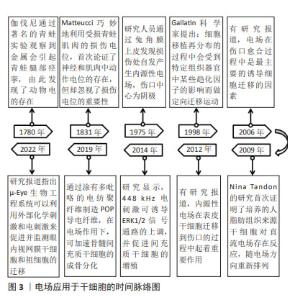
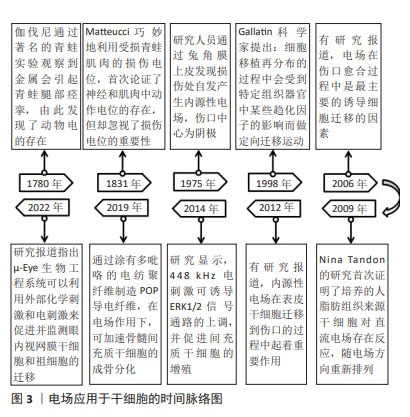
Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (23): 4984-4992.doi: 10.12307/2025.082
Previous Articles Next Articles
Electrotactic migration and mechanisms of stem cells
Han Fang1, 2, Shu Qing2, Jia Shaohui1, Tian Jun1, 2
- 1School of Sports Medicine, Wuhan Sports University, Wuhan 430079, Hubei Province, China; 2Department of Orthopedics and Rehabilitation, Zhongnan Hospital of Wuhan University, Wuhan 430071, Hubei Province, China
-
Received:2024-03-12Accepted:2024-05-21Online:2025-08-18Published:2024-09-30 -
Contact:Tian Jun, MD, Chief physician, Master’s supervisor, School of Sports Medicine, Wuhan Sports University, Wuhan 430079, Hubei Province, China; Department of Orthopedics and Rehabilitation, Zhongnan Hospital of Wuhan University, Wuhan 430071, Hubei Province, China -
About author:Han Fang, Master candidate, School of Sports Medicine, Wuhan Sports University, Wuhan 430079, Hubei Province, China; Department of Orthopedics and Rehabilitation, Zhongnan Hospital of Wuhan University, Wuhan 430071, Hubei Province, China -
Supported by:National Natural Science Foundation of China (General Program), No. 82174494 (to TJ)
CLC Number:
Cite this article
Han Fang, Shu Qing, Jia Shaohui, Tian Jun. Electrotactic migration and mechanisms of stem cells[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(23): 4984-4992.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
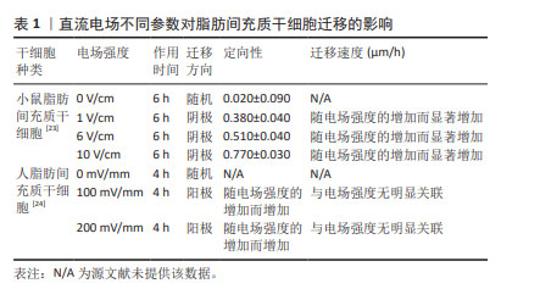
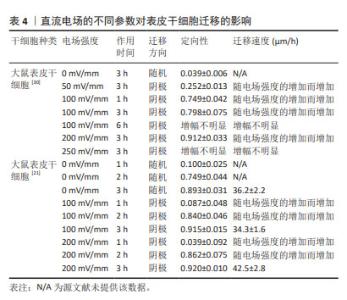
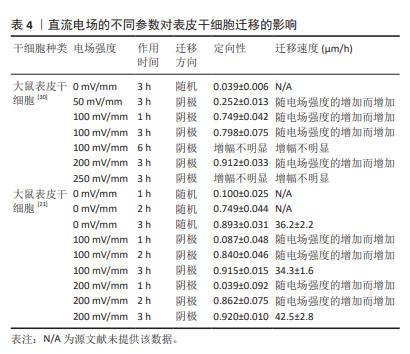
2.2 直流电场对干细胞迁移的影响 近年来,不断有学者对直流电场中干细胞的迁移特性进行量化研究。研究表明,干细胞迁移的定向性、迁移方向和迁移速度均不同程度受到直流电场强度及作用时间的影响。在电场极性反转后,干细胞的迁移方向也会发生相应的改变,这进一步证明了直流电场在干细胞迁移过程中扮演着至关重要的角色。 2.2.1 普遍量化方法(迁移方向、定向性、迁移速度、极性反转) 一般用Image J软件处理捕获的细胞迁移图像,获取细胞在XY坐标系下的位置,绘制细胞轨迹后即可进行量化。以细胞初始位置为原点,细胞迁移偏离原点的方向即为细胞迁移的方向(阴极/阳极)[19]。同时,细胞迁移在X轴的定向性可通过余弦函数表示,直接沿着电场线向阳/阴极移动的细胞将具有1/-1的方向性,而接近0的余弦值表示细胞进行随机移动。通过 (n表示细胞总数、θ表示干细胞迁移方向和电场方向的夹角)给出平均值产生平均定向指数[20-21],能够更直观显示电场对细胞迁移方向的定向能力。绘制细胞轨迹后,可得到运动轨迹长度,除以总观察时间,即可得出细胞在整个运动过程中的平均迁移速度[22]。此外,通过进行电场的极性反转,观察细胞是否会在电场正负极改变后向新阴极或新阳极重新进行迁移来进一步证实电场对细胞迁移方向的影响[20]。 2.2.2 直流电场不同参数对干细胞迁移的影响 小鼠脂肪间充质干细胞在电场强度分别为1,6,10 V/cm作用6 h的条件下,向阴极迁移的定向性逐渐增强,迁移速度随着电场强度的增加而增快[23]。此外,当电场强度为100 mV/mm时,人脂肪间充质干细胞已经表现出了趋电迁移行为。电场强度为200 mV/mm时,向阳极迁移的趋向性更为明显,且定向性与电场强度呈正比,但迁移速度、迁移距离、位移距离和位移速度与电场强度没有明显关联性[24],具体参数见表1。这一区别可能需要在相同的实验平台重复前人实验,探究不同物种来源干细胞对电场干预的反应,同时也是从动物实验转化到临床应用需要突破的一大难题。"
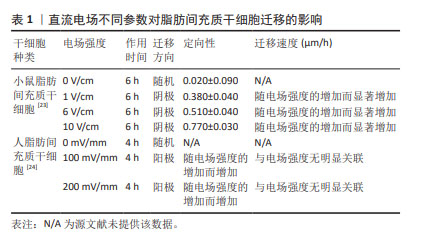
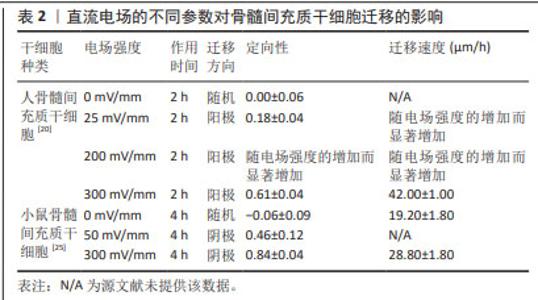
人骨髓间充质干细胞的迁移方向在无电流引导下是随机的,但小的生理直流电场(25 mV/mm)即可使其向阳极进行强劲的迁移,且迁移速率增加了1倍。电场强度与迁移定向性和迁移速度呈正相关[20]。在电场强度为300 mV/mm时,迁移速度进一步增加并达到峰值,接近对照组(无直流电场)迁移率的2倍。电场强度为200 mV/mm或600 mV/mm作用2 h后,都不会对其造成任何实测损伤,且200 mV/mm的电场强度不影响其表型和成骨潜能,同时200 mV/mm的电场强度作用3 h后进行电场极性反转,人骨髓间充质干细胞会朝向新阳极迁移。而小鼠骨髓间充质干细胞在电场的引导下迁移方向与人骨髓间充质干细胞相反,向阴极移动,同时巨噬细胞向阳极移动;电场强度为50 mV/mm作用4 h时,小鼠骨髓间充质干细胞已有向阴极移动的明显趋势,而300 mV/mm的电场强度作用4 h是小鼠骨髓间充质干细胞和巨噬细胞进行趋电性迁移的最佳电场参数[25],见表2。另外,在Transwell实验中,直流电场强度为200 mV/mm作用4 h是增强大鼠骨髓间充质干细胞向下室迁移的最佳选择[26]。筛选出趋电性迁移能力强的细胞用于后续的靶向迁移,这是提高干细胞治疗效率的又一途径。"

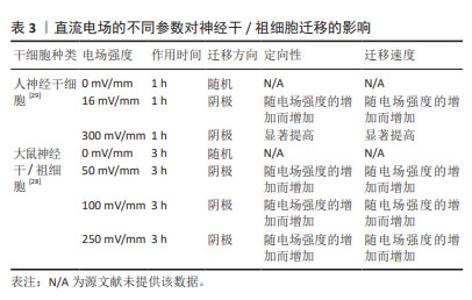
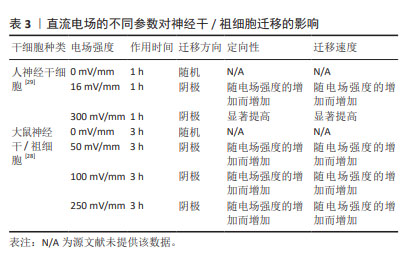
对神经干细胞趋电性迁移的研究显示,在无定向刺激下,神经干细胞的迁移形似变形虫运动,在细胞表面形成2个连续但方向相反的突起,先随机于某个方向进行运动,后向其反方向运动,使运动呈现为方向改变频繁的曲折轨迹。直流电场可抑制神经干细胞向阳极形成突起,引导神经干细胞向阴极迁移并增加其迁移速度,增加迁移方向的稳定性,减少了迁移轨迹的曲折程度[27]。因此,直流电场可能作为一个引导方向的线索,以控制和加速神经干细胞向阴极的迁移。研究报道,大鼠神经干/祖细胞在50,100,250 mV/mm电场强度作用3 h时,均向阴极迁移,同时迁移速度和定向性与电场强度呈正相关[28]。另外,人神经干细胞迁移的电场强度阈值在16 mV/mm以下,迁移方向为阴极。在电场强度为300 mV/mm作用1 h后表现出向阴极的强劲迁移,且平均定向性随电场强度的增加而增加[29],此时进行极性反转,人神经干细胞迁移方向随之改变,向新阴极移动,具体参数见表3。探索促进神经干细胞定向迁移的最佳参数可进一步提高其递送抗肿瘤药物的效率,为肿瘤治疗开辟新的途径。"
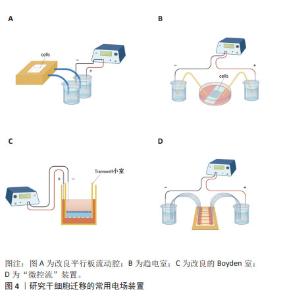
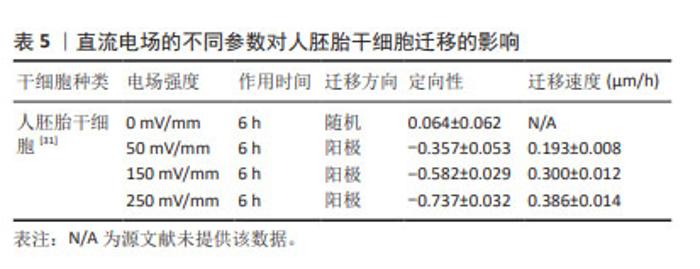
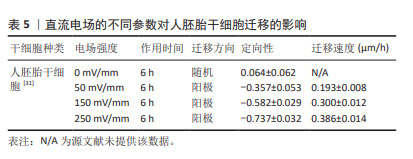
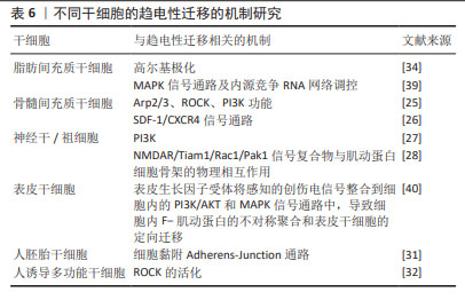
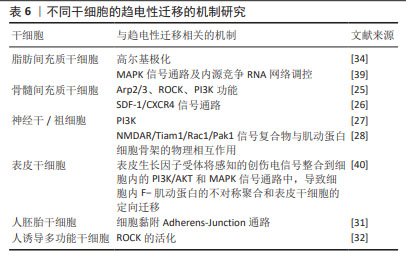
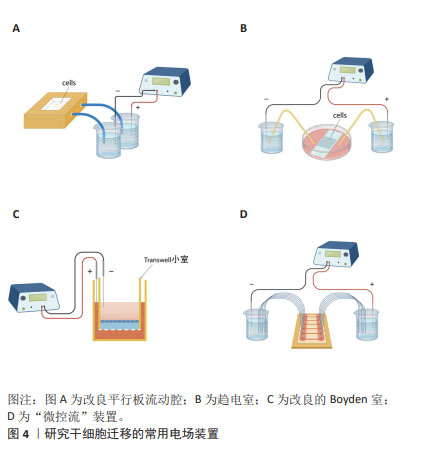
对人诱导性多能干细胞趋电性迁移的研究发现,其向阳极迁移所需的阈值电场强度小于30 mV/mm,较高电场强度会显著增加其定向性。在电场强度达到30 mV/mm作用3 h后,其定向性为?0.37±0.08,若此时进行极性反转,人诱导性多能干细胞则会向新阳极迁移。电场强度为50 mV/mm时,迁移速度提高20%。电场强度达到75 mV/mm时,定向性增加1倍,迁移速度增加了40%。在更高的电场强度下迁移速度开始趋于平稳,进入平台期[32]。人诱导性多功能干细胞在建立神经系统疾病和心脏病等模型中得到广泛应用。通过系统分析其趋电迁移情况,可以更深入地研究这些疾病的发病机制及治疗有效性。 以上研究表明,各类型干细胞在受到直流电场干预时,其迁移方向发生变化,且这种变化与直流电场的极性密切相关。随着直流电场极性的改变,干细胞的迁移方向也进行相应地调整。这可能与直流电场改变了细胞力学特点或者激活干细胞内的信号通路不同等因素有关[33-34]。同时,干细胞迁移的定向性与电场强度在一定范围内呈正相关。而干细胞的迁移速度是否受到电场强度的影响仍然存在争议,以及不同物种来源干细胞趋电性迁移特性不同的问题,需要之后在相同实验条件下重复研究,真正解决进入临床实际应用的困境。此外,除了定向性和速度,其他的迁移数值也可能受到直流电场强度的影响,例如:位移速率、X轴迁移速率等。但由于样本量较少,目前无法对这些参数进行全面的总结和分析。未来的研究可以进一步探索这些参数与直流电场强度之间的关系,以便更加全面地了解直流电场对于干细胞迁移的调控作用。 2.3 脉冲电场对干细胞迁移的影响 脉冲电场在细胞存活率方面相较于直流电场有着很大优势。LEE等[35]的研究显示,持续电流应用具有潜在的细胞毒性和电极降解等缺点,这些不良结果主要归因于单向离子流电荷的积累,并且细胞活性随着直流电场作用时间的增加而显著降低,而用作对照的脉冲电流组并没有观察到明显的细胞死亡,同时他们观察到单次1 200 μA电场作用3 h后诱导脂肪间充质干细胞的阳极定向迁移现象可维持6 h。JEZIERSKA-WO?NIAK等[36]的研究显示,在电场强度为80 mV/mm、频率130 Hz作用3 h的条件下,对骨髓间充质干细胞迁移速度的提高最为显著。目前暂无在相同条件下应用脉冲电场和直流电场作用于干细胞迁移的比较研究,在未来的研究中可以着重于此,探究一种高效省时节能且对细胞损伤较小的电场条件,进一步提高干细胞在组织工程中的应用。 2.4 研究干细胞迁移的常用电场装置 2.4.1 改良的平行板流动腔 如图4A所示,电源通过电解质和长度为35 cm的2%琼脂糖盐桥与细胞腔室相连,这个腔室由一个机械加工的聚碳酸酯块和玻璃窗组成,在腔室内将带有人脂肪间充质干细胞的玻片放置在厚度均匀的矩形硅酮垫片上,垫片中心有一个矩形开口,用螺丝和塑料盖(有一个矩形开口来容纳物镜)固定在一起。当该腔室适当关闭(足够紧密以防止泄漏,但不使玻片裂开)后,将创建一个尺寸为5 cm×1.3 cm×0.25 cm(分别为长、宽和高或间隙距离)的密封矩形通道。这种几何形状的腔室可以防止任何流体流动,并可以从外加电流中计算出电场强度或电流密度[37]。 2.4.2 趋电室 如图4B所示,趋电室是目前研究干细胞迁移最常用的装置,直流电源通过Steinberg’s溶液和琼脂盐桥与培养皿盖(提前打好相应大小的孔)相连,用真空硅脂胶形成开储液区与细胞区,中间带有人脂肪间充质干细胞的区域为单层玻片,两边为双层玻片,顶层为完全覆盖细胞的一块盖玻片,并用真空硅脂胶进行密封,既保证了细胞足够的迁移空间,又极大地减少了污染的风险,可通过捕捉图像观察分析出细胞迁移的完整过程[35]。 2.4.3 改良的Boyden室 改良的Boyden室是在Transwell迁移实验的基础上进行改造的。如图4C所示,正电极和负电极分别附着在上下腔体,其中未发生迁移的细胞可从上室移出,发生迁移的细胞可固定染色后进行观察。此装置不适用于观察迁移过程,但可作为筛选具备迁移能力细胞的一种方法[26]。 2.4.4 微控流装置 “微流控”(Micro-fluidics)是一种微米级别操纵流体的技术或平台,由于适用于细胞和蛋白等大分子,因此也被应用于生物领域。如图4D所示,是一种基于聚二甲基硅氧烷的微控流装置,用于观测人骨髓间充质干细胞的迁移情况。这个6通道装置允许同时刺激多达6个细胞迁移室,也可以使用单通道刺激。此装置更适用于观察更复杂的仿生环境下细胞的趋电行为[38]。"
| [1] SABA JA, LIAKATH-ALI K, GREEN R, et al. Translational control of stem cell function. Nat Rev Mol Cell Biol. 2021;22(10):671-690. [2] ZAKRZEWSKI W, DOBRZYŃSKI M, SZYMONOWICZ M, et al. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10(1):68. [3] DOW R, DELONG C, JIANG G, et al. Bipolar Patient-Specific In Vitro Diagnostic Test Reveals Underlying Cardiac Arrhythmia Phenotype Caused by Calcium Channel Genetic Risk Factor. Biol Psychiatry Glob Open Sci. 2024;4(3):100296. [4] LIU Y, BALAJI R, DE TOLEDO MAS, et al. The pain target NaV1.7 is expressed late during human iPS cell differentiation into sensory neurons as determined in high-resolution imaging. Pflugers Arch. 2024;476(6):975-992. [5] CALIXTO RD, FREITAS GP, SOUZA PG, et al. Effect of the secretome of mesenchymal stem cells overexpressing BMP-9 on osteoblast differentiation and bone repair. J Cell Physiol. 2023;238(11): 2625-2637. [6] GOMAA MA, ELHAWARY YM, BADR AE. Glycyrrhizin Enhances the Proliferation of Diabetic Bone Marrow-derived Mesenchymal Stem Cells: A Potential Therapeutic Agent in Endodontic Surgery. J Contemp Dent Pract. 2023;24(7):494-499. [7] CHOI SJ, PARK SY, SHIN YH, et al. Mesenchymal Stem Cells Derived from Wharton’s Jelly Can Differentiate into Schwann Cell-Like Cells and Promote Peripheral Nerve Regeneration in Acellular Nerve Grafts. Tissue Eng Regen Med. 2021;18(3):467-478. [8] 曹慧玲,汪小蓉,朱小飞,等.川芎嗪对脐带间充质干细胞移植缺血性脑卒中迁移率的影响及作用机制[J].实用医学杂志,2022, 38(6):726-730+737. [9] MESSI Z, BORNERT A, RAYNAUD F, et al. Traction Forces Control Cell-Edge Dynamics and Mediate Distance Sensitivity during Cell Polarization. Curr Biol. 2020;30(9):1762-1769.e5. [10] ASHRAF M, TIPPARAJU SM, KIM JW, et al. Chemokine/ITGA4 Interaction Directs iPSC-Derived Myogenic Progenitor Migration to Injury Sites in Aging Muscle for Regeneration. Cells. 2023;12(14): 1837. [11] ZHAO M, SONG B, PU J, et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442(7101):457-460. [12] HENG W, BHAVSAR M, HAN Z, et al. Effects of Electrical Stimulation on Stem Cells. Curr Stem Cell Res Ther. 2020;15(5):441-448. [13] LONG H, YANG G, MA K, et al. Effect of different electrical stimulation waves on orientation and alignment of adipose derived mesenchymal stem cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017;31(7): 853-861. [14] 刘兰,刘星可,刘梦嫦,等.直流电场对人脂肪间充质干细胞增殖和干细胞生物学特性的影响[J].第三军医大学学报,2021,43(11): 1010-1017. [15] 刘中,李克威,王敏,等.微电场对人脐带间充质干细胞增殖和成骨分化的影响[J].中国组织工程研究,2024,28(13):1983-1988. [16] SEFTON E, IWASA SN, MORRISON T, et al. Electric Field Application In Vivo Regulates Neural Precursor Cell Behavior in the Adult Mammalian Forebrain. eNeuro. 2020;7(4):ENEURO.0273-20.2020. [17] 张泽茜,伍佳琪,樊瑜波,等.干细胞心肌向分化成熟过程中的物理刺激方法[J].中国组织工程研究,2019,23(1):110-117. [18] HONG SH, LEE MH, KOO MA, et al. Stem cell passage affects directional migration of stem cells in electrotaxis. Stem Cell Res. 2019;38:101475. [19] 李力.生物电场促进皮肤创面修复的细胞机制[D].重庆:第三军医大学,2012. [20] ZHAO Z, WATT C, KARYSTINOU A, et al. Directed migration of human bone marrow mesenchymal stem cells in a physiological direct current electric field. Eur Cell Mater. 2011;22:344-358. [21] 王淞.生物电对表皮干细胞促进创面愈合作用的影响[D].重庆: 第三军医大学,2015. [22] CLANCY H, PRUSKI M, LANG B, et al. Glioblastoma cell migration is directed by electrical signals. Exp Cell Res. 2021;406(1):112736. [23] HAMMERICK KE, LONGAKER MT, PRINZ FB. In vitro effects of direct current electric fields on adipose-derived stromal cells. Biochem Biophys Res Commun. 2010;397(1):12-17. [24] 李时与.衰老对于人脂肪干细胞趋电性的影响及其机制探究[D].沈阳:中国医科大学,2022. [25] ZIMOLAG E, BOROWCZYK-MICHALOWSKA J, KEDRACKA-KROK S, et al. Electric field as a potential directional cue in homing of bone marrow-derived mesenchymal stem cells to cutaneous wounds. Biochim Biophys Acta Mol Cell Res. 2017;1864(2):267-279. [26] WANG X, GAO Y, SHI H, et al. Influence of the intensity and loading time of direct current electric field on the directional migration of rat bone marrow mesenchymal stem cells. Front Med. 2016;10(3): 286-296. [27] AROCENA M, ZHAO M, COLLINSON JM, et al. A time-lapse and quantitative modelling analysis of neural stem cell motion in the absence of directional cues and in electric fields. J Neurosci Res. 2010; 88(15):3267-3274. [28] LI L, EL-HAYEK YH, LIU B, et al. Direct-current electrical field guides neuronal stem/progenitor cell migration. Stem Cells. 2008;26(8): 2193-2200. [29] FENG JF, LIU J, ZHANG XZ, et al. Guided migration of neural stem cells derived from human embryonic stem cells by an electric field. Stem Cells. 2012;30(2):349-355. [30] 胡雪飞.电场对热损伤表皮干细胞迁移行为的影响[D].衡阳:南华大学,2016. [31] 江恒君.干细胞趋电性相关的microRNAs的表达量变化[D].杭州:浙江大学,2016. |
| [1] | Yuan Weibo, Liu Chan, Yu Limei. Potential application of liver organoids in liver disease models and transplantation therapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1684-1692. |
| [2] | Yu Ting, Lyu Dongmei, Deng Hao, Sun Tao, Cheng Qian. Icariin pretreatment enhances effect of human periodontal stem cells on M1-type macrophages [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1328-1335. |
| [3] | Yang Zhihang, Sun Zuyan, Huang Wenliang, Wan Yu, Chen Shida, Deng Jiang. Nerve growth factor promotes chondrogenic differentiation and inhibits hypertrophic differentiation of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1336-1342. |
| [4] | Hu Taotao, Liu Bing, Chen Cheng, Yin Zongyin, Kan Daohong, Ni Jie, Ye Lingxiao, Zheng Xiangbing, Yan Min, Zou Yong. Human amniotic mesenchymal stem cells overexpressing neuregulin-1 promote skin wound healing in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1343-1349. |
| [5] | Jin Kai, Tang Ting, Li Meile, Xie Yuan. Effects of conditioned medium and exosomes of human umbilical cord mesenchymal stem cells on proliferation, migration, invasion, and apoptosis of hepatocellular carcinoma cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1350-1355. |
| [6] | Li Dijun, Jiu Jingwei, Liu Haifeng, Yan Lei, Li Songyan, Wang Bin. Three-dimensional gelatin microspheres loaded human umbilical cord mesenchymal stem cells for chronic tendinopathy repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1356-1362. |
| [7] | Lou Guo, Zhang Min, Fu Changxi. Exercise preconditioning for eight weeks enhances therapeutic effect of adipose-derived stem cells in rats with myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1363-1370. |
| [8] | Liu Qi, Li Linzhen, Li Yusheng, Jiao Hongzhuo, Yang Cheng, Zhang Juntao. Icariin-containing serum promotes chondrocyte proliferation and chondrogenic differentiation of stem cells in the co-culture system of three kinds of cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1371-1379. |
| [9] | Huang Ting, Zheng Xiaohan, Zhong Yuanji, Wei Yanzhao, Wei Xufang, Cao Xudong, Feng Xiaoli, Zhao Zhenqiang. Effects of macrophage migration inhibitory factor on survival, proliferation, and differentiation of human embryonic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1380-1387. |
| [10] | Aikepaer · Aierken, Chen Xiaotao, Wufanbieke · Baheti. Osteogenesis-induced exosomes derived from human periodontal ligament stem cells promote osteogenic differentiation of human periodontal ligament stem cells in an inflammatory microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1388-1394. |
| [11] | Zhang Zhenyu, Liang Qiujian, Yang Jun, Wei Xiangyu, Jiang Jie, Huang Linke, Tan Zhen. Target of neohesperidin in treatment of osteoporosis and its effect on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1437-1447. |
| [12] | Xie Liugang, Cui Shuke, Guo Nannan, Li Aoyu, Zhang Jingrui. Research hotspots and frontiers of stem cells for Alzheimer’s disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1475-1485. |
| [13] | Li Jialin, Zhang Yaodong, Lou Yanru, Yu Yang, Yang Rui. Molecular mechanisms underlying role of mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1512-1522. |
| [14] | Sun Yuting, Wu Jiayuan, Zhang Jian. Physical factors and action mechanisms affecting osteogenic/odontogenic differentiation of dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1531-1540. |
| [15] | He Bo, Chen Wen, Ma Suilu, He Zhijun, Song Yuan, Li Jinpeng, Liu Tao, Wei Xiaotao, Wang Weiwei, Xie Jing . Pathogenesis and treatment progress of flap ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1230-1238. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||