in Severe Hypoxia after an Intermittent Hypoxic Protocol. Med Sci Sports Exerc. 2017;49(12):2422-2432. [4] ROWELL LB, O’LEARY DS, KELLOGG DL. Integration of cardiovascular control systems in dynamic exercise. Compr Physiol. 2010:770-838. [5] YOUNG AJ. Energy substrate utilization during exercise in extreme environments. Exerc Sport Sci Rev. 1990;18:65-117. [6] 周祎,赵凡.高温高湿对比一般环境有氧运动对摔跤运动员能量代谢和力量素质的影响[J].中国体育科技,2015,51(6):108-113. [7] GAGNON DD, PERRIER L, DORMAN SC, et al. Ambient temperature influences metabolic substrate oxidation curves during running and cycling in healthy men. Eur J Sport Sci. 2020;20(1):90-99. [8] RUÍZ-MORENO C, GUTIÉRREZ-HELLÍN J, GONZÁLEZ-GARCÍA J, et al. Effect of ambient temperature on fat oxidation during an incremental cycling exercise test. Eur J Sport Sci. 2021;21(8):1140-1147. [9] BOWTELL JL, COOKE K, TURNER R, et al. Acute physiological and performance responses to repeated sprints in varying degrees of hypoxia. J Sci Med Sport. 2014;17(4):399-403. [10] ZURBUCHEN A, LANZI S, VOIROL L, et al. Fat Oxidation Kinetics Is Related to Muscle Deoxygenation Kinetics During Exercise. Front Physiol. 2020;11:571. [11] DANDANELL S, MEINILD-LUNDBY AK, ANDERSEN AB, et al. Determinants of maximal whole-body fat oxidation in elite cross-country skiers: Role of skeletal muscle mitochondria. Scand J Med Sci Sports. 2018;28(12):2494-2504. [12] FRANDSEN J, VEST SD, LARSEN S, et al. Maximal Fat Oxidation is Related to Performance in an Ironman Triathlon. Int J Sports Med. 2017;38(13):975-982. [13] 车开萱,邱俊强.脂肪适应有助于提升运动表现吗?[J].体育科学,2022, 42(3):85-90+97. 14] CHENEVIÈRE X, MALATESTA D, PETERS EM, et al. A mathematical model to describe fat oxidation kinetics during graded exercise. Med Sci Sports Exerc. 2009;41(8):1615-1625. [15] SPENCER MD, MURIAS JM, PATERSON DH. Characterizing the profile of muscle deoxygenation during ramp incremental exercise in young men. Eur J Appl Physiol. 2012;112(9):3349-3360. [16] VIETH E. Fitting piecewise linear regression functions to biological responses. J Appl Physiol (1985). 1989;67(1):390-396. [17] LE MEUR Y, DOREL S, BAUP Y, et al. Physiological demand and pacing strategy during the new combined event in elite pentathletes. Eur J Appl Physiol. 2012; 112(7):2583-2593. [18] HOFFMANN B, FLATT AA, SILVA LEV, et al. A Pilot Study of the Reliability and Agreement of Heart Rate, Respiratory Rate and Short-Term Heart Rate Variability in Elite Modern Pentathlon Athletes. Diagnostics (Basel). 2020;10(10):833. [19] ARAÚJO CG, SCHARHAG J. Athlete: a working definition for medical and health sciences research. Scand J Med Sci Sports. 2016;26(1):4-7. [20] STÖGGL TL, STREPP T, BLUMKAITIS J, et al. Unraveling the mystery of isocaloric endurance training - Influence of exercise modality, biological sex, and physical fitness. Metabolism. 2023;144:155582. [21] SEDLOCK DA, LEE MG, FLYNN MG, et al. Excess postexercise oxygen consumption after aerobic exercise training. Int J Sport Nutr Exerc Metab. 2010;20(4):336-349. [22] 邱俊,陈文鹤.高住低训和高原训练对优秀现代五项运动员运动能力的影响[J].上海体育学院学报,2011,35(1):67-72. [23] YAMAGUCHI K, SUMI D, HAYASHI N, et al. Effects of combined hot and hypoxic conditions on muscle blood flow and muscle oxygenation during repeated cycling sprints. Eur J Appl Physiol. 2021;121(10):2869-2878. [24] SAITOH T, OOUE A, KONDO N, et al. Active muscle oxygenation dynamics measured during high-intensity exercise by using two near-infrared spectroscopy methods. Adv Exp Med Biol. 2010;662:225-230. [25] SORIA M, GONZÁLEZ-HARO C, LÓPEZ-COLÓN JL, et al. Submaximal exercise intensities do not provoke variations in plasma magnesium concentration in well-trained euhydrated endurance athletes with no magnesium deficiency. Magnes Res. 2011;24(2):36-44. [26] BEAVER WL, WASSERMAN K, WHIPP BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985). 1986;60(6):2020-2027. [27] ISLAM H, TOWNSEND LK, HAZELL TJ. Excess Postexercise Oxygen Consumption and Fat Utilization Following Submaximal Continuous and Supramaximal Interval Running. Res Q Exerc Sport. 2018;89(4):450-456. [28] JEUKENDRUP AE, WALLIS GA. Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int J Sports Med. 2005;26 Suppl 1:S28-37. [29] VALLERAND AL, JACOBS I. Rates of energy substrates utilization during human cold exposure. Eur J Appl Physiol Occup Physiol. 1989;58(8):873-878. [30] KAUFMANN S, ZIEGLER M, WERNER J, et al. Energetics of Floor Gymnastics: Aerobic and Anaerobic Share in Male and Female Sub-elite Gymnasts. Sports Med Open. 2022;8(1):3. [31] 章凌凌,黄鹏,吴雪萍,等.二种方法对男子轮椅竞速T54级比赛项目能量供应计算结果的比较[J].中国应用生理学杂志,2020,36(1):27-32. [32] BERTUZZI RC, FRANCHINI E, UGRINOWITSCH C, et al. Predicting MAOD using only a supramaximal exhaustive test. Int J Sports Med. 2010;31(7):477-481. [33] OZYENER F, ROSSITER HB, WARD SA, et al. Influence of exercise intensity on the on- and off-transient kinetics of pulmonary oxygen uptake in humans. J Physiol. 2001;533(Pt 3):891-902. [34] AZEVEDO RDA, J E BS, INGLIS EC, et al. Hypoxia equally reduces the respiratory compensation point and the NIRS-derived [HHb] breakpoint during a ramp-incremental test in young active males. Physiol Rep. 2020;8(12):e14478. [35] OSAWA T, KIME R, HAMAOKA T, et al. Attenuation of muscle deoxygenation precedes EMG threshold in normoxia and hypoxia. Med Sci Sports Exerc. 2011; 43(8):1406-1413. [36] LINNARSSON D, KARLSSON J, FAGRAEUS L, et al. Muscle metabolites and oxygen deficit with exercise in hypoxia and hyperoxia. J Appl Physiol. 1974;36(4):399-402. [37] OSAWA T, ARIMITSU T, TAKAHASHI H. Hypoxia affects tissue oxygenation differently in the thigh and calf muscles during incremental running. Eur J Appl Physiol. 2017;117(10):2057-2064. [38] BOWEN TS, KOGA S, AMANO T, et al. The Spatial Distribution of Absolute Skeletal Muscle Deoxygenation During Ramp-Incremental Exercise Is Not Influenced by Hypoxia. Adv Exp Med Biol. 2016;876:19-26. [39] AZEVEDO RA, BÉJAR SAONA JE, INGLIS EC, et al. The effect of the fraction of inspired oxygen on the NIRS-derived deoxygenated hemoglobin “breakpoint” during ramp-incremental test. Am J Physiol Regul Integr Comp Physiol. 2020; 318(2):R399-R409. [40] DENNIS MC, GOODS PSR, BINNIE MJ, et al. Increased air temperature during repeated-sprint training in hypoxia amplifies changes in muscle oxygenation without decreasing cycling performance. Eur J Sport Sci. 2023;23(1):62-72. [41] PÉRIARD JD, THOMPSON MW, CAILLAUD C, et al. Influence of heat stress and exercise intensity on vastus lateralis muscle and prefrontal cortex oxygenation. Eur J Appl Physiol. 2013;113(1):211-222. [42] RACINAIS S, COCKING S, PÉRIARD JD. Sports and environmental temperature: From warming-up to heating-up. Temperature (Austin). 2017;4(3):227-257. [43] TRINITY JD, PAHNKE MD, LEE JF, et al. Interaction of hyperthermia and heart rate on stroke volume during prolonged exercise. J Appl Physiol (1985). 2010; 109(3):745-751. [44] 季泰,袁伟琪,王坤,等.热环境下运动人体热感觉研究进展[J].中国体育科技,2022,58(4):73-80. [45] MORRISON SF. Central control of body temperature. F1000Res. 2016;5:F1000. [46] GORDAN R, GWATHMEY JK, XIE LH. Autonomic and endocrine control of cardiovascular function. World J Cardiol. 2015;7(4):204-214. [47] YAMAGUCHI K, IMAI T, YATSUTANI H, et al. A Combined Hot and Hypoxic Environment during Maximal Cycling Sprints Reduced Muscle Oxygen Saturation: A Pilot Study. J Sports Sci Med. 2021;20(4):684-689. [48] MURIAS JM, SPENCER MD, KEIR DA, et al. Systemic and vastus lateralis muscle blood flow and O2 extraction during ramp incremental cycle exercise. Am J Physiol Regul Integr Comp Physiol. 2013;304(9):R720-725. [49] IANNETTA D, OKUSHIMA D, INGLIS EC, et al. Blood flow occlusion-related O2 extraction “reserve” is present in different muscles of the quadriceps but greater in deeper regions after ramp-incremental test. J Appl Physiol (1985). 2018;125(2):313-319. [50] INGLIS EC, IANNETTA D, MURIAS JM. Evaluating the NIRS-derived microvascular O2 extraction “reserve” in groups varying in sex and training status using leg blood flow occlusions. PLoS One. 2019;14(7):e0220192. [51] MURRAY AJ. Energy metabolism and the high-altitude environment. Exp Physiol. 2016;101(1):23-27. [52] WORKMAN C, BASSET FA. Post-metabolic response to passive normobaric hypoxic exposure in sedendary overweight males: a pilot study. Nutr Metab (Lond). 2012;9(1):103. [53] 太获田.低压低氧环境下大强度训练对代谢能力及运动能力的影响[J].体育科研,2009,30(6):12-15. [54] GRIFFITHS A, DEIGHTON K, SHANNON OM, et al. Substrate oxidation and the influence of breakfast in normobaric hypoxia and normoxia. Eur J Appl Physiol. 2019;119(9):1909-1920. [55] GRIFFITHS A, SHANNON OM, MATU J, et al. The effects of environmental hypoxia on substrate utilisation during exercise: a meta-analysis. J Int Soc Sports Nutr. 2019;16(1):10. [56] CHENEVIÈRE X, MALATESTA D, GOJANOVIC B, et al. Differences in whole-body fat oxidation kinetics between cycling and running. Eur J Appl Physiol. 2010; 109(6):1037-1045. [57] JEUKENDRUP AE. Regulation of fat metabolism in skeletal muscle. Ann N Y Acad Sci. 2002;967:217-235. [58] STARRITT EC, HOWLETT RA, HEIGENHAUSER GJ, et al. Sensitivity of CPT I to malonyl-CoA in trained and untrained human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;278(3):E462-E468. [59] 魏婷,张永煜,张庆华.肉毒碱棕榈酰基转移酶1A的研究进展[J].生命科学,2013,25(6):614-620. [60] KELLY LP, BASSET FA. Acute Normobaric Hypoxia Increases Post-exercise Lipid Oxidation in Healthy Males. Front Physiol. 2017;8:293. [61] NYBO L, JENSEN T, NIELSEN B, et al. Effects of marked hyperthermia with and without dehydration on VO(2) kinetics during intense exercise. J Appl Physiol (1985). 2001;90(3):1057-1064. [62] LUNDBY C, VAN HALL G. Substrate utilization in sea level residents during exercise in acute hypoxia and after 4 weeks of acclimatization to 4100 m. Acta Physiol Scand. 2002;176(3):195-201. [63] MATU J, DEIGHTON K, ISPOGLOU T, et al. The effect of moderate versus severe simulated altitude on appetite, gut hormones, energy intake and substrate oxidation in men. Appetite. 2017;113:284-292. [64] PURGE P, LEHISMETS P, JÜRIMÄE J. A new method for the measurement of maximal fat oxidation: A pilot study. Acta Kinesiologiae Universitatis Tartuensis. 2014;20:90-99. [65] TAHARA Y, MOJI K, HONDA S, et al. Fat-free mass and excess post-exercise oxygen consumption in the 40 minutes after short-duration exhaustive exercise in young male Japanese athletes. J Physiol Anthropol. 2008;27(3):139-143. |
| [1] | Wang Qifei, Du Xingbin, Kong Jianda. Neural physiological basis and exercise-induced mechanism of central fatigue [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6979-6988. |
| [2] | Cao Panxia, Peng Zining, Liu Shanshan, Fei Tiantian, Liang Tengyun, Zhang Mengwen, Wu Hong. AMP-activated protein kinase mediates macrophage fatty acid oxidation: an approach to prevent and treat atherosclerosis with traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(18): 3906-3914. |
| [3] | Zhao Wenjing, Liu Baikun, Li Qiulian, Chen Xi. Effects of long-term subculture on biological characteristics of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(31): 4926-4930. |
| [4] | Liang Jiaqi, Liu Hengxu, Yang Jinxin, Yang Yi, Deng Xuhui, Tan Mingjian, Luo Jiong. Health benefit relationship between exercise and intestinal bacteria [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1292-1299. |
| [5] | Kong Fanming, Zhu Miaomiao, Mi Jing, Ma Jie. Application enlightenment of exercise and fat oxidation kinetic characteristics [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(29): 4709-4715. |
| [6] | Hao Wei, Sun Yue, Yang Anning, Liu Taiyang, Bao Rui, Liu Yaoyang, Wang Qiushi, Wang Meng, Chang Sirong, Li Yuanyuan, Liu Zhihong. Construction, phenotype and functional identification of vascular endothelial cell-specific PDHA1 knockout mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(20): 3207-3211. |
| [7] | Liu Jianguo, Pan Yong, Li Hanyu, Liu Yan, Zhang Zhentian, Lin Xiuping, Zhang Yuan, Liu Yajin, Zhang Fan, Zhang Leijun, Xiao Liehui, Xu Aimin, Zhu Cuifeng. Effect of microecological preparation combined with an improved low-carbon diet on fat metabolism and intestinal barrier function in obese patients [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(29): 4672-4679. |
| [8] | Wang Si-ya, Zhuang Jie, Zhu Zheng. SenseWear Pro Armband: a monitoring device of physical activity energy consumption [J]. Chinese Journal of Tissue Engineering Research, 2015, 19(42): 6843-6848. |
| [9] | Liu Jing, Li Li, Ran Jiang-hua, Zhang Sheng-ning, Li Lai-bang, Zhang Xi-bing, Gao Yang, Chen Yi-ming. Expression of hepatic energy proteins following reduced-size liver transplantation in rats [J]. Chinese Journal of Tissue Engineering Research, 2015, 19(18): 2874-2878. |
| [10] | Huang Le-lin. Effect of anesthetic methods on respiratory and lung functions of patients after sequential double lung transplantation [J]. Chinese Journal of Tissue Engineering Research, 2014, 18(27): 4304-4309. |
| [11] | Liu Jian-feng, Ding Yan-ping, Wang Jian-lin, Shao Bao-ping. Distribution, function and regulation mechanism of aquaporin in the brain [J]. Chinese Journal of Tissue Engineering Research, 2014, 18(2): 314-321. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||