Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (34): 7369-7375.doi: 10.12307/2025.499
Previous Articles Next Articles
Material characterization of finite element computational models of knee joints at different ages
Chen Jing1, Zhang Nan2, Meng Qinghua1, Bao Chunyu2
- 1School of Economics Management, 2Academy of Social Sports, Tianjin University of Sport, Tianjin 301617, China
-
Received:2024-07-01Accepted:2024-08-28Online:2025-12-08Published:2025-01-17 -
Contact:Meng Qinghua, Professor, School of Economics Management, Tianjin University of Sport, Tianjin 301617, China -
About author:Chen Jing, PhD, Associate professor, School of Economics Management, Tianjin University of Sport, Tianjin 301617, China -
Supported by:National Natural Science Foundation Project, No. 11372223, 11102135 (to MQH); Tianjin Natural Science Foundation Project, No. 17JCZDJC36000 (to BCY); Tianjin Natural Science Foundation Project, No. 18JCZDJC35900 (to MQH); Science and Technology Innovation Project of General Administration of Sport of China, No. 22KJCX077 (to BCY); Tianjin Graduate Innovation Project, No. 2022SKYZ318 (to ZN)
CLC Number:
Cite this article
Chen Jing, Zhang Nan, Meng Qinghua, Bao Chunyu. Material characterization of finite element computational models of knee joints at different ages[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7369-7375.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
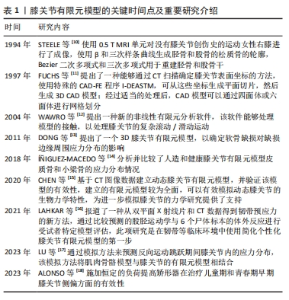
为获得有限元计算模型的机械参数,需要进行生物力学实验,生物力学实验的静态分析适用于瞬态变化或平衡状态,可用于研究软组织在某一时刻的特征变化。而受试者年龄的变化会严重影响组织结构和机械性能,影响膝关节中各个结构材料属性的选择。在有限元建模过程中,按照受试者年龄段选择合适的材料特性和材料参数可以更好地描述膝关节的力学特性,这对提高有限元的精度至关重要。下面将分别描述不同年龄阶段膝关节各结构的模型材料特性。 2.1 膝关节有限元模型研究情况 膝关节有限元模型的关键时间点及重要研究介绍,见表1。STEELE等[10]使用0.5 T MRI单元对没有膝关节创伤史的运动女性右膝进行了成像。FUCHS等[11]提出了一种能够通过CT扫描确定膝关节表面坐标的方法。WAWRO等[12]提出一种新的非线性有限元分析软件。DONG等[13]提出了一个3D膝关节有限元模型,以确定软骨缺损对缺损边缘周围应力分布的影响。í?IGUEZ-MACEDO等[14]分析并比较了人造和健康膝关节有限元模型皮质骨和小梁骨的应力分布情况。CHEN等[15]基于CT图像数据建立动态膝关节有限元模型,并验证该模型的有效性。LAHKAR等[16]报道了一种从双平面X射线片和CT数据得到韧带预应力的新方法。LU等[17]通过模拟方法来预测反向运动跳跃期间膝关节内的应力分布,该模拟方法将肌肉骨骼模型与膝关节的有限元模型相结合。ALONSO等[18]施加恒定的负荷提高矫形器在治疗儿童期和青春期早期膝关节侧偏方面的有效性。"
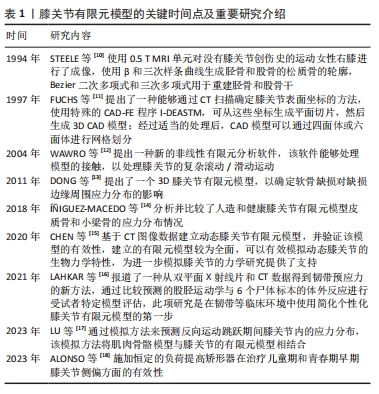
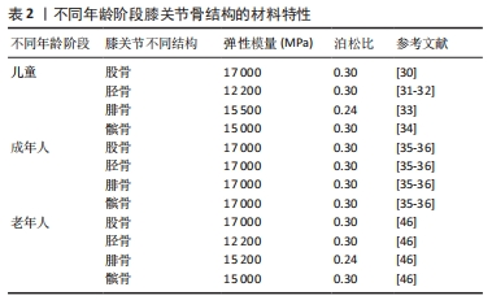
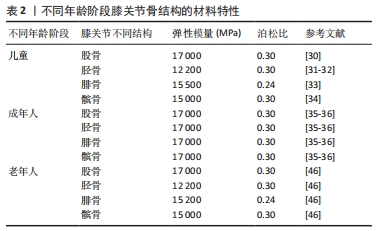
2.2 骨(股骨、胫骨、腓骨、髌骨) 膝关节中骨(股骨、胫骨、腓骨、髌骨)的解剖学、生理学、生化、矿化和病理学方面随年龄的变化已有大量文献,而关于膝关节骨材料特性随年龄变化的文献很少。 2.2.1 骨密度与年龄之间的关系 皮质骨是一种有机结构,矿物质含量约占人骨骼的80%。影响人类皮质骨质量的病理因素包括成人骨质疏松症[19]、儿童骨质疏松症[20]、克罗恩-S病或骨化症[21-22]。相关研究发现,由于人口老龄化的加剧,预测到2050年骨质疏松性骨折的直接成本将达到767亿欧元[23]。儿童时期的低骨量被认为是晚年骨质疏松的高危因素[24],相关研究认为评估儿童骨密度非常重要[25-26]。骨密度是评估矿物质状况的黄金标准参数之一,首先需要使用双能X射线吸收仪进来检测。相关研究使用霍洛奇双能X射线吸收仪(霍洛奇公司,Waltham,12 MA,USA)对7-11岁儿童进行骨密度扫描[27]。骨密度与骨微结构无关。多项研究表明,超声波测量能够评估探索骨区域(弹性和结构)的数量和质量[28-29]。KIM等[30]将儿童膝关节有限元计算模型中股骨材料的弹性模量与泊松比分别设置为17 000 MPa、0.3;LIMBERT等[31]与LANCIANESE等[32]将儿童胫骨材料的弹性模量与泊松比分别设置为12 200 MPa、0.3;BERTEAU等[33]将儿童腓骨材料的弹性模量与泊松比分别设置为15 500 MPa、0.24;KIAPOUR等[34]将儿童髌骨材料的弹性模量与泊松比分别设置为15 000 MPa、0.3。而有关成年人膝关节有限元计算模型中股骨、胫骨、腓骨、髌骨的弹性模量和泊松比设置为17 000 MPa、0.3[35-36]。这是由于骨密度随着年龄的增长而增加,在成年后达到峰值[37]。 2.2.2 骨密度与弹性模量之间的关系 王倞等[38]将10例成人新鲜尸体作为样本源,选取胫骨近端、大转子、股 骨颈、肱骨头和椎体5个部位的松质骨,采用CT扫描试样骨密度,通过力学测试分析分析不同部位松质骨的弹性模量,发现骨密度与弹性模量之间存在较高的线性相关性(0.850 > R2 > 0.785)和幂次相关性(0.871> R2 > 0.825)。袁嘉尧等[39]通过Pearson相关性分析发现,中老年人整体骨密度与身高、体质量、骨矿含量(整体、头部、主干、上肢、下肢)、肌肉质量(整体、头部、主干、下肢)、头部脂肪质量呈显著正相关,而与年龄呈显著负相关。从中年到老年阶段,膝关节中股骨、胫骨、腓骨、髌骨的弹性模量会随着年龄的增长而降低,进而回归到儿童时期的骨弹性模量。 2.2.3 年龄与弹性模量之间的关系 有关年轻骨骼强度和弹性模量的数据非常缺乏,多篇论文报道了超声轴向透射数据的年龄依赖性[40-42],只有2项体外机械研究对儿童皮质骨弹性模量进行了定量研究[43-44],这2项研究都是对干燥样本进行破坏性测试,儿童骨骼实验值都支持理论优化假设[45],即骨骼弹性模量会从新生儿值上升到成人值,这一假设目前被用于儿科计算方法。儿童骨骼体外超声波速度和弹性模量值与现有文献中的老年成人值接近[46-47]。 评估儿童皮质骨弹性特性是生物力学工程界及更广泛医疗保健专业人员面临的重大挑战[48]。即使采用X射线断层扫描、MRI成像和超声波成像等经典临床模式,由于缺乏儿童骨骼的参考值,也可能导致诊断不当。相关研究利用超声波和机械测量方法提供了儿童(4-16岁,平均年龄10岁)皮质骨的弹性特性值,并与老年人(75 岁以上)进行了比较,首先通过超声波方法评估动态弹性模量和泊松比,其次通过三点微弯曲测试估算静态弹性模量,结果显示:儿童样本在 10 MHz 频率下测得的纵向和横向波速平均值分别为(3.2±0.5) mm/μs 和(1.8±0.1) mm/μs,老年人样本的波速分别为(3.5±0.2) mm/μs 和(1.9±0.1) mm/μs,儿童样本的平均动态弹性模量和平均静态弹性模量分别为 (15.5±3.4) GPa 和(9.1±3.5) GPa;老年人样本的平均动态弹性模量和平均静态弹性模量分别为(16.7±1.9) GPa和(5.8±2.1) GPa,表明儿童和老年人骨骼的动态弹性模量、泊松比和静态弹性模量在相同范围内,没有统计差异[49]。 UEHIRA[50]发现成年人皮质骨的抗压和抗拉强度都随着年龄的增长而下降。抗压强度的下降归因于骨密度的下降,抗拉强度的下降归因于哈弗管横截面积的增加。根据SUGIYAMA[51]的说法,皮质骨的洛氏硬度随着年龄的增长而降低,这种降低是由骨质疏松症引起的,而不是由于骨骼中钙含量的变化。BLACK等[52]对8例胫骨中段的成活皮质骨动态力学性能进行了研究,发现35.4 Hz处的拉伸弹性模数随年龄的增加而下降,而353.6 Hz处的拉伸弹性模数则没有这种变化。WILSON[53]研究了35-89岁个体24个骨骼标本的压缩强度和单位体积骨矿物质的变化,35岁以后骨骼标本的最大抗压强度每10年下降约7%,单位体积骨矿含量每10年下降约3.3%。 不同年龄阶段骨结构的材料特性,见表2。"
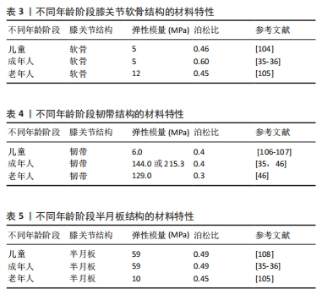
2.3 软骨 在膝关节有限元计算模型中,由于软骨结构光滑、柔软(刚性较小)、厚度较薄,因此在膝关节有限元建模过程中较为困难[54],也很难追踪软骨形态随年龄的演变。SI等[55]利用3-T超导扫描仪采集膝关节MRI图像,通过深度学习自动、精确地分割软骨,计算出14个解剖区域的软骨厚度,发现股骨软骨厚度始终大于胫骨软骨相应部位,除胫骨软骨内侧和外侧外,所有区域的软骨都随着年龄的增长而变薄。BLAZEK等[56]发现软骨厚度与体质量×身高呈正相关。相关研究发现,兔膝关节软骨弹性模量与关节软骨厚度呈负相关[57]。 由上可知,随着年龄的增加股骨软骨厚度在减少,而随着股骨软骨厚度的减少其弹性模量在增加。但是DéMARTEAU等[58]通过比较老年人股骨软骨与成年牛肱骨软骨发现,老年人股骨软骨具有更大的弹性模量。作者认为这是由于研究不同类型软骨得出的结论,不影响前文的结论。该文对后续学者研究软骨厚度与弹性模量直接的线性或非线性关系具有一定的指导意义。 2.4 韧带 膝关节韧带是由软组织组成的弹性带,具有复杂的微观结构和生物力学特性,对膝关节的运动学特性和应力承受能力至关重要[59-60]。从弹性一维单元到具有解剖逼真形状的复杂超弹性三维结构,已被广泛应用于膝关节韧带研究[61]。在膝关节3D模型中包含韧带的最直观方法是使用实体元素[62]。MRI扫描与3D重建软件相结合,提供了一种方便的方法来创建关节的详细模型,包括特定患者的韧带几何形状及其插入位置[63]。MRI扫描也可以很容易与CT相结合,CT提供了骨骼结构的高分辨率成像,这种方法还便于通过使用面对面接触来模拟韧带包裹,这在有限元程序中都可以实现[64]。韧带的材料特性(弹性模量、泊松比)选择对于计算精度非常重要,相关研究发现,青壮年人体标本中韧带的弹性模量、极限拉伸应力和破坏应变大约是60岁及以上人体标本的两三 倍[65],青壮年人体标本的主要破坏模式是韧带断裂,而老年人标本的主要破坏模式是韧带插入部位下方的骨骼撕裂,产生的差异和老年人标本韧带连接部位骨量减少有关。实验结果表明,随着年龄的增长,韧带的强度和刚度特性会显著降低,其程度超出预期。将年龄较大人体韧带标本实验结果应用于年轻或运动型个体的韧带破坏机制时,应谨慎解释,人们认为老年人的较差韧带生物力学特性减弱,同时关节固定也会导致韧带出现类似的功能性损伤。KASPERCZYK等[66]通过对6名年轻(平均年龄30岁)和6名年长(平均年龄64.7岁)活动水平相似供者的前交叉韧带和后交叉韧带进行了生物力学测试,发现年轻/年长前交叉韧带的最大应力为24/21 N/mm2)、年轻/年长前交叉韧带的弹性模量为144/129 MPa,年轻/年长前交叉韧带应变为0.25/0.28 mm。 综上所述,随着年龄的增长韧带弹性模量会出现一定程度的降低(出现减低的原因是由于随着年龄的增长韧带成纤维细胞的力学和生物学特性出现一定程度的退化),但是年轻人与老年人韧带之间的弹性模量无统计学差异,表明老年人并不一定会出现交叉韧带功能性损伤。 2.5 半月板 2.5.1 半月板的纳米力学特征随年龄的变化情况 半月板为人类膝关节提供基本支持并包含一系列功能,例如调节膝关节的负荷传递[67-69]。半月板含有沿圆周排列的胶原纤维,能够分布胫骨平台上的载荷,从而强化关节软骨并预防骨关节炎[70-73]。半月板通过称为“半月板附着物”的韧带结构与胫骨相连,其基质主要由蛋白多糖、弹性蛋白、糖脂、成纤维细胞以及60%-70%的水分组成[74-75]。这些强化的胶原纤维帮助半月板将载荷扩散到附着点,逐渐将软组织过渡到骨骼中,减少应力集中[76-78]。 随着年龄的增长,半月板的细胞外基质发生分子和结构变化,导致基质退化[79-80],这一退化过程的特征是合成代谢和分解代谢失衡,细胞减少了重要生长因子的产生,增加了基质降解酶和炎症因子的产生[81]。研究表明,衰老会导致半月板的胶原纤维排列紊乱、细胞减少等生化变化[77-78,82-84]。尽管衰老和骨关节炎引起的半月板退化已通过组织学和免疫组织化学分析研究,但尚未在微观结构层面详细研究其生物力学变化[85-87]。基于现有知识,衰老会改变半月板细胞外基质的生物力学特性,进而影响关节负荷时的反应,导致骨关节炎的发生[88]。研究还显示,随着年龄和疾病的进展,半月板的区域性机械性能逐渐模糊,机械完整性下降,这会破坏组织的生物力学功能,增加骨关节炎的风险。随着年龄增长,纳米力学性能的异质性逐渐增大[89-91]。 2.5.2 半月板的纳米力学特征与弹性模量之间的关系 正常、老年及骨关节炎患者的半月板组织在纳米力学特性上表现出显著差异[92]。年轻健康组织的弹性模量呈单峰分布,各区域表现出明确的峰值:外侧130-150 kPa、内侧70-90 kPa。老化组织同样呈现单峰分布,但其范围更宽,通常呈双峰,并且偏向较高的硬度值。整体上,双峰分布由一个较窄的第一峰和一个较宽的第二峰构成,表明退变相关的硬化区域有所增加。 骨关节炎不仅作为一种独立的疾病表现出特异的临床和生化特征,还随着组织的硬化而导致压缩刚度的增加,最终导致抗拉强度的下降,引发生物力学失效[93-95]。基质硬化源于细胞外基质中与年龄相关的分子变化,例如细胞外基质分子的异常沉积及交联,已知这些变化能够影响基质的机械特性,进而导致细胞功能异常[96-100]。半月板细胞对机械应力高度敏感,尤其是在关节受力时。细胞外基质中这些变化会促发或加剧与骨关节炎相关的细胞表型改变[101-103]。然而,基质力学与骨关节炎发展的确切关联仍有待进一步研究。 不同年龄阶段软骨、韧带与半月板结构的材料特性,见表3-5。"
| [1] TAPASVI S, SHEKHAR A, PATIL S, et al. Limb position influences component orientation in Oxford mobile bearing unicompartmental knee arthroplasty: an experimental cadaveric study. Bone Joint Res. 2020;9(6):272-278. [2] ARAUZ P, PENG Y, CASTILLO T, et al. In vitro kinematic analysis of single axis radius posterior-substituting total knee arthroplasty. J Knee Surg. 2021;34(11):1253-1259. [3] KONO K, TOMITA T, YAMAZAKI T, et al. Patellar resurfacing has minimal impact on in vitro tibiofemoral kinematics during deep knee flexion in total knee arthroplasty. Knee. 2021;30:163-169. [4] 潘正晔,马勇,郑伟涛.有限元分析立定跳远状态时不同摆臂动作和触地姿势的膝关节损伤[J].中国组织工程研究,2023,27(36): 5778-5783. [5] NAGHIBI H, JANSSEN D, VAN DEN BOOGAARD T, et al. The implications of non-anatomical positioning of a meniscus prosthesis on predicted human knee joint biomechanics. Med Biol Eng Comput. 2020;58(6): 1341-1355. [6] 韩广弢,李皓桓,高冯.创伤后膝骨关节炎发展中前交叉韧带损伤的作用与意义[J].中国组织工程研究,2020,24(15):2440-2446. [7] 王珂,张泽毅,张力文,等.不同年龄膝骨关节炎患者坐起生物力学特征的系统综述和Meta分析[J].中国组织工程研究,2024,28(18):2939-2946. [8] 张楠,孟庆华,鲍春雨.基于XCM模型预测排球运动员前交叉韧带应力[J].医用生物力学,2024,39(6):1146-1153. [9] KNOWLES NK, WHITTIER DE, BESLER BA, et al. Proximal tibia bone stiffness and strength in HR-pQCT-and QCT-based finite element models. Ann Biomed Eng. 2021;49(9):2389-2398. [10] STEELE JR, BASU A, JOB A. A three-dimensional representation of an athletic female knee joint using magnetic resonance imaging. Med Eng Phys. 1994;16(5): 363-369. [11] FUCHS S, KULLMER G, RICHARD H. Darstellung der Konstruktion eines FE-Modells am Beispiel des Kniegelenkes - Designing an FE Model as Exemplified by the Knee Joint. Biomedical Engineering. 1997;42(12): 347-351. [12] WAWRO M, FATHI-TORBAGHAN M. A parallel framework for the FE-based simulation of knee joint motion. IEEE Trans Biomed Eng. 2004;51(8):1490-1494. [13] DONG YF, HU GH, ZHANG LL, et al. Accurate 3D reconstruction of subject-specific knee finite element model to simulate the articular cartilage defects. J Shanghai Jiaotong Univ Sci. 2011;16:620-627. [14] ÍÑIGUEZ-MACEDO S, SOMOVILLA-GÓMEZ F, LOSTADO-LORZA R, et al. The process of designing a rotating platform artificial knee prosthesis with posterior stabilizers by finite element analysis. Int J Interact Des Manuf. 2018;12:853-864. [15] CHEN YF, LU C, ZHAO Y, et al. [Construction and simulation mechanical analysis of dynamic knee joint finite element model based on CT image]. Zhongguo Gu Shang. 2020;33(5):479-484. [16] LAHKAR BK, ROHAN PY, PILLET H, et al. Development and evaluation of a new procedure for subject-specific tensioning of finite element knee ligaments. Comput Methods Biomech Biomed Engin. 2021;24(11):1195-1205. [17] LU Z, SUN D, KOVÁCS B, et al. Case study: The influence of Achilles tendon rupture on knee joint stress during counter-movement jump - Combining musculoskeletal modeling and finite element analysis. Heliyon. 2023;9(8):e18410. [18] ALONSO MG, YAWNY A, BERTOLINO G. A numerical study towards shape memory alloys application in orthotic management of pediatric knee lateral deviations. Sci Rep. 2023;13(1):2134. [19] MCDONNELL P, MCHUGH PE, O’MAHONEY D. Vertebral osteoporosis and trabecular bone quality. Ann Biomed Eng. 2007;35(2):170-189. [20] MEHLMAN CT, SHEPHERD MA, NORRIS CS, et al. Diagnosis and Treatment of Osteopenic Fractures in Children. Curr Osteoporos Rep. 2012;10(4):317-321. [21] WALTERS TD. IBD: Is measuring bone age in children with Crohn’s disease useful. Nat Rev Gastroenterol Hepatol. 2012;9(11):620-622. [22] ENGIZ O, KARA S, BAGRUL D, et al. Infantile malignant osteopetrosis: a rare cause of neonatal hypocalcemia. J Pediatr Endocrinol Metab. 2012;25(11-12):1205-1207. [23] KANIS JA, COOPER C, HILIGSMANN M, et al. Partial adherence: a new perspective on health economic assessment in osteoporosis. Osteoporos Int. 2011;22(10):2565-2573. [24] JAVAID MK, COOPER C. Prenatal and childhood influences on osteoporosis. Best Pract Res Clin Endocrinol Metab. 2002;16(2):349-367. [25] BACHRACH LK. Osteoporosis in children: still a diagnostic challenge. J Clin Endocrinol Metab. 2007;92(6):2030-2032. [26] ZHANG C, LIU Z, KLEIN GL. Overview of pediatric bone problems and related osteoporosis. J Musculoskelet Neuronal Interact. 2012;12(3):174-182. [27] KALKWARF HJ, ZEMEL BS, GILSANZ V, et al. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab. 2007;92(6):2087-2099. [28] LAUGIER P. Instrumentation for in vivo ultrasonic characterization of bone strength. IEEE Trans Ultrason Ferroelectr Freq Control. 2008; 55(6):1179-1196. [29] CRETIN D, SUETOSHI R, UODOME A, et al. Assessment of cortical bone density and anisotropy in human femur using ultrasound and x-ray. J Acoust Soc Am. 2008;123(5):3785. [30] KIM JE, LI Z, ITO Y,e t al. Finite element model development of a child pelvis with optimization-based material identification. J Biomech. 2009;42:2191-2195. [31] LIMBERT G, ESTIVALÈZES E, HOBATHO MC, et al. In vivo determination of homogenized mechanical characteristics of human tibia: application to the study of tibial torsion in vivo. Clin Biomech. 1998;13:473-479. [32] LANCIANESE SL, GUSHUE DL, YAO J, et al. Risks of trabecular bone fatigue damage in children who are overweight.//52nd annual meeting of the Orthopaedic Research Society. 2005:0321. [33] BERTEAU JP, BARON C, PITHIOUX M, et al. Children cortical bone characterisation: the ultrasonic issue. Proceedings of acoustics 2012 nantes conference. 2012:217-221. [34] KIAPOUR A, KIAPOUR AM, KAUL V, et al. Finite element model of the knee for investi-gation of injury mechanisms: development and validation. J Biomech Eng. 2014;136:011002. [35] 鲍春雨,郭宝川,孟庆华.人体膝关节有限元模型建立及其有效性验证[J].应用力学学报,2017,34(3):559-563+615. [36] 杨骏良,路坦,徐彪,等.前交叉韧带部分断裂对膝关节应力影响的三维有限元分析[J].中国组织工程研究,2024,28(9):1347-1353. [37] 刘蒙飞,马鹏程,尹灿,等.不同骨密度对膝关节单髁置换后关节内各结构影响的三维有限元分析[J].中国组织工程研究,2024,28(24):3801-3806. [38] 王倞,李元超,汪方,等.人体松质骨矿质密度与弹性模量关系[J].医用生物力学,2014,29(5):465-470. [39] 袁嘉尧,林燕平,林贤灿,等.中老年人体成分与骨密度的关系[J].中国组织工程研究,2022,26(15):2394-2399. [40] WEISS M, BEN-SHLOMO AB, HAGAG P, et al. Reference database for bone speed of sound measurement by a novel quantitative multi-site ultrasound device. Osteoporos Int. 2000;11(8):688-696. [41] TALMANT M, KOLTA S, ROUX C, et al. In vivo performance evaluation of bi-directional ultrasonic axial transmission for cortical bone assessment. Ultrasound Med Biol. 2009;35(6):912-919. [42] KILAPPA V, MOILANEN P, XU L, et al. Low-frequency axial ultrasound velocity correlates with bone mineral density and cortical thickness in the radius and tibia in pre- and postmenopausal women. Osteoporos Int. 2011;22(4):1103-1113. [43] CURREY JD. Changes in the impact energy absorption of bone with age. J Biomech. 1979;12(6):459-469. [44] ÖHMAN C, BALEANI M, PANI C, et al.Compressive behaviour of child and adult cortical bone. Bone. 2011;49(4):769-776. [45] Child Occupant Protection Symposium, Society of Automotive Engineers, Association for the Advancement of Automotive Medicine, International Research Council on the Biomechanics of Impact, Stapp Car Crash Conference. Child Occupant Protection 2nd Symposium proceedings. Society of Automobile Engineers. 1997. [46] EVANS FG. Mechanical properties and histology of cortical bone from younger and older men. Anat Rec. 1976;185(1):1-11. [47] LOTZ JC, GERHART TN, HAYES WC. Mechanical properties of metaphyseal bone in the proximal femur. J Biomech. 1991;24(5):317-329. [48] ALBERT CI, JAMESON J, HARRIS G. Design and validation of bending test method for characterization of miniature pediatric cortical bone specimens. Proc Inst Mech Eng H. 2013;227(2):105-113. [49] BERTEAU JP, BARON C, PITHIOUX M, et al. In vitro ultrasonic and mechanic characterization of the modulus of elasticity of children cortical bone. Ultrasonics. 2014;54(5):1270-1276. [50] UEHIRA T. On the relation between the chemical components and the strength of the compact bone. J Kyoto Prefect Led. Univ. 1960;68:923-940. [51] SUGIYAMA Y. A study on the hardness of huinan bones. J Kyoto Prefect Med Univ. 1960;68:557-569. [52] BLACK J, KOROSTOFF E. Dynamic mechanical properties of viable human cortical bone. J Biomech. 1973;6(5):435-438. [53] WILSON CR. Age related changes in the compressive strength and bone mineral per unit volume of compact bone. Phys Med Biol. 1973;18:587. [54] SHAH RF, MARTINEZ AM, PEDOIA V, et al. Variation in the thickness ofknee cartilage. The use ofa novel machine learning algorithm for cartilage segmentation of magnetic resonance images. J Arthropl. 2019;34:2210-2215. [55] SI L, XUAN K, ZHONG J, et al. Knee Cartilage Thickness Differs Alongside Ages: A 3-T Magnetic Resonance Research Upon 2,481 Subjects via Deep Learning. Front Med (Lausanne). 2021;7:600049. [56] BLAZEK K, FAVRE J, ASAY J, et al. Age and obesity alter the relationship between femoral articular cartilage thickness and ambulatory loads in individuals without osteoarthritis. J Orthop Res. 2014;32:394-402. [57] RÄSÄNEN T. MESSNER K. Regional variations of indentation stiffness and thickness of normal rabbit knee articular cartilage. J Biomed Mater Res. 1996;31:519-524. [58] DÉMARTEAU O, PILLET L, INAEBNIT A, et al. Biomechanical characterization and in vitro mechanical injury of elderly human femoral head cartilage: comparison to adult bovine humeral head cartilage. Osteoarthritis Cartilage. 2006;14(6):589-596. [59] TRENT PS, WALKER PS, WOLF B. Ligament length patterns, strength, and rotational axes of the knee joint. Clin Orthop Relat Res. 1976;(117): 263-270. [60] VERONDA DR, WESTMANN RA. Mechanical characterization of skin-finite deformations. J Biomech. 1970;3(1):111-124. [61] VICECONTI M, OLSEN S, NOLTE LP, et al. Extracting clinically relevant data from finite element simulations. Clin Biomech (Bristol, Avon). 2005;20(5):451-454. [62] WANG Y, FAN Y, ZHANG M. Comparison of stress on knee cartilage during kneeling and standing using finite element models. Med Eng Phys. 2014;36(4):439-447. [63] PIERRAT B, MURPHY JG, MACMANUS DB, et al. Finite element implementation of a new model of slight compressibility for transversely isotropic materials. Comput Methods Biomech Biomed Engin. 2016;19(7):745-758. [64] WEISS JA, GARDINER JC. Computational modeling of ligament mechanics. Crit Rev Biomed Eng. 2001;29(3):303-371. [65] NOYES FR, GROOD ES. The strength of the anterior cruciate ligament in humans and Rhesus monkeys. J Bone Joint Surg Am. 1976;58(8):1074-1082. [66] KASPERCZYK WJ, ROSOCHA S, BOSCH U, et al. Alter, Aktivität und die Belastbarkeit von Kniebändern [Age, activity and strength of knee ligaments]. Unfallchirurg. 1991;94(7):372-375. [67] ALHALKI MM, HOWELL SM. How three methods for fixing a medial meniscal autograft affect tibial contact mechanics. Am J Sports Med. 1999;27(3):320-358. [68] BENJAMIN M, EVANS EJ. Quantitative differences in the histology of the attachment zones of the meniscal horns in the knee joint of man. J Anat. 1991; 177:127-134. [69] BENJAMIN M, RALPHS JR. Fibrocartilage in tendons and ligaments--an adaptation to compressive load. J Anat. 1998;193(Pt 4):481-494. [70] AHN JH, LEE YS. Arthroscopic all inside repair of the lateral meniscus root tear. Knee. 2009;16(1):77-80. [71] GAO J, OQVIST G. The attachments of the rabbit medial meniscus. A morphological investigation using image analysis and immunohistochemistry. J Anat. 1994;185(Pt 3):6639-6667. [72] GARDINER JC, WEISS JA. Subject-specific finite element analysis of the human medial collateral ligament during valgus knee loading. J Orthop Res. 2003; 21(6):1098-1106. [73] GOERTZEN D, GILLQUIST J. Tensile strength of the tibial meniscal attachments in the rabbit. J Biomed Mater Res. 1996;30(1):125-128. [74] HAUCH KN, VILLEGAS DF. Geometry time-dependent and failure properties of human meniscal attachments. J Biomech. 2010;43(3):463-468. [75] HAUT DONAHUE TL, HULL ML. How the stiffness of meniscal attachments and meniscal material properties affect tibio-femoral contact pressure computed using a validated finite element model of the human knee joint. J Biomech. 2003;36(1):19-34. [76] ADAMS ME, BILLINGHAM ME, MUIR H. The glycosaminoglycans in menisci in experimental and natural osteoarthritis. Arthritis Rheum. 1983;26:69-76. [77] HELLIO L, GRAVERAND MP, VIGNON E, et al. Early changes in lapine menisci during osteoarthritis development: Part I: cellular and matrix alterations. Osteoarthritis Cartilage. 2001;9:56-64. [78] HELLIO LE GRAVERAND MP, VIGNON E, et al. Early changes in lapine menisci during osteoarthritis development Part II: Molecular alterations. Osteoarthritis Cartilage. 2001;9:65-72. [79] HERWIG J, EGNER E, BUDDECKE E. Chemical changes of human knee joint menisci in various stages of degeneration. Ann Rheum Dis. 1984;43:635-640. [80] ISHIHARA G, KOJIMA T, SAITO Y,et al. Roles of metalloproteinase-3 and aggrecanase 1 and 2 in aggrecan cleavage during human meniscus degeneration. Orthop Rev (Pavia). 2009;1(2):e14. [81] PAULI C, GROGAN S, PATIL S, et al. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthritis Cartilage. 2011;19:1132-1141. [82] BURMAN MS, SUTRO CJ. A study of the degenerative changes of the menisci of the knee joint, and the clinical significance thereof. Bone Joint Surg. 1933;15: 835-861. [83] ENGLUND M. The role of the meniscus in osteoarthritis genesis. Med Clin North Am.2009; 93:37-43. [84] LOESER RF. Age-related changes in the musculoskeletal system and the development of osteoarthritis. Clin Geriatr Med. 2010;26:371. [85] BHATTACHARYYA T, GALE D, DEWIRE P, et al. The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. J Bone Joint Surg Am. 2003;85(1):4-9. [86] ENGLUND M, GUERMAZI A, GALE D,et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008;359(11):1108-1115. [87] MAKRIS EA, HADIDI P, ATHANASIOU KA. The knee meniscus: structure–function, pathophysiology,current repair techniques, and prospects for regeneration. Biomaterials. 2011;32:7411-7431. [88] FELSON DT, LAWRENCE RC, DIEPPE PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635-646. [89] WANG X, SHEN X, LI X, et al. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31:1-7. [90] VERZIJL N, DEGROOT J, OLDEHINKEL E, et al. Age-related accumulation of Maillard reaction products in human articular cartilage collagen. Biochem J. 2000;350: 381-387. [91] VERZIJL N, DEGROOT J, ZAKEN CB, et al. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002;46:114-123. [92] KWOK J, GROGAN S, MECKES B, et al. Atomic force microscopy reveals age-dependent changes in nanomechanical properties of the extracellular matrix of native human menisci: implications for joint degeneration and osteoarthritis. Nanomedicine. 2014;10(8):1777-1785. [93] FELSON DT, LAWRENCE RC, DIEPPE PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635-646. [94] WANG X, SHEN X, LI X, et al. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31:1-7. [95] INGBER DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575-599. [96] INGBER DE. Mechanobiology and diseases of mechanotransduction. Annu Rev Physiol. 2003;35:564-577. [97] WANG N, BUTLER JP, INGBER DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124-1127. [98] WANG N, TYTELL JD, INGBER DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10:75-82. [99] DISCHER DE, JANMEY P, WANG YL. Tissue cells feel and respond to the stiffness of their substrate.Science. 2005;310:1139-1143. [100] FINK C, FERMOR B, WEINBERG J, et al. The effect of dynamic mechanical compression on nitric oxide production in the meniscus. Osteoarthritis Cartilage. 2001;9:481-487. [101] FERMOR B, JEFFCOAT D, HENNERBICHLER A, et al. The effects of cyclic mechanical strain and tumor necrosis factor alpha on the response of cells of the meniscus. Osteoarthritis cartilage. 2004;12:956-962. [102] UPTON ML, CHEN J, GUILAK F, et al. Differential effects of static and dynamic compression on meniscal cell gene expression. J Orthop Res. 2003;21:963-969. [103] UPTON ML, GUILAK F, LAURSEN TA, et al. Finite element modeling predictions of region-specific cell-matrix mechanics in the meniscus. Biomech Model Mechanobiol. 2006;5:140-149. [104] LI G, LOPEZ O, RUBASH H. Variability of a three-dimensional finite element model constructed using magnetic resonance images of a knee for joint contact stress analysis. J Biomech Eng. 2001;123(4):341-346. [105] MESFAR W, SHIRAZI-ADL A. Biomechanics of the knee joint in flexion under various quadriceps forces. Knee. 2005;12(6):424-434. [106] SIEGLER S, BLOCK J, SCHNECK CD. The mechanical characteristics of the collateral ligaments of the human ankle joint. Foot Ankle. 1998;8(5):234-242. [107] PEÑA E, CALVO B, MARTÍNEZ MA, et al. Finite element analysis of the effects of meniscal tears and meniscetomies on human knee biomechanics. Clin Biomech. 2005;20:498-507. [108] LEROUX MA, SETTON LA. Experimental and biphasic FEM determinations of the material properties and hydraulic permeability of meniscus in tension. J Biomech Eng. 2002;124(3):315-321. |
| [1] | Zhong Caihong, Xiao Xiaoge, Li Ming, Lin Jianhong, Hong Jing. Biomechanical mechanism of sports-related patellar tendinitis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1417-1423. |
| [2] | Zhou Jian, Zhang Tao, Zhou Weili, Zhao Xingcheng, Wang Jun, Shen Jie, Qian Li, Lu Ming. Effects of resistance training on quadriceps mass and knee joint function in patients with osteoporosis and sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1081-1088. |
| [3] | Zhou Feng, Fu Pengfei, Qian Yufan, Xu Pingcheng, Guo Jiongjiong, Zhang Lei. Correlation between spinal sagittal imbalance and knee joint parameters detected by whole-body EOS imaging [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 596-603. |
| [4] | Jiang Kan, Alimujiang·Abudourousuli, Shalayiding·Aierxiding, Aikebaierjiang·Aisaiti, Kutiluke·Shoukeer, Aikeremujiang·Muheremu. Biomaterials and bone regeneration: research hotspots and analysis of 500 influential papers [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 528-536. |
| [5] | Zhang Qian, Wang Fuxia, Wang Wen, Zhang Kun. Characteristic analysis of nanogel composite system and its application strategies in visualization of diagnostic imaging and therapy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 480-488. |
| [6] | Dong Chao, Zhao Mohan, Liu Yunan, Yang Zeli, Chen Leqin, Wang Lanfang. Effects of magnetic nano-drug carriers on exercise-induced muscle injury and inflammatory response in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 345-353. |
| [7] | Li Congcong, Wufanbieke·Baheti, Zhao Li, Chen Xiaotao, Kong Chuifan, Yu Min. Physicochemical properties and biocompatibility of hydroxyapatite/graphene oxide/interleukin-4 composite coating materials [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 404-413. |
| [8] | Guo Jingwen, Wang Qingwei, He Zijun, Hu Zihang, Chen Zhi, Zhu Rong, Wang Yuming, Liu Wenfei, Luo Qinglu. Intra-articular injection of different concentrations of silicon-based bioceramics in treatment of knee osteoarthritis in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 288-295. |
| [9] | Zhou Jinhai, Li Jiangwei, Wang Xuquan, Zhuang Ying, Zhao Ying, Yang Yuyong, Wang Jiajia, Yang Yang, Zhou Shilian. Three-dimensional finite element analysis of anterior femoral notching during total knee arthroplasty at different bone strengths [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1775-1782. |
| [10] | Wang Juan, Wang Guanglan, Zuo Huiwu. Efficacy of exercise therapy in the treatment of anterior cruciate ligament reconstruction patients: #br# a network meta-analysis #br# [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1714-1726. |
| [11] | Que Meng, Gao Fengqing, He Yang, Ruan Changgeng. Patent technology analysis of platelet-rich plasma preparation devices [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7461-7469. |
| [12] | Ma Yucong, Ouyang Zhengzheng, Liu Xiaojie, Yang Sifei. Tracking of research trends and hotspots in medical magnesium alloy materials [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7470-7480. |
| [13] | Zhang Fei, Zuo Jun. Inhibition of hypertrophic scar in rats by beta-sitosterol-laden mesoporous silica nanoparticles [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7301-7309. |
| [14] | Pan Chun, Fan Zhencheng, Hong Runyang, Shi Yujie, Chen Hao. Effect and mechanism of polystyrene microplastics on prostate in male mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7353-7361. |
| [15] | Wang Guanyuan, Li Wenli, Liu Jinglin, Zhang Jie. Development and application of fully automated intelligent intravenous medication dispensing robot ML300 in Pharmacy Intravenous Admixture Services [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7362-7368. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||

