Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (32): 6858-6865.doi: 10.12307/2025.934
Previous Articles Next Articles
tert-Butyl hydroperoxide can induce ferroptosis in nucleus pulposus cells
Chen Chao, Hu Yaoquan, Lyu Zhengpin, He Qicong, Yangyang Zijiu, Luo Haoyan, Wu Guishuai, Zuo Qianlin, Wang Xuenan, Zhang Fan
- Department of Orthopedics, The First Affiliated Hospital of Kunming Medical University, Kunming 650032, Yunnan Province, China
-
Received:2024-10-18Accepted:2024-11-28Online:2025-11-18Published:2025-04-25 -
Contact:Zhang Fan, MD, Associate professor, Department of Orthopedics, The First Affiliated Hospital of Kunming Medical University, Kunming 650032, Yunnan Province, China -
About author:Chen Chao, MD candidate, Department of Orthopedics, The First Affiliated Hospital of Kunming Medical University, Kunming 650032, Yunnan Province, China -
Supported by:the National Natural Science Foundation of China, No. 82160428 (to ZF); 535 Talent Project of First Affiliated Hospital of Kunming Medical University, No. 2022535D10 (to ZF); Yunnan Province “Xingdian Talent Support Plan” Project for Medical and Health Talent Training (No project number available) (to ZF); The “Rising Star” Talent Training Program for Young and Middle-Aged Discipline Leaders and Reserve Candidates at Kunming Medical University, No. J13397034 (to ZF); First-class Academic Team of Kunming Medical University, No. 2024XKTDYS05 (to ZF [project participant])
CLC Number:
Cite this article
Chen Chao, Hu Yaoquan, Lyu Zhengpin, He Qicong, Yangyang Zijiu, Luo Haoyan, Wu Guishuai, Zuo Qianlin, Wang Xuenan, Zhang Fan. tert-Butyl hydroperoxide can induce ferroptosis in nucleus pulposus cells[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6858-6865.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

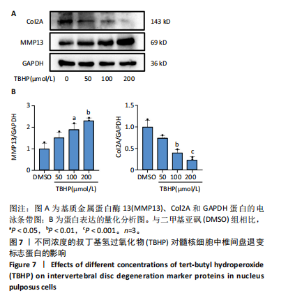
2.1 髓核细胞形态及细胞活力水平 为了模拟椎间盘退变进展期间氧化应激的作用,二甲基亚砜作为溶剂,用不同浓度的TBHP(25,50,100和200 μmol/L)处理髓核细胞 3 h。细胞形态学分析显示,TBHP处理的髓核细胞出现细胞死亡的形态变化,呈现“气球”样表型,这与铁死亡的形态特征一致,且与铁死亡诱导剂RSL3诱导的结果相似,见图1A。 此外,细胞活力测定结果表明,髓核细胞的活力随着TBHP浓度的增加而降低。在100 μmol/L TBHP处理后,细胞活力显著下降(P < 0.001),且接近IC50值,见图1B。 因此,后续实验中选择100 μmol/L TBHP作为药物处理浓度。 2.2 髓核细胞增殖水平 与二甲基亚砜对照组相比,TBHP组与RSL3组的细胞增殖水平明显下降 (P < 0.001),见图2,这表明TBHP能够显著降低髓核细胞的增殖水平,其效果与RSL3相似。 2.3 铁死亡标志蛋白表达情况 2.3.1 Western blot结果 随着TBHP浓度的增加,GPX4和FTH1的表达水平呈现下降趋势,而ACSL4和PTGS2的表达水平则上升,见图3。 2.3.2 免疫荧光结果 TBHP和RSL3组的FTH1荧光强度较对照组明显下降,表明其表达水平下调,见图4。 结果提示,TBHP可引发髓核细胞中铁死亡标志蛋白的异常表达,初步表明TBHP可能诱导髓核细胞发生铁死亡。 2.4 活性氧与脂质过氧化水平 见图5。 2.4.1 活性氧测定结果 TBHP和RSL3处理均可诱导髓核细胞中活性氧的生成,相较于对照组,TBHP显著诱导了髓核细胞的氧化应激状态,见图5A,C。 2.4.2 脂质过氧化测定结果 TBHP处理显著提高了髓核细胞的脂质过氧化水平,其效果与RSL3组相似,见图5B,D。 2.5 线粒体形态变化 电镜检测结果显示,TBHP和RSL3处理的髓核细胞线粒体呈现收缩变小、膜密度增加及嵴减少的变化,这些特征符合铁死亡的线粒体形态变化,见图6。说明铁死亡可能在TBHP诱导的髓核细胞死亡过程中起着关键作用。 2.6 椎间盘退变标志蛋白表达情况 Western blotting结果显示,随着TBHP浓度的增加,髓核细胞中Col2A的表达水平呈现下降趋势,而基质金属蛋白酶13的表达水平则上升,见图7。结果提示,TBHP可引发髓核细胞中椎间盘退变标志蛋白的异常表达,初步表明TBHP可能诱导髓核细胞发生退变。"

| [1] FRANCISCO V, PINO J, GONZáLEZ-GAY M, et al. A new immunometabolic perspective of intervertebral disc degeneration. Nat Rev Rheumatol. 2022;18(1):47-60. [2] LI G, ZHANG W, LIANG H, et al. Epigenetic regulation in intervertebral disc degeneration. Trends Mol Med. 2022;28(10):803-805. [3] ZHANG GZ, LIU MQ, CHEN HW, et al. NF-κB signalling pathways in nucleus pulposus cell function and intervertebral disc degeneration. Cell Prolif. 2021;54(7):e13057. [4] SWAHN H, MERTENS J, OLMER M, et al. Shared and Compartment-Specific Processes in Nucleus Pulposus and Annulus Fibrosus During Intervertebral Disc Degeneration. Adv Sci (Weinh). 2024;11(17): e2309032. [5] SUN K, YAN C, DAI X, et al. Catalytic Nanodots-Driven Pyroptosis Suppression in Nucleus Pulposus for Antioxidant Intervention of Intervertebral Disc Degeneration. Adv Mater. 2024;36(19):e2313248. [6] SUN Y, LYU M, LU Q, et al. Current Perspectives on Nucleus Pulposus Fibrosis in Disc Degeneration and Repair. Int J Mol Sci. 2022;23(12):6612. [7] JIANG X, STOCKWELL BR, CONRAD M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22(4):266-282. [8] LI J, CAO F, YIN HL, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):8. [9] TANG D, CHEN X, KANG R, et al. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31(2):107-125. [10] DIXON SJ, OLZMANN JA. The cell biology of ferroptosis. Nat Rev Mol Cell Biol. 2024;25(6):424-442. [11] POPE LE, DIXON SJ. Regulation of ferroptosis by lipid metabolism.Trends Cell Biol. 2023;33(12):1077-1087. [12] YAO Z, JIAO Q, DU X, et al. Ferroptosis in Parkinson’s disease -- The iron-related degenerative disease. Ageing Res Rev. 2024;101:102477. [13] TONG L, YU H, HUANG X, et al. Current understanding of osteoarthritis pathogenesis and relevant new approaches. Bone Res. 2022;10(1):60. [14] HE DL, FAN YG, WANG ZY. Energy Crisis Links to Autophagy and Ferroptosis in Alzheimer’s Disease: Current Evidence and Future Avenues. Curr Neuropharmacol. 2023;21(1):67-86. [15] FAN X, ZHANG X, LIU LC, et al. Hemopexin accumulates in kidneys and worsens acute kidney injury by causing hemoglobin deposition and exacerbation of iron toxicity in proximal tubules. Kidney Int. 2022; 102(6):1320-1330. [16] CHEN Y, ZHAO W, HU A, et al. Type 2 diabetic mellitus related osteoporosis: focusing on ferroptosis. J Transl Med. 2024;22(1):409. [17] ZHOU LP, ZHANG RJ, JIA CY, et al. Ferroptosis: A potential target for the intervention of intervertebral disc degeneration. Front Endocrinol (Lausanne). 2022;13:1042060. [18] CHEN J, YANG X, FENG Y, et al. Targeting Ferroptosis Holds Potential for Intervertebral Disc Degeneration Therapy. Cells. 2022;11(21):3508. [19] WANG W, JING X, DU T, et al. Iron overload promotes intervertebral disc degeneration via inducing oxidative stress and ferroptosis in endplate chondrocytes. Free Radic Biol Med. 2022;190:234-246. [20] JIA C, XIANG Z, ZHANG P, et al. Selenium-SelK-GPX4 axis protects nucleus pulposus cells against mechanical overloading-induced ferroptosis and attenuates senescence of intervertebral disc. Cell Mol Life Sci. 2024;81(1):49. [21] LU X, LI D, LIN Z, et al. HIF-1α-induced expression of the m6A reader YTHDF1 inhibits the ferroptosis of nucleus pulposus cells by promoting SLC7A11 translation. Aging Cell. 2024;23(9):e14210. [22] CHEN X, ZHANG A, ZHAO K, et al. The role of oxidative stress in intervertebral disc degeneration: Mechanisms and therapeutic implications. Ageing Res Rev. 2024;98:102323. [23] FAN C, CHU G, YU Z, et al. The role of ferroptosis in intervertebral disc degeneration. Front Cell Dev Biol. 2023;11:1219840. [24] WANG J, YANG J, FANG Y, et al. Vinpocetine protects against osteoarthritis by inhibiting ferroptosis and extracellular matrix degradation via activation of the Nrf2/GPX4 pathway. Phytomedicine. 2024;135:156115. [25] MA X, NI J, WANG W, et al. Protective Effect of Epigallocatechin-3-gallate against Hepatic Oxidative Stress Induced by tert-Butyl Hhydroperoxide in Yellow-Feathered Broilers. Antioxidants (Basel). 2024;13(10):1153. [26] GAO L, HUA W, TIAN L, et al. Molecular Mechanism of Ferroptosis in Orthopedic Diseases. Cells. 2022;11(19):2979. [27] COSTA I, BARBOSA DJ, BENFEITO S, et al. Molecular mechanisms of ferroptosis and their involvement in brain diseases. Pharmacol Ther. 2023;244:108373. [28] RU Q, LI Y, CHEN L, et al. Iron homeostasis and ferroptosis in human diseases: mechanisms and therapeutic prospects. Signal Transduct Target Ther. 2024;9(1):271. [29] LAI L, TAN M, HU M, et al. Important molecular mechanisms in ferroptosis. Mol Cell Biochem. 2025;480(2):639-658. [30] KONG Y, LI J, LIN R, et al. Understanding the unique mechanism of ferroptosis: a promising therapeutic target. Front Cell Dev Biol. 2023;11:1329147. [31] YANG Q, XIA Y, CHEN K, et al. Blue light induced ferroptosis via STAT3/GPX4/SLC7A11/FTH1 in conjunctiva epithelium in vivo and in vitro. J Photochem Photobiol B. 2024;255:112908. [32] LV QK, TAO KX, YAO XY, et al. Melatonin MT1 receptors regulate the Sirt1/Nrf2/Ho-1/Gpx4 pathway to prevent α-synuclein-induced ferroptosis in Parkinson’s disease. J Pineal Res. 2024;76(2):e12948. [33] RAO Y, LI J, SHI L, et al. Silencing CK19 regulates ferroptosis by affecting the expression of GPX4 and ACSL4 in oral squamous cell carcinoma in vivo and in vitro. Sci Rep. 2024;14(1):15968. [34] LOU T, WU H, FENG M, et al. Integration of metabolomics and transcriptomics reveals that Da Chuanxiong Formula improves vascular cognitive impairment via ACSL4/GPX4 mediated ferroptosis. J Ethnopharmacol. 2024;325:117868. [35] HAN J, ZHAN LN, HUANG Y, et al. Moderate mechanical stress suppresses chondrocyte ferroptosis in osteoarthritis by regulating NF-κB p65/GPX4 signaling pathway. Sci Rep. 2024;14(1):5078. [36] LEI M, ZHANG YL, HUANG FY, et al. Gankyrin inhibits ferroptosis through the p53/SLC7A11/GPX4 axis in triple-negative breast cancer cells. Sci Rep. 2023;13(1):21916. [37] JIANG W, YU L, MU N, et al. MG53 inhibits ferroptosis by targeting the p53/SLC7A11/GPX4 pathway to alleviate doxorubicin-induced cardiotoxicity. Free Radic Biol Med. 2024;223:224-236. [38] PANDA SK, PENG V, SUDAN R, et al. Repression of the aryl-hydrocarbon receptor prevents oxidative stress and ferroptosis of intestinal intraepithelial lymphocytes. Immunity. 2023;56(4):797-812.e794. [39] SU W, GAO W, ZHANG R, et al. TAK1 deficiency promotes liver injury and tumorigenesis via ferroptosis and macrophage cGAS-STING signalling. JHEP Rep. 2023;5(5):100695. [40] CHEN Y, GUO X, ZENG Y, et al. Oxidative stress induces mitochondrial iron overload and ferroptotic cell death. Sci Rep. 2023;13(1):15515. [41] CAZZANELLI P, WUERTZ-KOZAK K. MicroRNAs in Intervertebral Disc Degeneration, Apoptosis, Inflammation, and Mechanobiology. Int J Mol Sci. 2020;21(10):3601. [42] YANG RZ, XU WN, ZHENG HL, et al. Involvement of oxidative stress-induced annulus fibrosus cell and nucleus pulposus cell ferroptosis in intervertebral disc degeneration pathogenesis. J Cell Physiol. 2021; 236(4):2725-2739. |
| [1] | Zhao Jiyu, Wang Shaowei. Forkhead box transcription factor O1 signaling pathway in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1923-1930. |
| [2] | Zhao Nannan, Li Yanjie, Qin Hewei, Zhu Bochao, Ding Huimin, Xu Zhenhua. Changes in ferroptosis in hippocampal neurons of vascular dementia model rats treated with Tongmai Kaiqiao Pill [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1401-1407. |
| [3] | Zhang Mingyang, Yang Xinling. Verbascoside inhibits Erastin-induced ferroptosis of dopaminergic nerve cell line MN9D cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1408-1413. |
| [4] | Wang Mi, Ma Shujie, Liu Yang, Qi Rui. Identification and validation of characterized gene NFE2L2 for ferroptosis in ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1466-1474. |
| [5] | He Guanghui, Yuan Jie, Ke Yanqin, Qiu Xiaoting, Zhang Xiaoling. Hemin regulates mitochondrial pathway of oxidative stress in mouse chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1183-1191. |
| [6] | He Bo, Chen Wen, Ma Suilu, He Zhijun, Song Yuan, Li Jinpeng, Liu Tao, Wei Xiaotao, Wang Weiwei, Xie Jing . Pathogenesis and treatment progress of flap ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1230-1238. |
| [7] | Gao Yang, Qin Hewei, Liu Dandan. ACSL4 mediates ferroptosis and its potential role in atherosclerotic cardiovascular disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1239-1247. |
| [8] | Lu Ranran, Zhou Xu, Zhang Lijie, Yang Xinling. Dimethyl fumarate alleviates nerve damage in a mouse model of Parkinson’s disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 989-994. |
| [9] | Sima Xinli, Liu Danping, Qi Hui. Effect and mechanism of metformin-modified bone marrow mesenchymal stem cell exosomes on regulating chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7728-7734. |
| [10] | Wang Chen, Zhang Weinan, Shen Jining, Liu Fan, Yuan Jishan, Liu Yake. Inhibitory effect of ferroptosis inhibitor toxicity induced by cobalt nanoparticles through reactive oxygen species [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7310-7317. |
| [11] | Zhang Xiaoyu, Wei Shanwen, Fang Jiawei, Ni Li. Prussian blue nanoparticles restore mitochondrial function in nucleus pulposus cells through antioxidation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7318-7325. |
| [12] | Su Yongkun, Sun Hong, Liu Miao, Yang Hua, Li Qingsong. Development of novel antioxidants and antioxidant combination carried by nano-hydrogel systems in treatment of intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7376-7384. |
| [13] | Wu Qingyun, Su Qiang. Antioxidant nanomedicine-mediated targeted therapy for myocardial ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7431-7438. |
| [14] | Wang Ziheng, Wu Shuang. Oxidative stress-related genes and molecular mechanisms after spinal cord injury: data analysis and verification based on GEO database [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6893-6904. |
| [15] | Tian Yushi, Fu Qiang, Li Ji . Bioinformatics identification and validation of mitochondrial genes related to acute myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6697-6707. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||