Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (23): 4975-4983.doi: 10.12307/2025.092
Previous Articles Next Articles
Application and problems in targeted delivery of antitumor drugs by exosomes derived from engineered mesenchymal stem cells
Wang Shuangmin1, Wang Xianyao1, 2, 3, He Zhixu2, 3, 4
- 1Department of Immunology, Zunyi Medical University, Zunyi 563000, Guizhou Province, China; 2Key Cell Engineering Laboratory, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China; 3Ministry of Tissue Damage Repair and Regenerative Medicine Jointly Established a Collaborative Innovation Center, Zunyi Medical University, Zunyi 563000, Guizhou Province, China; 4Department of Pediatrics, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China
-
Received:2024-03-13Accepted:2024-05-24Online:2025-08-18Published:2024-09-30 -
Contact:He Zhixu, Chief physician, Professor, Doctoral supervisor, Key Cell Engineering Laboratory, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China; Ministry of Tissue Damage Repair and Regenerative Medicine Jointly Established a Collaborative Innovation Center, Zunyi Medical University, Zunyi 563000, Guizhou Province, China; Department of Pediatrics, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China -
About author:Wang Shuangmin, Master candidate, Department of Immunology, Zunyi Medical University, Zunyi 563000, Guizhou Province, China -
Supported by:National Natural Science Foundation of China, No. 32270848 (to HZX); National Natural Science Foundation of China, No. 82360044 (to WXY); Guizhou Provincial Science and Technology Plan Project, No. ZK[2022]607 (to WXY)
CLC Number:
Cite this article
Wang Shuangmin, Wang Xianyao, He Zhixu. Application and problems in targeted delivery of antitumor drugs by exosomes derived from engineered mesenchymal stem cells[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(23): 4975-4983.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
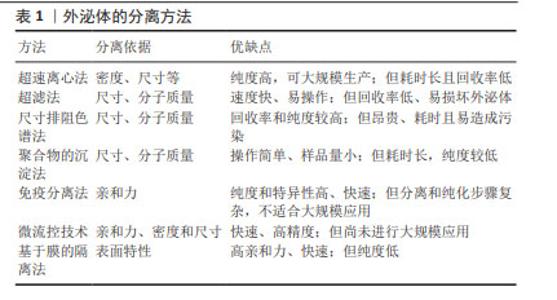
2.2 MSC-EXO的特点 2.2.1 外泌体的简介 外泌体是一种含有蛋白质、核酸和脂质等多种成分的细胞外囊泡,广泛存在于生物体液中,具有参与细胞间信息传递、免疫反应和组织修复等功能,还可作为载体靶向肿瘤递送,提高治疗效果[5]。外泌体的形成始于质膜内陷形成内体囊泡[6],通过多种复杂的机制调节mRNA和蛋白质等物质在内体积聚,形成多泡体[7]。多泡体可与溶酶体融合并被降解,也可通过与质膜融合释放外泌体[8]。外泌体通过直接膜融合、膜内陷形成内体包裹后再与膜融合、膜向外突出吞噬以及受体-配体等结合方式被靶细胞摄取[9]。目前有多种方法分离外泌体[10],主要包括超速离心法、基于分子质量的可大规模使用的超滤法、尺寸排阻色谱法、聚合物沉淀法、高纯度的免疫分离法、高效的基于微流控的技术、高亲和力的基于膜的隔离方法等[11]。这些方法的优缺点如表1所示,不同的分离方法会影响外泌体的纯度、数量和理化性质。因此,优化外泌体分离和纯化技术十分有必要。"
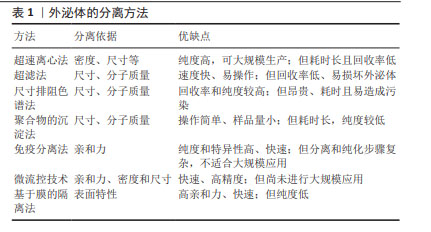

2.2.2 MSC-EXO的递送优势 间充质干细胞作为最具潜力的药物递送的活细胞载体,已在许多递送药物靶向治疗方面取得了巨大进展。然而间充质干细胞治疗仍具有许多安全性问题,如潜在的致瘤性、免疫排斥、细胞聚集促进栓塞和感染传播等[12],间充质干细胞在患者体内的活力、效力、转化难以监测和维持[13]。而外泌体作为一种旁分泌递质,不仅具有与间充质干细胞相似的作用,而且具有比间充质干细胞更稳定的膜结构、更低的免疫原性、更小的尺寸和更好的耐受性[14-15]。此外,外泌体含有跨膜和膜锚定蛋白,可增强内吞作用,从而促进药物的递送[16],这种“无细胞疗法”克服了间充质干细胞的不足,为靶向递送药物治疗带来新希望。外泌体发生过程以及被靶细胞摄取如图4所示。 2.3 工程化MSC-EXO 外泌体靶向递送效率易受亲代细胞和靶细胞的影响[17]。此外,由于免疫系统的防御机制、肝脏及肾脏的过滤作用,外泌体可能会在到达目标组织前被迅速清除,同时外泌体的组成和释放机制复杂且生物分布和运输受到多种因素影响,其组织特异性并不明显。与天然外泌体相比,工程化外泌体通过生物工程技术,以更精确的方式修饰外泌体或供体细胞,使其具有较长的循环半衰期和更强的靶向能力,并减少药物全身分布和不良反应,从而增强治疗效果[18]。目前,可通过装载工程提高外泌体的药物装载率和通过表面工程加强靶向特异性,从而提高外泌体的递送效率和治疗效果。 2.3.1 外泌体装载的工程化技术 为了实现高效率的药物靶向输送,开发有效的外泌体装载策略至关重要。装载工程主要是通过直接和间接法对外泌体进行修饰[2]。直接修饰是通过物理或化学方法改变外泌体的组成或结构,以增强外泌体与药物的结合能力[19]。物理方法(超声处理、电穿孔、挤压、冻融、pH 梯度等)通常是利用外力对膜的瞬时破坏,将药物加载进外泌体中[20]。电穿孔技术利用电场在外泌体膜上形成临时亲水孔使药物进入外泌体,是较常用的方法。GOMARI等[21]使用电穿孔技术成功地将阿霉素加载到MSC-EXO 中,通过分光光度计测量负载效率较高。与冻融方法相比,挤压方法装载率更高,可使药物在一定参数下反复挤出后均匀加载到外泌体中[22]。尽管物理方法可在一定程度上提高负载率,但会损害外泌体的完整性,影响外泌体的功能。而化学方法利用转染试剂或透化剂(如皂苷)发生化学反应来促进药物进入外泌体,不会破坏膜结构[23]。PARADA等[24]利用点击化学反应,成功将药物和质粒加载到外泌体中。此外,疏水性药物可通过与外泌体共孵育,附着在膜表面,是最简单的装载方式,也可保留外泌体的最大活性,但装载效率受多种因素影响,且药物毒性也可影响外泌体功能[25]。 间接修饰则是在外泌体分泌前,通过基因工程技术修饰亲本细胞产生含有特定结构的外泌体,可与药物更好地结合。利用细胞转染,使亲本细胞过表达治疗性药物,随后将其封装到外泌体中[2]。LOU 等[26]成功将miR-122转染到脂肪组织来源间充质干细胞中,采用PCR法检测miR-122在外泌体中的表达水平升高。LI等[27]构建CD9-HuR融合蛋白,将miR-155选择性装载到外泌体中,该方法不破坏RNA结构,装载效率显著提高,有望成为体内基因递送临床试验的新策略。此外,也可直接将药物与亲本细胞一起孵育,从而产生含药物的外泌体。如将褪黑素与间充质干细胞孵育,可产生含有褪黑激素的外泌体[28]。这种方法相对简单,但操作耗时且装载效率低。 2.3.2 外泌体的表面工程技术 外泌体内部的装载工程是实现高效率靶向递送的首要策略,而表面修饰则是提高外泌体靶向递送效率的主要方法。表面工程的主要目标是赋予外泌体靶向特异性并增加外泌体的血液循环时间,还可通过降低外泌体中促肿瘤分子的表达来进一步提高外泌体的递送效率。修饰方法主要见图5。"
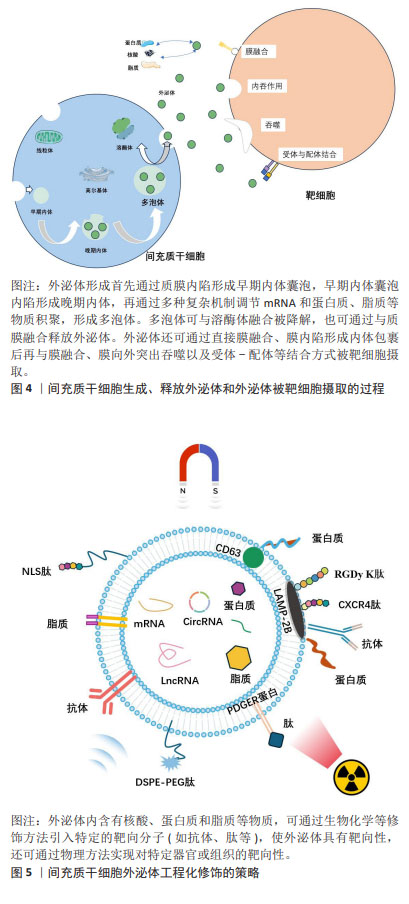
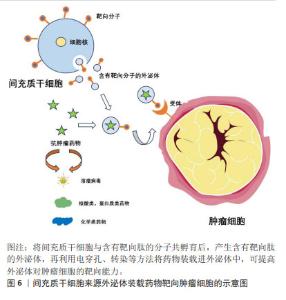
(1)增强器官或组织靶向性的工程化技术:可通过生物修饰方法在供体细胞表面采用基因编辑或转染技术引入特定的靶向分子(如抗体、靶向肽等),产生含有目标蛋白的外泌体,实现对特定器官或组织的靶向递送[29]。细胞膜上溶酶体相关膜蛋白2B(lysosomal-associated membrane protein 2B,LAMP-2B)可作为跨膜结构与靶向分子融合[30],如LAMP-2B对靶向蛋白或抗体片段具有较高亲和力,可进行基因融合。白细胞介素3在慢性白血病细胞中高表达,研究发现,将白细胞介素3基因融合到LAMP-2B的N端,可使外泌体靶向白血病细胞的能力显著提高[31]。WANG等[32]开发了在间充质干细胞中表达缺血性心肌靶向肽的外泌体,其特异性定位于缺血性心肌区域,具有保护心脏作用。在某些情况下,靶向肽可能会被降解,为了增强其稳定性,可以在肽-LAMP-2B融合的N 末端添加糖基化基序[33]。此外,跨膜蛋白血小板来源生长因子受体和跨膜蛋白CD63也可与靶向分子融合,发挥特异性靶向功能。 靶向分子也可在外泌体分离后通过化学修饰附着在膜表面,其中点击化学反应可在多种溶剂中进行,反应时间短且共轭反应对外泌体的大小和摄取均没有影响,是一种十分高效的反应[34]。整合素avβ3与一种肽c(RGDy K)具有高亲和力,在缺血后反应性脑血管内皮细胞中表达,有研究通过点击化学将其偶联到间充质干细胞衍生外泌体表面,发现c(RGDy K)肽标记的外泌体对小鼠缺血性脑损伤区域的靶向性比未修饰的外泌体高11倍[35]。LI等[36]将外泌体和羧基Fe3O4超顺磁性纳米颗粒表面涂覆A33抗体进行偶联,得到具有治疗功能且可靶向结直肠癌的特定外泌体。适配体作为一种人工合成的单链核苷酸,对癌细胞具有高亲和力和特异性靶向能力。WU等[37]发现,AS1411适配体修饰的外泌体在胶质母细胞瘤中具有高效的血脑屏障穿透力和癌细胞靶向能力。 此外,利用物理手段(如磁场、超声波等)也可引导外泌体定向到达目标位置。研究发现,含有超顺磁性纳米颗粒的外泌体在外部磁力作用下,对肿瘤的靶向性明显增强并抑制了肿瘤的生长[38]。有研究将磁性纳米粒子涂布在装载多柔比星的外泌体表面,通过外部磁场引导外泌体在肿瘤部位富集,用红外辐射诱发局部高热触发外泌体内装载的药物释放,为肿瘤精准治疗提供了新的思路[39]。同样,也可在来源于肿瘤细胞的外泌体上加载华卟啉钠,利用体外超声设备无创地提高肿瘤靶向性[40]。 (2)延长外泌体血液循环时间工程技术:研究发现,除了直接增强器官或组织靶向性,还可通过改变外泌体的大小、表面电荷和性质等特性来增加其在血液循环中的停留时间。其中聚合物修饰是最常用的一种表面修饰方法,通过将聚合物分子,如聚乙二醇与外泌体表面结合可显著增加外泌体的尺寸和电荷,还可通过形成水合层避免外泌体被清除,从而延长其在体内的循环时间[41]。外泌体还可与脂质体通过疏水作用连接,再通过直接膜融合使外泌体的膜表面性质改变,从而提高外泌体在血液中的半衰期。LI等[42]将外泌体与脂质体融合,再用肿瘤靶向的 cRGD肽修饰,发现该融合体不仅富集于肿瘤组织内,还可避免体内单核吞噬细胞系统的消除。此外,通过模仿肿瘤细胞免疫逃逸的机制,可激活“不要吃我”信号等方式使外泌体逃脱巨噬细胞的清除。如CD47在体内大多数细胞中表达,可与免疫抑制受体SIRPα相互作用保护细胞免受吞噬。YANG等[43]发现,CD47在外泌体中的过表达使其在血液中的半衰期延长了3倍,且在不同剂量和时间下,对小鼠无毒性作用。同样,BELHADJ等[44]也观察到,在外泌体中CD47会减少巨噬细胞的内吞作用以及向肝脏和脾脏的非特异性分布,并增加肿瘤细胞内的积累。 (3)降低外泌体中促肿瘤分子工程技术:相较于通过各种修饰方法提高外泌体靶向递送药物的效率,直接降低外泌体中促肿瘤分子的表达抑制肿瘤进展更能达到治疗效果。外泌体中存在一些促肿瘤分子,如生长因子、趋化因子和miRNA 等,这些分子可促进肿瘤的生长和扩散,降低外泌体的药物递送效率[45],可利用CRISPR-Cas9等基因编辑技术,通过敲除或降低外泌体中促肿瘤分子的表达,从而降低其促肿瘤作用,提高治疗的安全性[46]。MCANDREWS等[47]将载有CRISPR/Cas9 的外泌体靶向突变体KrasG12D系列胰腺癌细胞中的致癌等位基因,发现其可显著抑制肿瘤生长。DUAN等[48]将与sgRNA结合的Cas9核酸酶递送至肿瘤细胞中,可在目标位置修饰基因,并去除现有基因或进行体内编辑,再选择合适的递送载体释放CRISPR/Cas9用于体内基因治疗。此外,将促肿瘤分子的siRNA 转导入细胞,可实现基因沉默,通过抑制促肿瘤分子的表达,降低其在外泌体中的含量,提高外泌体的药物递送效率。XING等[49]发现,用 siGARP转染的MSC-EXO中GARP表达显著降低,且siGARP-MSC-EXO可通过阻碍白细胞介素6分泌和使JAK1/STAT3通路失活来抑制结肠癌细胞的增殖、迁移和侵袭。此外,将外泌体与其他抗肿瘤药物或治疗手段联合使用,如化疗药物、免疫治疗等,也可减少促肿瘤分子的负面影响。 2.4 MSC-EXO在肿瘤治疗中靶向递送的应用 靶向递送可以提高药物对肿瘤组织的疗效,减少药物的不良反应。目前已对多种抗肿瘤药物通过外泌体的递送进行研究,治疗效果显著。外泌体递送药物至肿瘤细胞示意图,见图6。"

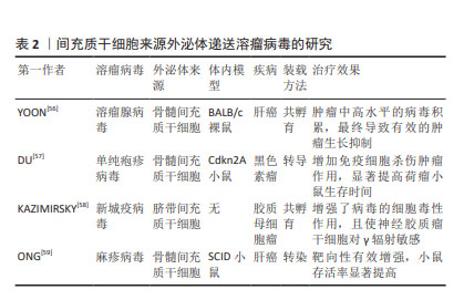
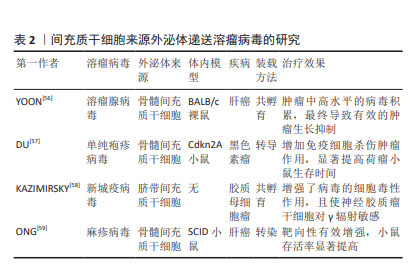
2.4.1 递送溶瘤病毒 溶瘤病毒是一种可诱导肿瘤细胞溶解死亡并激活抗肿瘤免疫反应,而对正常细胞无杀伤作用的病毒,是一种很有前途的癌症免疫疗法,然而由于病毒具有免疫原性,全身给药时可被抗体中和,使到达靶细胞积累量减少[50],导致治疗效果不佳。间充质干细胞由于其自身优势,可通过外泌体直接向肿瘤细胞释放药物[51],被视为溶瘤病毒的理想载体,不仅可增强病毒在生物体内的稳定性,还可促进病毒的扩散和释放[52]。GAROFALO等[53]通过研究发现,与直接使用溶瘤腺病毒相比,外泌体装载显著增强了溶瘤腺病毒的肿瘤趋向性,治疗效果显著提高。通过进一步研究发现,将溶瘤病毒和紫杉醇同时装载进外泌体中,对肺癌进行联合治疗,与单独包载溶瘤腺病毒、紫杉醇相比,外泌体同时包载两者显著提高了转导率和感染滴度[54],证明了外泌体装载溶瘤病毒和紫杉醇联合用药能够降低肺癌模型的肿瘤生长。此外,装载溶瘤病毒的间充质干细胞的抗肿瘤功效增强不仅得益于其规避体液免疫归巢的能力,还得益于免疫细胞的浸润和间充质干细胞的分化能力。RINCóN等[55]认为,使用间充质干细胞作为溶瘤腺病毒的载体不仅可将腺病毒靶送至肿瘤,还可募集 T 细胞发挥抗肿瘤免疫。目前关于MSC-EXO递送溶瘤病毒的相关研究较少,多数为其母细胞递送溶瘤病毒的研究,见表2。"
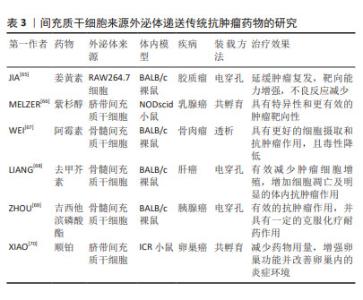
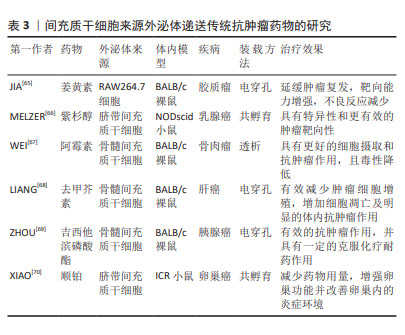
2.4.2 递送传统抗肿瘤药物 传统抗肿瘤药物(如姜黄素、紫杉醇、阿霉素等)由于药物溶解度差、半衰期和体内循环时间短以及靶向性低等导致治疗效果欠佳,且还具有耐药性和各种毒副作用等问题。MSC-EXO作为一种天然的递送工具,具有完美的纳米尺寸和稳定的膜结构,可以稳定携带和保护药物,防止其降解和失活[60]。一些研究表明,将药物通过不同方式加载到外泌体中,可表现出更好的抗肿瘤效应,如姜黄素与外泌体直接孵育的抗炎活性是单独给药的3倍以上[61]。紫杉醇通过与间充质干细胞共孵育,递送至靶细胞可介导强烈抗肿瘤反应,为外泌体运载抗肿瘤药物进行体内治疗奠定了基础[62],GOMARI等[63]发现MSC-EXO递送的阿霉素可显著降低小鼠乳腺癌模型中肿瘤的生长速度。最重要的是,外泌体具有生物相容性,可有效降低化疗药物的毒性和不良反应。在TIAN等[64]研究中,负载阿霉素的外泌体可显著抑制乳腺肿瘤细胞生长,而对小鼠无毒性作用。一些使用小鼠建模的研究和治疗效果见表3。"
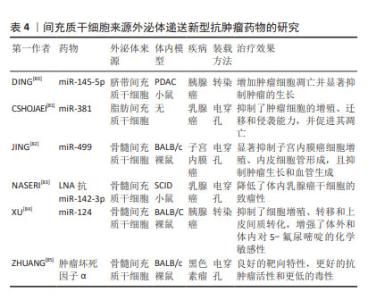
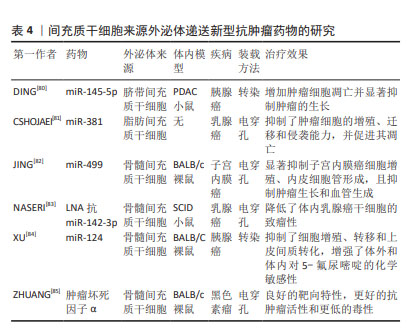
2.4.3 递送新型抗肿瘤药物 目前,出现许多新型技术治疗肿瘤,其中基因治疗可以通过编辑遗传物质改变患病细胞中的基因,从根本上治疗疾病,具有巨大前景。但由于基因易被降解、不稳定以及难以被靶细胞摄取等,需要载体克服这些弊端。近年来,外泌体以相对安全、易调节的理化性质、可大量生产、成本低且装载能力强等优势,逐渐被应用于基因递送领域[71]。miRNA作为一种非编码RNA,可异常表达而引起疾病的发生,通过外泌体包装递送至靶细胞中可恢复异常基因表达,进而抑制肿瘤生长[72]。siRNA可通过破坏异常基因治疗癌症,FARUQU 等[73]通过研究发现,将siRNA加载到外泌体中,其装载和递送效率提高,治疗癌症效果显著。将半乳糖凝集素9 siRNA通过电穿孔装载进骨髓间充质干细胞来源外泌体,与奥沙利铂联合应用,显著抑制了胰腺癌的肿瘤生长[74]。此外,有研究证实大分子核酸物质mRNA和DNA等也可通过转染或电穿孔方法加载到外泌体中[75-76],递送至肿瘤细胞抑制其活性并促进凋亡,提高了小鼠存活率。蛋白质、多肽等大分子药物在体内环境中易被降解失活,且由于缺乏天然构象而无法发挥预期的功能[77]。外泌体可以保护蛋白质免受各种酶和免疫系统的侵害。研究发现,过氧化氢酶通过不同装载方式(皂苷孵育、冻融循环、超声处理和挤出)至外泌体中,显着减少了神经炎症,提供了有效的神经保护作用[78]。目前,MSC-EXO负载蛋白应用于抗癌治疗的研究较少,但已有研究发现肿瘤坏死因子相关细胞凋亡诱导配体作为一种抗癌蛋白可装载到MSC-EXO中,诱导肺癌、肾癌、乳腺癌等癌症的细胞凋亡[79]。关于具体的研究和治疗效果见表4。"
| [1] PITTENGER MF, DISCHER DE, PÉAULT BM, et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. [2] WENG Z, ZHANG B, WU C, et al. Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. J Hematol Oncol. 2021;14(1):136. [3] WANG J, CHEN D, HO EA. Challenges in the development and establishment of exosome-based drug delivery systems. J Control Release. 2021;329:894-906. [4] SADEGHI S, TEHRANI FR, TAHMASEBI S, et al. Exosome engineering in cell therapy and drug delivery. Inflammopharmacology. 2023;31(1): 145-169. [5] RAJPUT A, VARSHNEY A, BAJAJ R, et al. Exosomes as New Generation Vehicles for Drug Delivery: Biomedical Applications and Future Perspectives. Molecules. 2022;27(21):7289. [6] VAN NIEL G, PORTO-CARREIRO I, SIMOES S, et al. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140(1):13-21. [7] THÉRY C, ZITVOGEL L, AMIGORENA S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569-579. [8] MONDAL J, PILLARISETTI S, JUNNUTHULA V, et al. Hybrid exosomes, exosome-like nanovesicles and engineered exosomes for therapeutic applications. J Control Release. 2023;353:1127-1149. [9] KRYLOVA SV, FENG D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int J Mol Sci. 2023;24(2):1337. [10] LIANGSUPREE T, MULTIA E, RIEKKOLA ML. Modern isolation and separation techniques for extracellular vesicles. J Chromatogr A. 2021;1636:461773. [11] KIMIZ-GEBOLOGLU I, ONCEL SS. Exosomes: Large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release. 2022;347:533-543. [12] SU Y, ZHANG T, HUANG T, et al. Current advances and challenges of mesenchymal stem cells-based drug delivery system and their improvements. Int J Pharm. 2021;600:120477. [13] TOH WS, LAI RC, ZHANG B, et al. MSC exosome works through a protein-based mechanism of action. Biochem Soc Trans. 2018;46(4): 843-853. [14] LOU G, CHEN Z, ZHENG M, et al. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med. 2017;49(6):e346. [15] TANG Y, ZHOU Y, LI HJ. Advances in mesenchymal stem cell exosomes: a review. Stem Cell Res Ther. 2021;12(1):71. [16] VADER P, MOL EA, PASTERKAMP G, et al. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106(Pt A):148-156. [17] CHEN H, WANG L, ZENG X, et al. Exosomes, a New Star for Targeted Delivery. Front Cell Dev Biol. 2021;9:751079. [18] DENG S, CAO H, CUI X, et al. Optimization of exosome-based cell-free strategies to enhance endogenous cell functions in tissue regeneration. Acta Biomater. 2023;171:68-84. [19] HAN Y, JONES TW, DUTTA S, et al. Overview and Update on Methods for Cargo Loading into Extracellular Vesicles. Processes (Basel). 2021; 9(2):356. [20] RAYAMAJHI S, ARYAL S. Surface functionalization strategies of extracellular vesicles. J Mater Chem B. 2020;8(21):4552-4569. [21] GOMARI H, FOROUZANDEH MOGHADAM M, SOLEIMANI M, et al. Targeted delivery of doxorubicin to HER2 positive tumor models. Int J Nanomedicine. 2019;14:5679-5690. [22] ANTIMISIARIS SG, MOURTAS S, MARAZIOTI A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics. 2018;10(4):218. [23] HANEY MJ, KLYACHKO NL, HARRISON EB, et al. TPP1 Delivery to Lysosomes with Extracellular Vesicles and their Enhanced Brain Distribution in the Animal Model of Batten Disease. Adv Healthc Mater. 2019;8(11):e1801271. [24] PARADA N, ROMERO-TRUJILLO A, GEORGES N, et al. Camouflage strategies for therapeutic exosomes evasion from phagocytosis. J Adv Res. 2021;31:61-74. [25] FU S, WANG Y, XIA X. Exosome engineering: Current progress in cargo loading and targeted delivery. NanoImpact. 2020;20:100261. [26] LOU G, SONG X, YANG F, et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. 2015;8:122. [27] LI Z, ZHOU X, WEI M, et al. In Vitro and in Vivo RNA Inhibition by CD9-HuR Functionalized Exosomes Encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 2019;19(1):19-28. [28] ALZAHRANI FA. Melatonin improves therapeutic potential of mesenchymal stem cells-derived exosomes against renal ischemia-reperfusion injury in rats. Am J Transl Res. 2019;11(5): 2887-2907. [29] LIANG Y, DUAN L, LU J, et al. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11(7):3183-3195. [30] LIU Q, LI D, PAN X, et al. Targeted therapy using engineered extracellular vesicles: principles and strategies for membrane modification. J Nanobiotechnology. 2023;21(1):334. [31] BELLAVIA D, RAIMONDO S, CALABRESE G, et al. Interleukin 3- receptor targeted exosomes inhibit in vitro and in vivo Chronic Myelogenous Leukemia cell growth. Theranostics. 2017;7(5): 1333-1345. [32] WANG X, CHEN Y, ZHAO Z, et al. Engineered Exosomes With Ischemic Myocardium-Targeting Peptide for Targeted Therapy in Myocardial Infarction. J Am Heart Assoc. 2018;7(15):e008737. [33] HUNG ME, LEONARD JN. Stabilization of exosome-targeting peptides via engineered glycosylation. J Biol Chem. 2015;290(13):8166-8172. [34] 黄春满,李丽薇,黄勇彬,等.外泌体靶向修饰的研究进展[J].科学通报,2023,68(33):4532-4543. [35] TIAN T, ZHANG HX, HE CP, et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137-149. [36] LI Y, GAO Y, GONG C, et al. A33 antibody-functionalized exosomes for targeted delivery of doxorubicin against colorectal cancer. Nanomedicine. 2018;14(7):1973-1985. [37] WU T, LIU Y, CAO Y, et al. Engineering Macrophage Exosome Disguised Biodegradable Nanoplatform for Enhanced Sonodynamic Therapy of Glioblastoma. Adv Mater. 2022;34(15):e2110364. [38] MALEKIAN F, SHAMSIAN A, KODAM SP, et al. Exosome engineering for efficient and targeted drug delivery: Current status and future perspective. J Physiol. 2023;601(22):4853-4872. [39] WANG J, CHEN P, DONG Y, et al. Designer exosomes enabling tumor targeted efficient chemo/gene/photothermal therapy. Biomaterials. 2021;276:121056. [40] LIU Y, BAI L, GUO K, et al. Focused ultrasound-augmented targeting delivery of nanosonosensitizers from homogenous exosomes for enhanced sonodynamic cancer therapy. Theranostics. 2019;9(18): 5261-5281. [41] FERRERO-ANDRÉS A, CLOSA D, ROSELLÓ-CATAFAU J, et al. Polyethylene Glycol 35 (PEG35) Modulates Exosomal Uptake and Function. Polymers (Basel). 2020;12(12):3044. [42] LI L, HE D, GUO Q, et al. Exosome-liposome hybrid nanoparticle codelivery of TP and miR497 conspicuously overcomes chemoresistant ovarian cancer. J Nanobiotechnology. 2022; 20(1):50. [43] YANG Z, SHI J, XIE J, et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat Biomed Eng. 2020;4(1):69-83. [44] BELHADJ Z, HE B, DENG H, et al. A combined “eat me/don’t eat me” strategy based on extracellular vesicles for anticancer nanomedicine. J Extracell Vesicles. 2020;9(1):1806444. [45] KONG YW, FERLAND-MCCOLLOUGH D, JACKSON TJ, et al. microRNAs in cancer management. Lancet Oncol. 2012;13(6):e249-258. [46] LIANG Y, XU X, XU L, et al. Chondrocyte-specific genomic editing enabled by hybrid exosomes for osteoarthritis treatment. Theranostics. 2022;12(11):4866-4878. [47] MCANDREWS KM, XIAO F, CHRONOPOULOS A, et al. Exosome-mediated delivery of CRISPR/Cas9 for targeting of oncogenic KrasG12D in pancreatic cancer. Life Sci Alliance. 2021;4(9):e202000875. [48] DUAN L, OUYANG K, WANG J, et al. Exosomes as Targeted Delivery Platform of CRISPR/Cas9 for Therapeutic Genome Editing. Chembiochem. 2021;22(24):3360-3368. [49] XING H, LIANG C, XU X, et al. Mesenchymal stroma/stem-like cells of GARP knockdown inhibits cell proliferation and invasion of mouse colon cancer cells (MC38) through exosomes. J Cell Mol Med. 2020; 24(23):13984-13990. [50] 李雪,王华.溶瘤病毒和自然杀伤细胞来源外泌体杀伤肿瘤作用的研究进展[J].中国药理学与毒理学杂志,2023,37(9):711-718. [51] NA Y, NAM JP, HONG J, et al. Systemic administration of human mesenchymal stromal cells infected with polymer-coated oncolytic adenovirus induces efficient pancreatic tumor homing and infiltration. J Control Release. 2019;305:75-88. [52] JACKMAN JA, LEE J, CHO NJ. Nanomedicine for Infectious Disease Applications: Innovation towards Broad-Spectrum Treatment of Viral Infections. Small. 2016;12(9):1133-1139. [53] GAROFALO M, VILLA A, RIZZI N, et al. Systemic Administration and Targeted Delivery of Immunogenic Oncolytic Adenovirus Encapsulated in Extracellular Vesicles for Cancer Therapies. Viruses. 2018;10(10):558. [54] GAROFALO M, SAARI H, SOMERSALO P, et al. Antitumor effect of oncolytic virus and paclitaxel encapsulated in extracellular vesicles for lung cancer treatment. J Control Release. 2018;283:223-234. [55] RINCÓN E, CEJALVO T, KANOJIA D, et al. Mesenchymal stem cell carriers enhance antitumor efficacy of oncolytic adenoviruses in an immunocompetent mouse model. Oncotarget. 2017;8(28): 45415-45431. [56] YOON AR, HONG J, LI Y, et al. Mesenchymal Stem Cell-Mediated Delivery of an Oncolytic Adenovirus Enhances Antitumor Efficacy in Hepatocellular Carcinoma. Cancer Res. 2019;79(17):4503-4514. [57] DU W, SEAH I, BOUGAZZOUL O, et al. Stem cell-released oncolytic herpes simplex virus has therapeutic efficacy in brain metastatic melanomas. Proc Natl Acad Sci U S A. 2017;114(30):E6157-E6165. [58] KAZIMIRSKY G, JIANG W, SLAVIN S, et al. Mesenchymal stem cells enhance the oncolytic effect of Newcastle disease virus in glioma cells and glioma stem cells via the secretion of TRAIL. Stem Cell Res Ther. 2016;7(1):149. [59] ONG HT, FEDERSPIEL MJ, GUO CM, et al. Systemically delivered measles virus-infected mesenchymal stem cells can evade host immunity to inhibit liver cancer growth. J Hepatol. 2013;59(5):999-1006. [60] SHAMS F, POURJABBAR B, HASHEMI N, et al. Current progress in engineered and nano-engineered mesenchymal stem cells for cancer: From mechanisms to therapy. Biomed Pharmacother. 2023; 167:115505. [61] SUN D, ZHUANG X, XIANG X, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18(9):1606-1614. [62] PASCUCCI L, COCCÈ V, BONOMI A, et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release. 2014;192:262-270. [63] GOMARI H, FOROUZANDEH MOGHADAM M, SOLEIMANI M. Targeted cancer therapy using engineered exosome as a natural drug delivery vehicle. Onco Targets Ther. 2018;11:5753-5762. [64] TIAN Y, LI S, SONG J, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383-2390. [65] JIA G, HAN Y, AN Y, et al. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials. 2018;178:302-316. [66] MELZER C, REHN V, YANG Y, et al. Taxol-Loaded MSC-Derived Exosomes Provide a Therapeutic Vehicle to Target Metastatic Breast Cancer and Other Carcinoma Cells. Cancers (Basel). 2019;11(6):798. [67] WEI H, CHEN F, CHEN J, et al. Mesenchymal Stem Cell Derived Exosomes as Nanodrug Carrier of Doxorubicin for Targeted Osteosarcoma Therapy via SDF1-CXCR4 Axis. Int J Nanomedicine. 2022;17:3483-3495. [68] LIANG L, ZHAO L, WANG Y, et al. Treatment for Hepatocellular Carcinoma Is Enhanced When Norcantharidin Is Encapsulated in Exosomes Derived from Bone Marrow Mesenchymal Stem Cells. Mol Pharm. 2021;18(3):1003-1013. [69] ZHOU Y, ZHOU W, CHEN X, et al. Bone marrow mesenchymal stem cells-derived exosomes for penetrating and targeted chemotherapy of pancreatic cancer. Acta Pharm Sin B. 2020;10(8):1563-1575. [70] XIAO Y, PENG Y, ZHANG C, et al. hucMSC-derived exosomes protect ovarian reserve and restore ovarian function in cisplatin treated mice. J Biomed Res. 2022;37(5):382-393. [71] DUAN L, XU L, XU X, et al. Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale. 2021;13(3):1387-1397. [72] ELL B, MERCATALI L, IBRAHIM T, et al. Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer Cell. 2013;24(4):542-556. [73] FARUQU FN, XU L, AL-JAMAL KT. Preparation of Exosomes for siRNA Delivery to Cancer Cells. J Vis Exp. 2018;(142):10.3791/58814. [74] ZHOU W, ZHOU Y, CHEN X, et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials. 2021;268:120546. [75] RÄDLER J, GUPTA D, ZICKLER A, et al. Exploiting the biogenesis of extracellular vesicles for bioengineering and therapeutic cargo loading. Mol Ther. 2023;31(5):1231-1250. [76] WANG Y, DING N, GUAN G, et al. Rapid Delivery of Hsa-miR-590-3p Using Targeted Exosomes to Treat Acute Myocardial Infarction Through Regulation of the Cell Cycle. J Biomed Nanotechnol. 2018;14(5): 968-977. [77] XING H, LU M, YANG T, et al. Structure-function relationships of nonviral gene vectors: Lessons from antimicrobial polymers. Acta Biomater. 2019;86:15-40. [78] HANEY MJ, KLYACHKO NL, ZHAO Y, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207: 18-30. [79] YUAN Z, KOLLURI KK, GOWERS KH, et al. TRAIL delivery by MSC-derived extracellular vesicles is an effective anticancer therapy. J Extracell Vesicles. 2017;6(1):1265291. [80] DING Y, CAO F, SUN H, et al. Exosomes derived from human umbilical cord mesenchymal stromal cells deliver exogenous miR-145-5p to inhibit pancreatic ductal adenocarcinoma progression. Cancer Lett. 2019;442:351-361. [81] CSHOJAEI S, HASHEMI SM, GHANBARIAN H, et al. Delivery of miR-381-3p Mimic by Mesenchymal Stem Cell-Derived Exosomes Inhibits Triple Negative Breast Cancer Aggressiveness; an In Vitro Study. Stem Cell Rev Rep. 2021;17(3):1027-1038. [82] JING L, HUA X, YUANNA D, et al. Exosomal miR-499a-5p Inhibits Endometrial Cancer Growth and Metastasis via Targeting VAV3. Cancer Manag Res. 2020;12:13541-13552. [83] NASERI Z, OSKUEE RK, FOROUZANDEH-MOGHADAM M, et al. Delivery of LNA-antimiR-142-3p by Mesenchymal Stem Cells-Derived Exosomes to Breast Cancer Stem Cells Reduces Tumorigenicity. Stem Cell Rev Rep. 2020;16(3):541-556. [84] XU Y, LIU N, WEI Y, et al. Anticancer effects of miR-124 delivered by BM-MSC derived exosomes on cell proliferation, epithelial mesenchymal transition, and chemotherapy sensitivity of pancreatic cancer cells. Aging (Albany NY). 2020;12(19):19660-19676. [85] ZHUANG M, CHEN X, DU D, et al. SPION decorated exosome delivery of TNF-α to cancer cell membranes through magnetism. Nanoscale. 2020;12(1):173-188. |
| [1] | Lai Pengyu, Liang Ran, Shen Shan. Tissue engineering technology for repairing temporomandibular joint: problems and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [2] | Yang Zhihang, Sun Zuyan, Huang Wenliang, Wan Yu, Chen Shida, Deng Jiang. Nerve growth factor promotes chondrogenic differentiation and inhibits hypertrophic differentiation of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1336-1342. |
| [3] | Hu Taotao, Liu Bing, Chen Cheng, Yin Zongyin, Kan Daohong, Ni Jie, Ye Lingxiao, Zheng Xiangbing, Yan Min, Zou Yong. Human amniotic mesenchymal stem cells overexpressing neuregulin-1 promote skin wound healing in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1343-1349. |
| [4] | Jin Kai, Tang Ting, Li Meile, Xie Yuan. Effects of conditioned medium and exosomes of human umbilical cord mesenchymal stem cells on proliferation, migration, invasion, and apoptosis of hepatocellular carcinoma cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1350-1355. |
| [5] | Li Dijun, Jiu Jingwei, Liu Haifeng, Yan Lei, Li Songyan, Wang Bin. Three-dimensional gelatin microspheres loaded human umbilical cord mesenchymal stem cells for chronic tendinopathy repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1356-1362. |
| [6] | Liu Qi, Li Linzhen, Li Yusheng, Jiao Hongzhuo, Yang Cheng, Zhang Juntao. Icariin-containing serum promotes chondrocyte proliferation and chondrogenic differentiation of stem cells in the co-culture system of three kinds of cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1371-1379. |
| [7] | Aikepaer · Aierken, Chen Xiaotao, Wufanbieke · Baheti. Osteogenesis-induced exosomes derived from human periodontal ligament stem cells promote osteogenic differentiation of human periodontal ligament stem cells in an inflammatory microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1388-1394. |
| [8] | Zhang Zhenyu, Liang Qiujian, Yang Jun, Wei Xiangyu, Jiang Jie, Huang Linke, Tan Zhen. Target of neohesperidin in treatment of osteoporosis and its effect on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1437-1447. |
| [9] | Lyu Liting, Yu Xia, Zhang Jinmei, Gao Qiaojing, Liu Renfan, Li Meng, Wang Lu. Bibliometric analysis of research process and current situation of brain aging and exosomes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1457-1465. |
| [10] | Li Jialin, Zhang Yaodong, Lou Yanru, Yu Yang, Yang Rui. Molecular mechanisms underlying role of mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1512-1522. |
| [11] | Cao Yue, Ye Xinjian, Li Biyao, Zhang Yining, Feng Jianying. Effect of extracellular vesicles for diagnosis and therapy of oral squamous cell carcinoma [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1523-1530. |
| [12] | He Bo, Chen Wen, Ma Suilu, He Zhijun, Song Yuan, Li Jinpeng, Liu Tao, Wei Xiaotao, Wang Weiwei, Xie Jing . Pathogenesis and treatment progress of flap ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1230-1238. |
| [13] | Sun Xianjuan, Wang Qiuhua, Zhang Jinyi, Yang Yangyang, Wang Wenshuang, Zhang Xiaoqing. Adhesion, proliferation, and vascular smooth muscle differentiation of bone marrow mesenchymal stem cells on different electrospinning membranes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 661-669. |
| [14] | Ma Weibang, Xu Zhe, Yu Qiao, Ouyang Dong, Zhang Ruguo, Luo Wei, Xie Yangjiang, Liu Chen. Screening and cytological validation of cartilage degeneration-related genes in exosomes from osteoarthritis synovial fluid [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7783-7789. |
| [15] | Zhang Min, Zhang Nini, Huang Guilin, Li Zhuangzhuang, Wang Xue, Wang Huike. Human amniotic mesenchymal stem cell exosomes repair radiation-induced submandibular gland damage in rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7804-7815. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||