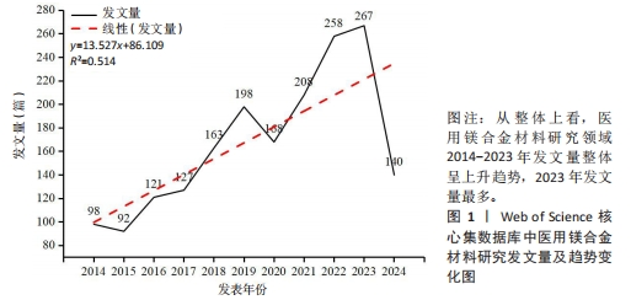
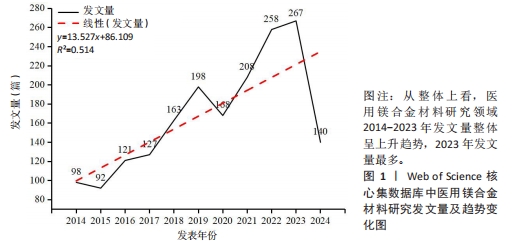
Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (34): 7470-7480.doi: 10.12307/2025.882
Tracking of research trends and hotspots in medical magnesium alloy materials
Ma Yucong, Ouyang Zhengzheng, Liu Xiaojie, Yang Sifei
- National Science Library (Chengdu), Chinese Academy of Sciences, Chengdu 610299, Sichuan Province, China
-
Received:2024-08-09Accepted:2024-10-08Online:2025-12-08Published:2025-01-18 -
Contact:Ouyang Zhengzheng, MS, Associate research librarian, National Science Library (Chengdu), Chinese Academy of Sciences, Chengdu 610299, Sichuan Province, China -
About author:Ma Yucong, PhD, National Science Library (Chengdu), Chinese Academy of Sciences, Chengdu 610299, Sichuan Province, China -
Supported by:2023 Innovation Fund Youth Project of National Science Library (Chengdu) of Chinese Academy of Sciences, No. E3Z00008 (to MYC)
CLC Number:
Cite this article
Ma Yucong, Ouyang Zhengzheng, Liu Xiaojie, Yang Sifei. Tracking of research trends and hotspots in medical magnesium alloy materials[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7470-7480.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

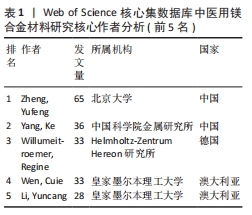
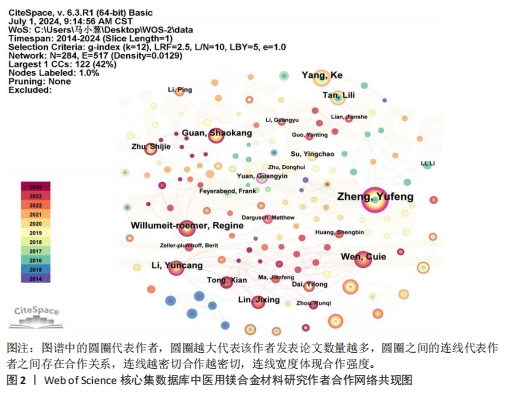
2.2 作者分析 分析发文作者及其合作网络,是评估领域研究主体及核心团队成熟度的重要指标[11-12]。运用CiteSpace软件得到作者合作网络图谱(图2),图谱中的圆圈及标签大小代表作者发表论文的数量,圆圈之间的连线代表作者之间存在合作关系,连线越密切合作越密切,连线宽度体现合作强度,圆圈节点若存在紫色的外圈,表明具有高中介中心性(中心性大于0.1)。医用镁合金材料领域发文作者对应的圆圈数、连线数、网络密度分别为284,517和0.012 9。由图2可知,该领域已形成以郑玉峰、Willumeit-roemer Regine、Wen Cuie为代表的科研合作网络呈现成果产出多、科研合作密切的特点。对纳入的发文作者和发文量进行分析,其中包括284名作者,发表论文数最多的是来自北京大学的郑玉峰教授,发表相关文章65篇。按照普赖斯公式,核心作者的最低发文量M≈0.749×1/2Nmax(其中Nmax为最高发文量)[13],带入该领域最高发文量可得M≈24,即发文量在24篇及以上的作者为核心作者,共9位,总发文量为294篇,形成了该领域的核心作者群。但核心作者的总发文量尚未达到该领域总发文量的50%,表明核心作者的贡献率还较低,有待进一步提高[10]。"

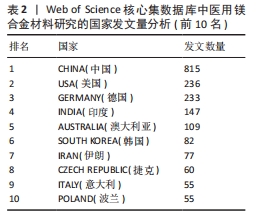
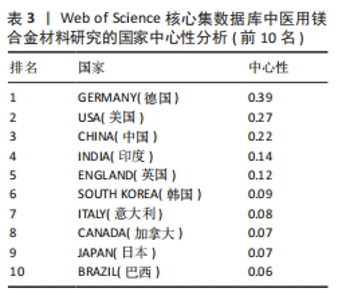
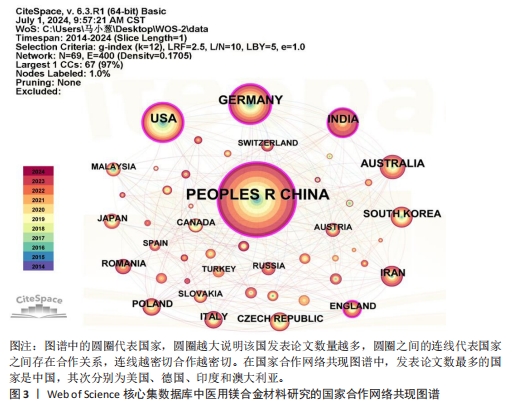
2.3 国家分析 图3及表2,3分别展示了医用镁合金材料研究的国家共现图谱、论文数量和中心性排名前10位的国家。在国家共现网络图谱中,发表论文数最多的国家是中国(815篇),其次分别为美国(236篇)、德国(233篇)、印度(147篇)和澳大利亚(109篇),说明这些国家在该领域的研究较为活跃,研究成果可观。进一步分析中心性数据可知,中心性超过0.1的国家有5个,分别是德国(0.39)、美国(0.27)、中国(0.22)、印度(0.14)和英国(0.12),表明这些国家在该领域具有较高的合作度和影响力。尽管中国在发文量上遥遥领先,但是中心性排名第三,还需要在提升论文质量上发力,通过加强国际间合作,争取在高水平期刊上发表更多高质量论文,进一步提高国际影响力。"
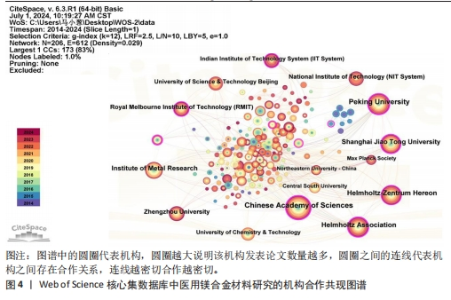
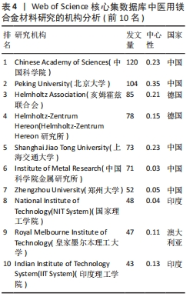
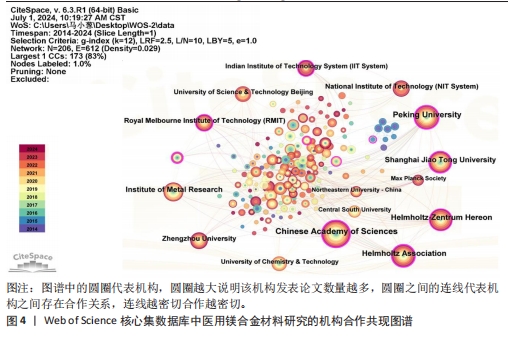
2.4 机构分析 研究机构是专注于执行一项乃至多项科研任务的专门机构,在某种程度上可被视为学术社群或研究共同体的象征性表述[14]。图4为医用镁合金材料领域重要机构合作共现图谱,共纳入206个机构,连线数、网络密度分别为612和0.029。表4罗列了该领域发文数排名前10的机构,其中有一半机构来自中国,它们的总发文数量达420篇,在该研究领域内处于显著领先地位。发文量第一的机构是中国科学院,发文量为120篇,中心性为0.23。最大的合作网络是以北京大学为中心的合作机构,其构成的合作机构群体包括中国科学院、Helmholtz-Zentrum Hereon研究所、皇家墨尔本理工大学、上海交通大学等科研院所和高校,其中该领域研究的先锋机构为北京大学和中国科学院。北京大学主要围绕镁基可降解金属的高生物可降解性、良好的机械性能和生物相容性,深入研究了对镁合金在骨科、心脑血管、抗肿瘤治疗等领域的临床应用[15-17];中国科学院主要基于“医用金属材料生物功能化”这一概念,利用镁合金在生理环境中降解形成的碱性环境以及持续释放镁离子的特点,开展赋予可降解镁合金抗菌、促成骨、促血管化等多重生物医学功能的研究[18-19]。"

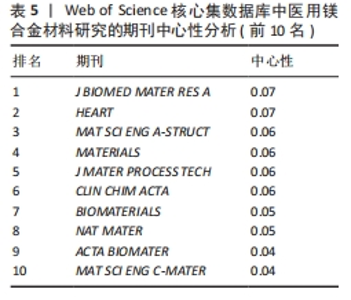
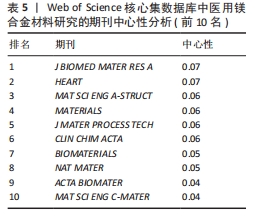
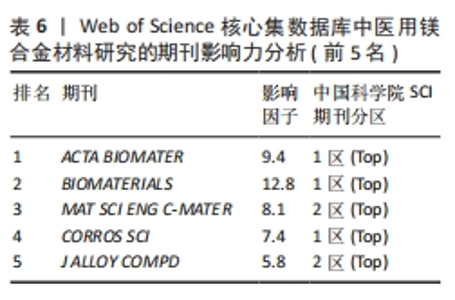
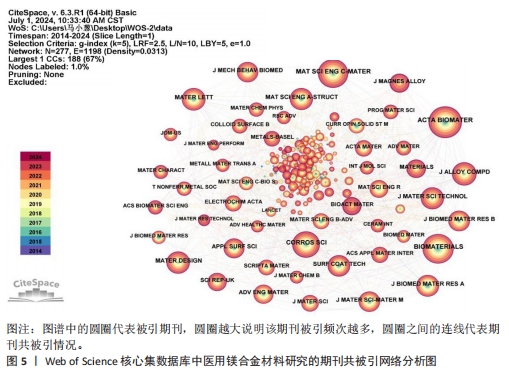
2.5 期刊共被引网络分析 图5和表5分别为医用镁合金材料研究领域的期刊共被引网络分析图和中心性排名前5位的期刊。发文期刊对应的圆圈数、连线数、网络密度分别为277,1 198和0.031 3。通过分析该领域发文期刊被引频次与发文量的平均值得出,该领域有影响力的期刊包括《ACTA BIOMATER》《BIOMATERIALS》《MAT SCI ENG C-MATER》《CORROS SCI》和《J ALLOY COMPD》,这些期刊也确实具有较高的影响因子(表6)。分析被引用期刊之间的中心性可知,中心性排名前5位的期刊分别为《J BIOMED MATER RES A》《HEART》《MAT SCI ENG A-STRUCT》《MATERIALS》和《J MATER PROCESS TECH》,这些期刊在一定程度上连接了其他期刊,但是中心性都未超过0.1,说明未来该领域各期刊之间还应加强科研合作,组织更多跨学科、跨领域的学术活动。"
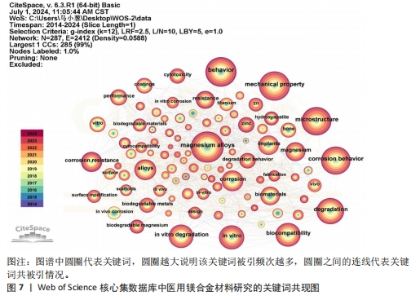
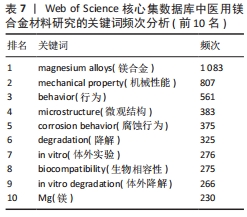
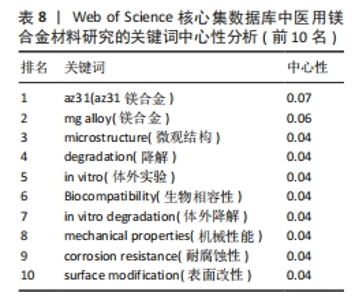
2.6 关键词分析 关键词是文章精髓的精炼表达,在特定领域内能一定程度上映射出当前研究的热点方向[20]。关键词在文中被提及的次数为频次,频次愈高往往代表研究更为详尽和深入;此外,关键词在整体研究架构中的位置,以中心性来衡量,中心性越强,意味着该关键词在构建知识体系或研究网络时占据更为核心和关键的位置[21]。图7为医用镁合金材料研究的关键词共现图,图谱中的节点及标签大小反映关键词的频次,节点越大说明该关键词词频越大,与主题的相关性越大。表7和表8分别为频次和中心性排名前10位的关键词。剔除与论文主题高度相关的关键词,如magnesium alloys(镁合金)、az31(az31镁合金)、mg alloy(镁合金)外,频次最高的关键词是mechanical property(机械性能,807次),表明医用镁合金作为极具潜力的生物医用材料,其机械性能受到研究人员的高度关注。就中心性而言,排名前列的microstructure(微观结构)、degradation(降解)、in vitro(体外实验)、Biocompatibility(生物相容性)、in vitro degradation(体外降解)、mechanical properties(机械性能)、corrosion resistance(耐腐蚀性)、surface modification(表面改性)的中心性一样(0.04)且都不高,说明研究人员对医用镁合金材料的微观结构、降解行为、生物相容性、机械性能以及耐腐蚀性能等都较为关注,但关注点相对较散,将其关联起来研究的程度还不够,未来应该进行更深入的探索。"
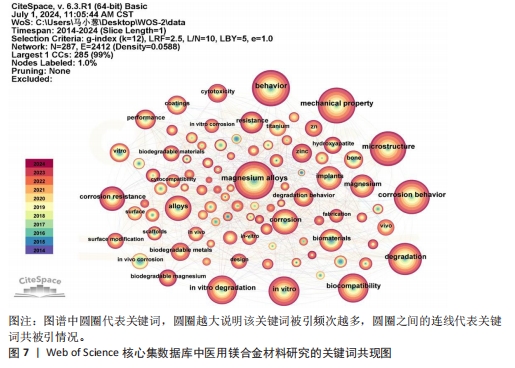
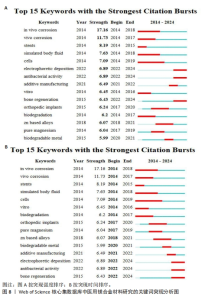
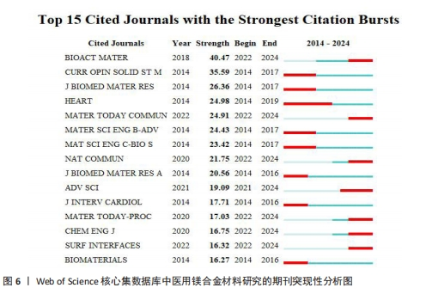
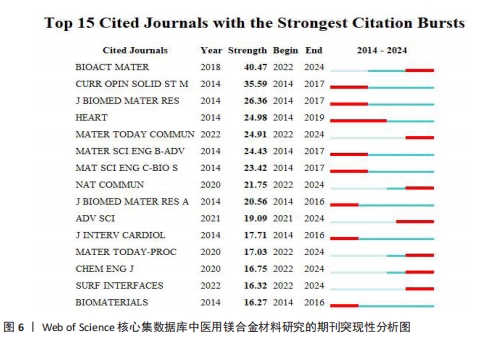
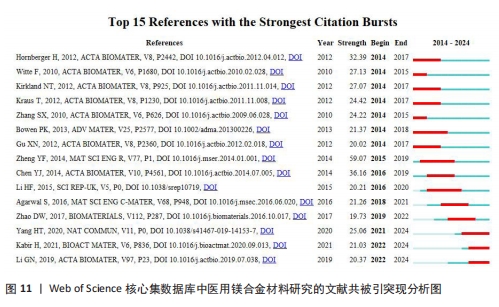
关键词中的突现词不仅能够揭示该学科领域当前的研究热点与前沿趋势,同时还能动态地勾勒出这些研究热点随时间推进的演变历程[22]。使用CiteSpace软件对文献关键词进行突现分析。图8A展示了按照突现强度排序的前15个关键词,“Strength”为关键词的突现强度,其中in vivo corrosion(体内腐蚀)的爆发强度最大,这说明医用镁合金材料在植入活体后的腐蚀情况始终是研究人员的观察重点,因为镁合金可降解生物材料的一个重要性能指标是降解速率,即腐蚀速率,这一指标不仅直接关系到组织愈合期,还影响到降解过程中生物材料机械性能的损失[4]。图8B展示了按照时间顺序排列的前15个突现关键词,“Begin”为开始时间,“End”为结束时间。据图可大概梳理近10年医用镁合金材料研究热点的变化情况,2014-2018年研究人员主要关注医用镁合金材料作为支架、骨钉等植入物时的腐蚀情况,2019-2021年对于医用镁合金材料制备方法的研究较多,如增材制造、电泳沉积等,2022至2024年6月,除了力学性能和耐腐蚀性能,医用镁合金材料的抗菌活性以及促进骨再生性能等成为了研究人员的关注焦点。"
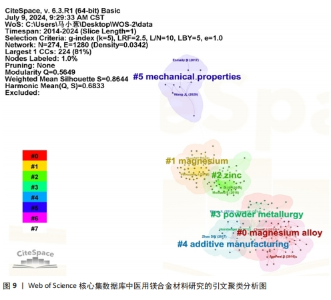
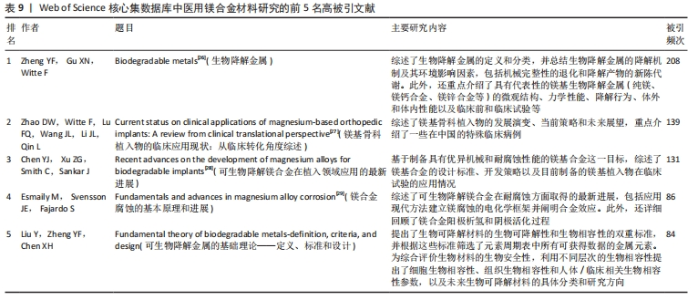
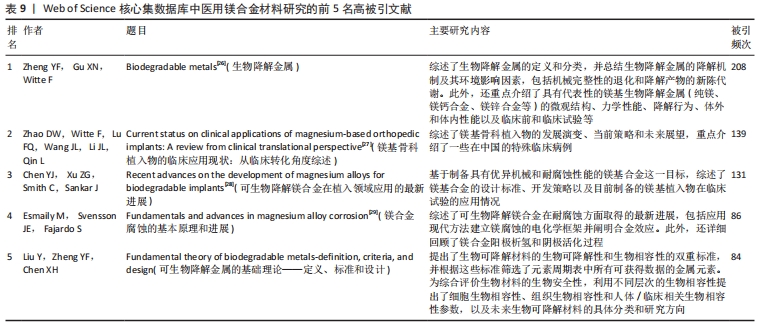
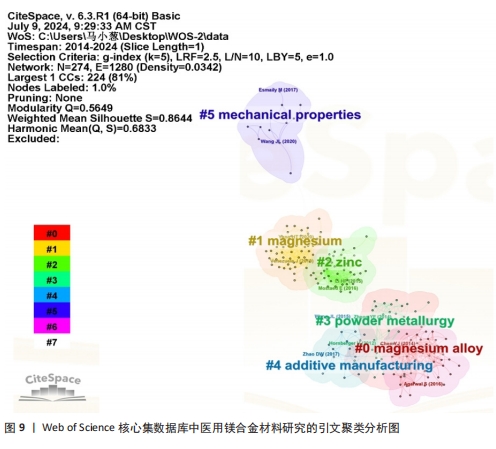
2.7 高被引文献分析 高引用论文被认为是该领域最具影响力的研究论文[23]。共被引分析可用于精选引文中的重要参考文献,揭示领域前沿演变,预测未来研究方向[24]。使用CiteSpace软件进行文献共被引分析,图9为使用似然估计算法对被引文献聚类后的图谱,从图谱中可知共形成6个聚类,聚类模块化值和聚类平均轮廓值分别为0.564 9和0.864 4。通常情况下,聚类模块化值> 0.5表明该聚类结构显著,聚类平均轮廓值> 0.7则被认为该聚类结果是有价值的,说明文献具有较高的同质性,反映研究人员对医用镁合金材料的研究较为专注和深入[25]。聚类标签从0开始依次变大,标签序号越小表明对应聚类中文献规模越大。对聚类标签进一步分析,剔除与论文主题高度相关的聚类(#0 magnesium alloys、#1 magnesium)外,剩下的四大聚类为#2 zinc(锌)、#3 powder metallurgy(粉末冶金)、#4 additive manufacturing(增材制造)和#5 mechanical properties(机械性能)。通过以上聚类可以看出,研究最广泛的是镁合金材料的制备方法以及该材料在生物医用领域具体应用时相关性能的变化情况,例如怎样利用合金化提升镁合金各项性能,特别是添加锌(Zn)元素对镁合金性能的影响。表9罗列了共被引频次前5位的文献[26-30],其中被引次数最多的文献是一篇综述,主要论述了镁合金材料的降解机制及其降解环境的影响因素,包括机械完整性的退化和降解产物的新陈代谢过程[26]。"
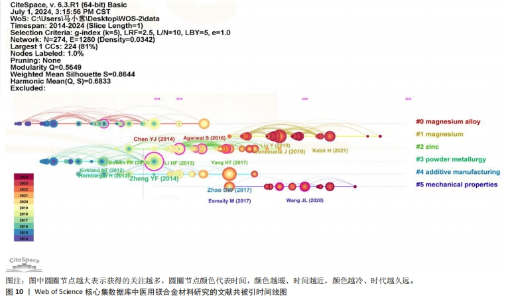
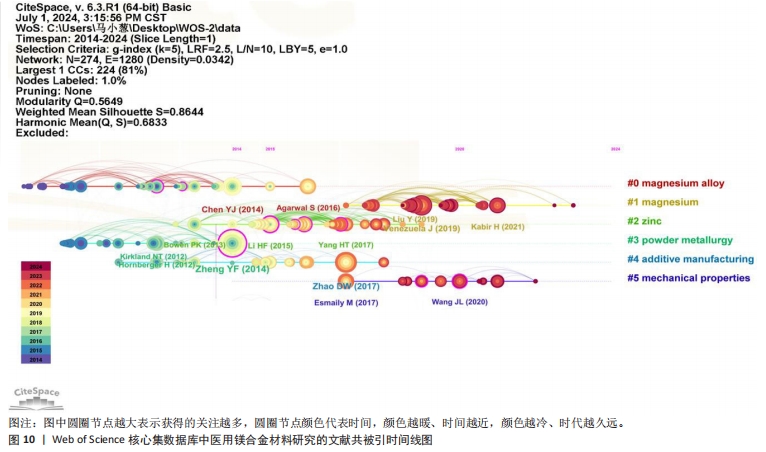
为了深入洞察医用镁合金材料研究的焦点演变与未来发展趋势,针对前6个聚类绘制了时间线图和突现图,如图10和图11所示。在时间线图中,圆圈节点越大表示获得的关注越多;圆圈节点颜色代表时间,颜色越暖、时间越近,颜色越冷、时代越久远。结合共被引聚类图谱发现,在该领域的相关研究中,对医用镁合金材料制备和植入体内后的机械性能转变以及腐蚀行为的研究较为深入,呈现出研究时间早、持续时间长的特点。而近年来针对纯镁材料在机械强度不足与降解速率过快方面的固有局限,通过合金化和表面改性等技术增强其机械强度及耐腐蚀性能的研究成为热点。这一趋势在图10的突现性分析中得到了鲜明体现,特别是在2021-2024年间,多篇高被引文献都探讨了利用合金化提升生物可降解材料的抗压强度[30-32],如通过添加钙(Ca)元素可以细化镁的微观结构,提高其在高温下的强度和蠕变性能,同时添加锌(Zn)和锶(Sr)等元素时能提高镁合金的强度和延展性。"
| [1] WATANABE K, FUKUZAKI S, SUGINO A, et al. Cobalt-Chromium Alloy Has Superior Antibacterial Effect Than Titanium Alloy In Vitro and In Vivo Studies. Spine (Phila Pa 1976). 2021;46(17):E911-E915. [2] BERNHARDT A, HELMHOLZ H, KILIAN D, et al. Impact of Degradable Magnesium Implants on Osteocytes in Single and Triple Cultures. Biomater Adv. 2022;134:112692. [3] ANGRISANI N, VON DER AHE C, WILLUMEIT-RÖMER R, et al. Treatment of Osteoarthritis by Implantation of Mg-and WE43-cylinders-A Preclinical Study on Bone and Cartilage Changes and Their Influence on Pain Sensation in Rabbits. Bioact Mater. 2024;40:366-377. [4] BERNHARDT A, HELMHOLZ H, KILIAN D, et al. Impact of Degradable Magnesium Implants on Osteocytes in Single and Triple Cultures. Biomater Adv. 2022;134:112692. [5] ZHANG L, JIA GZ, TANG M, et al. Simultaneous Enhancement of Anti-corrosion, Biocompatibility, and Antimicrobial Activities by Hierarchically-structured Brushite/Ag3PO4-Coated Mg-based Scaffolds. Mater Sci Eng C. 2020;111:110779. [6] 刘凯,马江,何新.基于Citespace的巴戟天研究进展和热点可视化分析[J].药物评价研究,2024,47(10):2399-2411. [7] 张子旋,安逸,张馨文,等.基于CiteSpace对青藤碱在中医药研究应用现状的可视化分析[J].中国医药导报,2022,19(36):29-35. [8] 陈悦,陈超美,刘则渊,等.CiteSpace知识图谱的方法论功能[J].科学学研究,2015, 33(2):242-253. [9] 贺雅洁,王延博,杨硕.基于CiteSpace对中医药治疗子宫肌瘤的可视化分析[J].世界中西医结合杂志, 2022,17(12):2374-2380. [10] 党小雯,黄海量,黄雷,等.生物医学领域碳纳米材料10年研究前沿与热点[J].中国组织工程研究,2025,29(4):752-760. [11] 文跃然,王丹阳,刘颖.国内外医院薪酬研究分析对我国公立医院薪酬制度设计的启示:基于Citespace和Vosviewer的文献计量[J].中国卫生政策研究,2022,15(12):1-8. [12] 李晓陵,高瑞雪,李昂,等.基于VOSviewer与CiteSpace的fMRI研究血管性认知障碍可视化分析[J]. 磁共振成像,2022,13(11):99-104. [13] 谢恩礼,陶慧敏.血流限制训练在临床康复医学中的应用趋势[J].中国组织工程研究, 2024,28(2): 258-262. [14] 梁杉,吉晓鹏,康英豪,等.基于CiteSpace的絮凝作用改善污泥脱水性能研究进展[J].应用化工,2023,52(11):3199-3204. [15] HSU YC, LU YP, WANG SY, et al. Magnesium Alloys in Tumor Treatment: Current Research Status, Challenges and Future Prospects. J Magnesium Alloys. 2023;11(10):3399-3426. [16] 郑玉峰,夏丹丹,谌雨农,等.增材制造可降解金属医用植入物[J].金属学报,2021, 57(11):1499-1520. [17] TONG X, DONG Y, ZHOU R, et al. Enhanced Mechanical Properties, Corrosion Resistance, Cytocompatibility, Osteogenesis, and Antibacterial Performance of Biodegradable Mg-2Zn-0.5Ca-0.5Sr/Zr Alloys for Bone-implant Application. Adv Healthc Mater. 2024;13(12): e2303975. [18] ZHANG X, YANG C, YANG K. Novel Antibacterial Metals as Food Contact Materials: A review. Materials (Basel). 2023;16(8):3029. [19] ERISEN DE, ZHANG YQ, ZHANG BC, et al. Biosafety and Biodegradation Studies of AZ31B Magnesium Alloy Carotid Artery stent in vitro and in vivo. J Biomed Mater Res B Appl Biomater. 2022;110(1):239-248. [20] 李一飞,周开宇,沈建通,等.基于关键词共现分析的我国先心病介入诊疗发展的可视化研究[J].临床儿科杂志,2012,30(7):631-637. [21] 郭文,冷军,刘会敏,等.脑卒中后尿失禁10年研究文献的可视化分析[J].中国组织工程研究,2021,25(35):5676-5681. [22] 李曌嫱,秦义,田元祥,等.针灸治疗腰椎间盘突出症的CiteSpace知识图谱可视化分析[J].中国针灸,2017,37(5):545-548. [23] 陈文娟.数字资源评价研究特征、前沿与展望: 基于WOS数据库的文献计量学分析[J].图书馆学研究,2022(7):2-14. [24] 高楠,周庆山.基于共被引方法的情报学前沿领域识别与演进趋势分析[J].现代情报, 2024,44(5):3-19. [25] 王瑾茜,喻嵘,黄娟,等.基于CiteSpace的中性粒细胞在糖尿病领域研究进展可视化分析[J].中国比较医学杂志,2024,34(6):28-39. [26] ZHENG YF, GU XN, WITTE F. Biodegradable Metals. Mater Sci Eng R. 2014;77:1-34. [27] ZHAO DZ, WITTE F, LU FQ, et al. Current Status on Clinical Applications of Magnesium-based Orthopedic Implants: A Review from Clinical Translational Perspective. Biomaterials. 2017;112:287-302. [28] CHEN Y, XU Z, SMITH C, et al. Recent Advances on The Development of Magnesium alloys for biodegradable implants. Acta Biomater. 2014;10(11):4561-4573. [29] ESMAILY M, SVENSSON JE, FAJARDO S, et al. Fundamentals and Advances in Magnesium Alloy Corrosion. Prog Mater Sci. 2017;89:92-193. [30] YANG H, JIA B, ZHANG Z, et al. Alloying Design of Biodegradable Zinc as Promising Bone Implants for Load-bearing Applications. Nat Commun. 2020;11(1):401. [31] KABIR H, MUNIR K, WEN C, et al. Recent Research and Progress of Biodegradable Zinc Alloys and Composites for Biomedical Applications: Biomechanical and Biocorrosion Perspectives. Bioact Mater. 2021;6(3):836-879. [32] ROUT PK, ROY S, GANGULY S, et al. A Review on Properties of Magnesium-Based Alloys for Biomedical Applications. Biomed Phys Eng Express. 2022;8(4):042002. [33] 王晶华,陈祺琪,顾金科.我国农业科技创新研究热点及演进态势:基于CiteSpace的可视化分析[J].科技管理研究,2022,42(22): 8-16. [34] 邹婧,楚尧娟,杜秋争,等.酪氨酸激酶抑制剂在HER2阳性乳腺癌中应用的可视化分析[J].中国药房,2023,34(24):3036-3041. [35] AGARWAL S, CURTIN J, DUFFY B, et al. Biodegradable Magnesium Alloys for Orthopedic Applications A Review on Corrosion, Biocompatibility and Surface Modifications. Mater Sci Eng C. 2016;68:948-963. [36] LIU B, ZHANG X, XIAO GY, et al. Phosphate Chemical Conversion Coatings on Metallic Substrates for Biomedical Application: A review. Mater Sci Eng C. 2015;47:97-104. [37] GARIMELLA A, RAMYA M, GHOSH SB, et al. Bioactive Fluorcanasite Reinforced Magnesium Alloy-based Porous Bio-nanocomposite Scaffolds with Tunable Mechanical Properties. J Biomed Mater Res B Appl Biomater. 2023; 111(2):463-477. [38] MARTYNENKO NS, ANISIMOVA NY, KISELEVSKIY MV, et al. In Vitro Biodegradation of Resorbable Magnesium Alloys Promising for Implant Development. Sovrem Tekhnologii Med. 2021;12(6):47-52. [39] GNEDENKOV AS, FILONINA VS, SINEBRYUKHOV SL, et al. A Superior Corrosion Protection of Mg Alloy via Smart Nontoxic Hybrid Inhibitor-Containing Coatings. Molecules. 2023;28(6):2538. [40] ANGRISANI N, AHE CVD, WILLUMEIT-RMER R, et al. Treatment of Osteoarthritis by Implantation of Mg-and WE43-cylinders-A Preclinical Study on Bone and Cartilage Changes and Their Influence on Pain Sensation in Rabbits. Bioact Mater. 2024;40:366-377. [41] 许雅南,王伟强,杨帅康,等.铁基材料在生物可降解血管支架领域的研究进展[J].钢铁钒钛,2023, 44(4):158-166. [42] 韩伟,张兴凯,郑玉峰,等.可降解铁基支架材料的研究进展[J].材料导报,2013, 27(S2):205-208. [43] SU Y, COCKERILL I, WANG Y, et al. Zinc-Based Biomaterials for Regeneration and Therapy. Trends Biotechnol. 2019;37(4):428-441. [44] JOSHI A, DIAS GJ, STAIGER MP. Surgically-Induced Deformation in Biodegradable Orthopaedic Implant Devices. Acta Biomater. 2022;154:667-675. [45] LI W, QIAO W, LIU X, et al. Biomimicking Bone-Implant Interface Facilitates the Bioadaption of A New Degradable Magnesium Alloy to The Bone Tissue Microenvironment. Adv Sci. 2021;8(23):e2102035. [46] CHEN Y, XIAO M, ZHAO H, et al. On the Antitumor Properties of Biomedical Magnesium Metal. J Mater Chem B. 2015;3(5): 849-858. [47] 郑玉峰.可降解金属研究前沿进展[J].材料科学与工艺,2023,31(2):1-14. [48] 孟帅举,王孟璐,宋金龙,等.Cu对铸态Mg-Bi-Sn系合金微观组织与力学性能的影响[J].中国有色金属学报,2024,34(10):3352-3365. [49] 贾澳,魏珂正,徐媛媛,等.Al、Zn元素添加对Mg-Cu合金微观组织和力学性能的影响[J].材料热处理学报,2024,45(8):58-65. [50] RAPIEJKO C, MIKUSEK D, JANUSZEWICZ B, et al. Refinement of The Magnesium-Aluminium Alloy Microstructure with Zirconium. Materials (Basel). 2022;15(24):8982. [51] ALATEYAH AL, ALAWAD MO, AUOHANI TA, et al. Influence of UItra Fine-grained Microstructure and Texture Evolution of ECAP ed ZK30 Magnesium Alloy on The Corrosion Behavior in Different Corrosive Agents. Materials. 2022;15(16):5515. [52] MOHAMED A, ELAZIZ A, BREITINGER HG. Study of The Degradation Behavior and The Biocompatibility of Mg-0.8Ca Alloy for Orthopedic Implant Applications. J Magnesium Alloys. 2019;7(2):249-257. [53] YANG X, CHEN S, ZHANG S, et al. Intracellular Zinc Protects Kv7 K+ Channels from Ca2+ Calmodulin-Mediated Inhibition. J Biol Chem. 2023;299(2):102819. [54] GONG XL, CHEN JH, YAN HG, et al. Effects of Minor Sr Addition on Biocorrosion and Stress Corrosion Cracking of As-Cast Mg-4Zn Alloys. Corrosion. 2020;76(1):71-81. [55] TALTAVULL C, TORRES B, LOPEZ A J, et al. Selective Laser Surface Melting of a Magnesium-Aluminum Alloy. Mater Lett. 2012;85:98-101. [56] HE M, LU W, YU D, et al. Corrosion Behavior and Biocompatibility of Na2EDTA-Induced Nacre Coatings on AZ91D Alloys Prepared via Hydrothermal Treatment. Front Chem. 2022; 9:810886. [57] KOZINA I, KRAWIEC H, STAROWICZ M, et al. Corrosion Resistance of MgZn Alloy Covered by Chitosan-Based Coatings. Int J Mol Sci. 2021;22(15):8301. [58] WU Y, ZHU B, ZHANG X, et al. Preparation and Characterization of Y-doped Microarc Oxidation Coating on AZ31 Magnesium Alloys. J Biomater Appl. 2022;37(5):930-941. [59] 王嘉昀,吴俏兰,高祖,等.基于科学知识图谱的桔梗研究热点与趋势分析[J].中医药导报,2023,29(10):110-118. [60] LI W, QIAO W, LIU X, et al. Biomimicking Bone-Implant Interface Facilitates the Bioadaption of a New Degradable Magnesium Alloy to the Bone Tissue Microenvironment. Adv Sci. 2021;8(23):e2102035. [61] HWANG SW, TAO H, KIM DH, et al. A Physically Transient Form of Silicon Electronics. Science. 2012;337(6102):1640-1644. [62] WANG XH, NI JS, CAO NL, et al. In Vivo Evaluation of Mg-6Zn and Titanium Alloys on Collagen Metabolism in the Healing of Intestinal Anastomosis. Sci Rep. 2017;7:44919. [63] BAO G, FAN Q, GE D, et al. In Vitro and In Vivo Studies to Evaluate the Feasibility of Zn-0.1Li and Zn-0.8Mg Application in the Uterine Cavity Microenvironment Compared to Pure Zinc. Acta Biomater. 2021;123:393-406. [64] YU T, ZHENG L, CHEN G, et al. A Study to Compare the Efficacy of a Biodegradable Dynamic Fixation System with Titanium Devices in Posterior Spinal Fusion between Articular Processes in a Canine Model. J Biomech Eng. 2021;143(3):031010. [65] REYES RL, GHIM MS, KANG NU, et al. Development and Assessment of Modified-Honeycomb-Structure Scaffold for Bone Tissue Engineering. Addit Manuf. 2022;54:102740. [66] TORRONI A, XIANG CC, WITEK L, et al. Histo-Morphologic Characteristics of Intra-osseous Implants of WE43 Mg Alloys With and Without Heat Treatment in an in Vivo Cranial Bone Sheep Model. J Craniomaxillofac Surg. 2018;3(46):473-478. |
| [1] | Xu Canli, He Wenxing, Wang Yuping, Ba Yinying, Chi Li, Wang Wenjuan, Wang Jiajia. Research context and trend of TBK1 in autoimmunity, signaling pathways, gene expression, tumor prevention and treatment [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(在线): 1-11. |
| [2] | Lai Yu, Chen Yueping, Zhang Xiaoyun. Research hotspots and frontier trends of bioactive materials in treating bone infections [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2132-2144. |
| [3] | Zhang Haiwen, Zhang Xian, Xu Taichuan, Li Chao. Bibliometric and visual analysis of the research status and trends of senescence in osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1580-1591. |
| [4] | Huang Jie, Zeng Hao, Wang Wenchi, Lyu Zhucheng, Cui Wei. Visualization analysis of literature on the effect of lipid metabolism on osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1558-1568. |
| [5] | Yang Zeyu, Zhi Liang, Wang Jia, Zhang Jingyi, Zhang Qingfang, Wang Yulong, Long Jianjun. A visualized analysis of research hotspots in high-frequency repetitive transcranial magnetic stimulation from the macroscopic perspective [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1320-1330. |
| [6] | Peng Hao, Chen Qigang, Shen Zhen. A visual analysis of research hotspots of H-type vessels in various bone diseases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 760-769. |
| [7] | Zhang Anqi, Hua Haotian, Cai Tianyuan, Wang Zicheng, Meng Zhuo, Zhan Xiaoqian, Chen Guoqian . Pain after total knee arthroplasty: current status and trend analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 795-804. |
| [8] | Dong Chao, Zhao Mohan, Liu Yunan, Yang Zeli, Chen Leqin, Wang Lanfang. Effects of magnetic nano-drug carriers on exercise-induced muscle injury and inflammatory response in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 345-353. |
| [9] | Li Congcong, Wufanbieke·Baheti, Zhao Li, Chen Xiaotao, Kong Chuifan, Yu Min. Physicochemical properties and biocompatibility of hydroxyapatite/graphene oxide/interleukin-4 composite coating materials [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 404-413. |
| [10] | Zhang Qian, Wang Fuxia, Wang Wen, Zhang Kun. Characteristic analysis of nanogel composite system and its application strategies in visualization of diagnostic imaging and therapy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 480-488. |
| [11] | Jiang Kan, Alimujiang·Abudourousuli, Shalayiding·Aierxiding, Aikebaierjiang·Aisaiti, Kutiluke·Shoukeer, Aikeremujiang·Muheremu. Biomaterials and bone regeneration: research hotspots and analysis of 500 influential papers [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 528-536. |
| [12] | Zhao Qianwei, Sun Guangyuan . Intestinal organoids: a bibliometric analysis of the latest trends in tissue/organ biology, disease modeling, and clinical applications [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 238-247. |
| [13] | Ao Xiaojing, Li Kun, Liu Yuhang, Yang Xiaoxuan, Wang Xing, Li Zhijun, Ren Xiaoyan, Zhang Shaojie. Development and application of a three-dimensional digital visualization system for children’s neck acupoints [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1834-1840. |
| [14] | Liang Haobo, Wang Zeyu, Ma Wenlong, Liu Hao, Liu Youwen. Hot issues in the field of joint revision: infection, rehabilitation nursing, bone defect, and prosthesis loosening [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1963-1971. |
| [15] | Lyu Liting, Yu Xia, Zhang Jinmei, Gao Qiaojing, Liu Renfan, Li Meng, Wang Lu. Bibliometric analysis of research process and current situation of brain aging and exosomes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1457-1465. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||