Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (19): 4000-4010.doi: 10.12307/2025.047
Previous Articles Next Articles
Effect and mechanisms of highly active umbilical cord mesenchymal stem cells on aging spleen in elderly tree shrews
Ye Li1, 2, Tian Chuan2, Zhao Xiaojuan2, Chen Mengdie2, Ye Qianqian1, 2, Li Qiang2, Liao Zhuyin2, Li Ye2, Zhu Xiangqing2, Ruan Guangping2, He Zhixu3, Shu Liping1, Pan Xinghua2
- 1Guizhou Medical University, Department of Immunology of Basic Medical College, Tissue Engineering and Stem Cell Experiment Center, Guiyang 550004, Guizhou Province, China; 2National and Local Joint Engineering Laboratory of Stem Cell and Immune Cell Biomedical Technology, 920 Hospital of China People’s Liberation Army Joint Logistics Support Force, Yunnan Provincial Key Laboratory of Cell Therapy Technology Translational Medicine, Kunming Key Laboratory of Stem Cell and Regenerative Medicine, Basic Medical Laboratory, Kunming 650032, Yunnan Province, China; 3Department of Pediatrics, Affiliated Hospital of Zunyi Medical University, Zunyi 563100, Guizhou Province, China
-
Received:2024-01-08Accepted:2024-03-16Online:2025-07-08Published:2024-09-12 -
Contact:Pan Xinghua, PhD, Chief physician, National and Local Joint Engineering Laboratory of Stem Cell and Immune Cell Biomedical Technology, 920 Hospital of China People’s Liberation Army Joint Logistics Support Force, Yunnan Provincial Key Laboratory of Cell Therapy Technology Translational Medicine, Kunming Key Laboratory of Stem Cell and Regenerative Medicine, Basic Medical Laboratory, Kunming 650032, Yunnan Province, China; Co-corresponding author: Shu Liping, PhD, Professor, Guizhou Medical University, Department of Immunology of Basic Medical College, Tissue Engineering and Stem Cell Experiment Center, Guiyang 550004, Guizhou Province, China -
About author:Ye Li, Master candidate, Guizhou Medical University, Department of Immunology of Basic Medical College, Tissue Engineering and Stem Cell Experiment Center, Guiyang 550004, Guizhou Province, China; National and Local Joint Engineering Laboratory of Stem Cell and Immune Cell Biomedical Technology, 920 Hospital of China People’s Liberation Army Joint Logistics Support Force, Yunnan Provincial Key Laboratory of Cell Therapy Technology Translational Medicine, Kunming Key Laboratory of Stem Cell and Regenerative Medicine, Basic Medical Laboratory, Kunming 650032, Yunnan Province, China -
Supported by:Major Science and Technology Special Project of Yunnan Provincial Science and Technology Plan Project, No. 2018ZF007 (to PXH); Yunnan Province Basic Research Special General Project, No. 202301AT070302 (to TC)
CLC Number:
Cite this article
Ye Li, Tian Chuan, Zhao Xiaojuan, Chen Mengdie, Ye Qianqian, Li Qiang, Liao Zhuyin, Li Ye, Zhu Xiangqing, Ruan Guangping, He Zhixu, Shu Liping, Pan Xinghua. Effect and mechanisms of highly active umbilical cord mesenchymal stem cells on aging spleen in elderly tree shrews[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(19): 4000-4010.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
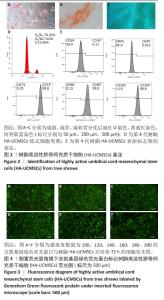
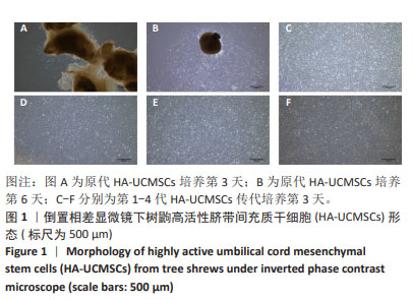
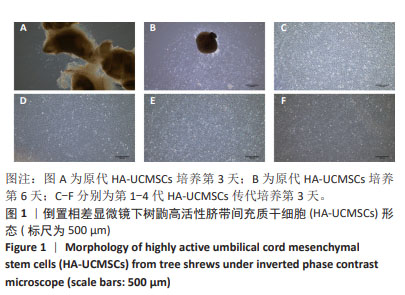
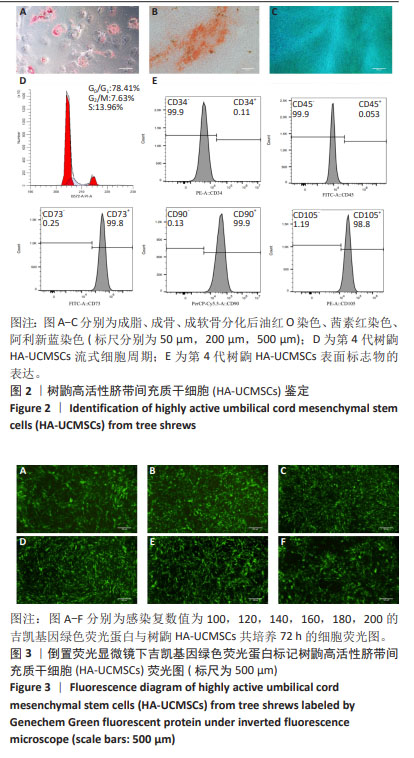
2.1.2 树鼩HA-UCMSCs鉴定结果 第4代树鼩HA-UCMSCs成脂诱导培养14 d,油红O染色显示胞浆内有红色脂肪泡,见图2A;成骨诱导培养21 d,茜素红染色显示橙橘色钙化结节,见图2B;成软骨诱导培养14 d,阿利新蓝染色显示片状蓝色软骨胶原基质,见图2C。上述结果表明HA-UCMSCs具有成脂、成骨、成软骨分化潜能。用流式细胞仪检测细胞周期,第4代HA-UCMSCs G0/G1的表达量为78.41%,见图2D,表明HA-UCMSCs具有长效分化潜能。此外,流式细胞仪检测HA-UCMSCs的生物标志物,观察到造血相关标志物CD34、CD45阴性表达,间充质干细胞表面标志物CD73、CD90、CD105强阳性表达,阳性率分别为0.11%,0.053%,99.8%,99.9%,98.8%,见图2E。 2.1.3 吉凯基因绿色荧光蛋白标记树鼩HA-UCMSCs 吉凯基因绿色荧光蛋白是较增强型绿色荧光蛋白的荧光强度高60%的一种强荧光载体蛋白。感染复数值分别为100,120,140,160,180,200的吉凯基因绿色荧光蛋白与树鼩HA-UCMSCs共培养24 h时,无荧光表达;共培养48 h时,可见绿色荧光,但表达量极弱,且转染细胞较少;共培养72 h时,观察到强烈的绿色荧光表达,转染率高达85%及以上,见图3A-F。感染复数值为140时,细胞形态较好、细胞增殖较快、荧光强度较均一,表明感染复数值140且共培养72 h是吉凯基因绿色荧光蛋白标记树鼩HA-UCMSCs的最佳转染条件。"
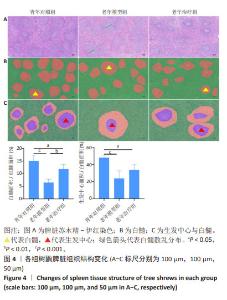
2.2 实验动物数量分析 共使用60只树鼩,每组20只,实验分组的动物数量符合统计学研究,饲养期间因环境等客观因素致死5只不计入实验研究,其余动物均纳入实验研究。同时,除白细胞介素1β和转化生长因子β1酶联免疫吸附实验分别为每组10个和9个样本外,其余检测指标均为每组4个样本并纳入统计学分析。 2.3 HA-UCMSCs改善衰老树鼩脾脏组织结构 HA-UCMSCs治疗4个月时,树鼩安乐死后取出脾脏组织。大体解剖可见青年对照组树鼩脾脏多为鲜红色,富有弹性;老年模型组树鼩脾脏呈黑红色,质地稍硬,少有韧性。苏木精-伊红染色结果显示,青年对照组树鼩脾脏组织边缘完整,结构排列紧密,白髓大小均一、形态多规则似球形,红、白髓界线清晰;而老年模型组树鼩脾脏组织红白髓界线模糊,白髓排列稀疏、间隙增宽,白髓散乱分布且形变较大(绿色箭头),多为不规则形,多见白髓区生发中心丢失,见图4A;与青年对照组相比,老年模型组脾脏白髓/红髓面积减小(P < 0.001),与老年模型组相比,HA-UCMSCs治疗后白髓/红髓面积增加(P < 0.01),见图4B;与青年对照组相比,老年模型组脾脏组织生发中心/白髓面积减小(P < 0.001),与老年模型组相比,HA-UCMSCs治疗后脾脏生发中心/白髓面积增加,但差异无显著性意义(P > 0.05),见图4C。"

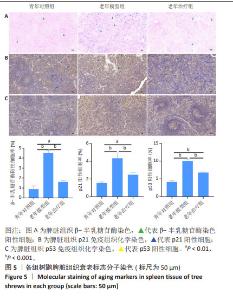
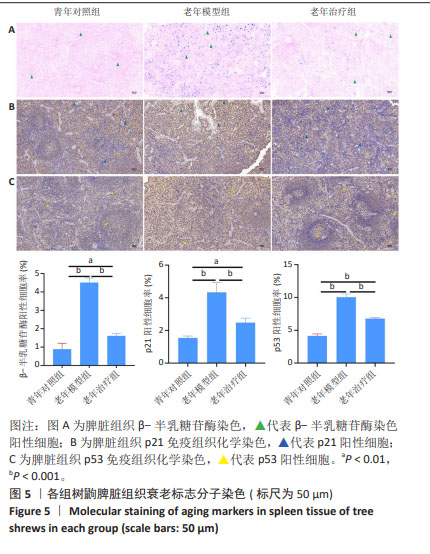
2.4 HA-UCMSCs抑制脾脏细胞衰老 衰老相关β-半乳糖苷酶活性是衰老细胞的重要标志。衰老细胞数量增加是组织衰老的主要标志,衰老细胞的结构和功能变化是衰老器官功能下降的主要特征。β-半乳糖苷酶染色结果显示,与青年对照组相比,老年模型组β-半乳糖苷酶染色深度增加,阳性率增加(P < 0.001),与老年模型组相比,HA-UCMSCs治疗后β-半乳糖苷酶阳性率下降(P < 0.001),见图5A。提示老年树鼩脾脏组织衰老细胞增加,而HA-UCMSCs治疗抑制脾脏细胞衰老。免疫组织化学染色结果显示,与青年对照组相比,老年模型组脾脏组织中衰老蛋白分子p21和p53显著上调(P < 0.001),与老年模型组相比,HA-UCMSCs治疗后p21和p53表达下调(P < 0.001),见图5B,C。提示HA-UCMSCs治疗抑制脾脏细胞衰老,下调衰老标志分子表达。"

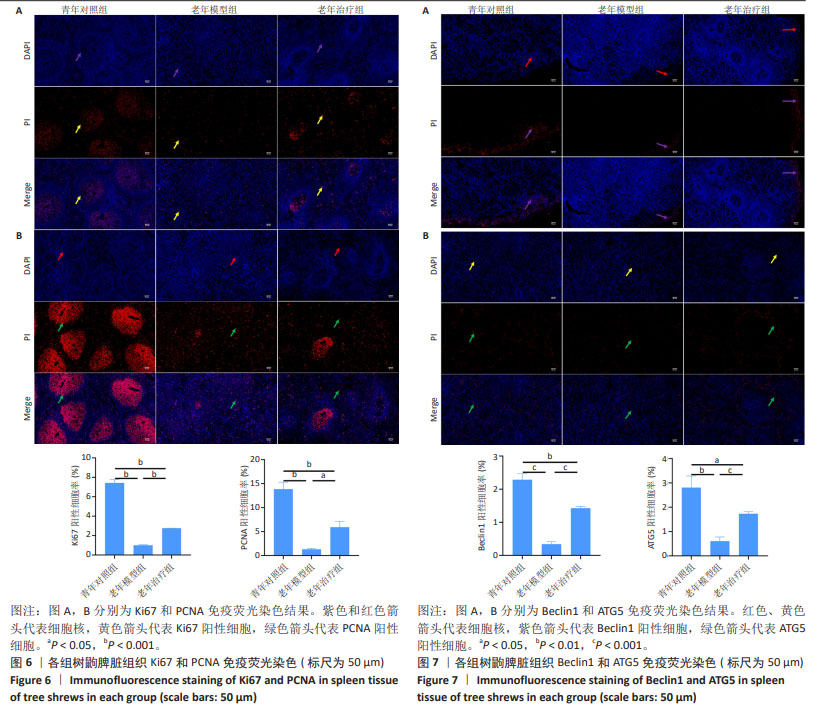
2.5 HA-UCMSCs促进脾脏细胞增殖 为了深入研究HA-UCMSCs治疗脾脏衰老的潜在机制,检测脾脏组织增殖细胞标记分子表达的变化。由免疫荧光染色结果可知,青年对照组脾脏白髓区可见大量阳性增殖细胞,且细胞主要分布在生发中心内;而老年模型组脾脏组织少见阳性增殖细胞(均P < 0.001),且分布不规律;HA-UCMSCs治疗后脾脏Ki67和PCNA阳性细胞增加(P < 0.001和P < 0.05),且呈白髓区聚集性分布,与青年对照组增殖型免疫细胞的分布位置相似,以PCNA阳性细胞的分布位置变化最明显,见图6。提示HA-UCMSC促进脾脏细胞增殖,增加增殖细胞标记分子表达。 2.6 HA-UCMSCs诱导脾脏细胞自噬 与年龄相关的自噬水平降低影响T细胞功能。由免疫荧光染色结果可知,与青年对照组相比,老年模型组自噬相关Beclin1分子表达减少(P < 0.001),ATG5分子表达也减少(P < 0.01),与老年模型组相比,HA-UCMSCs治疗后自噬蛋白分子表达均增加(P < 0.001),见图7。表明衰老后脾脏组织自噬水平降低,自噬功能障碍,而HA-UCMSCs治疗激活细胞自噬。 "
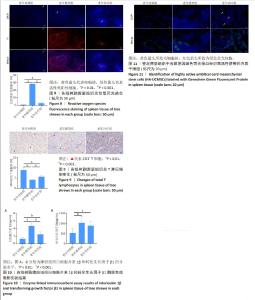
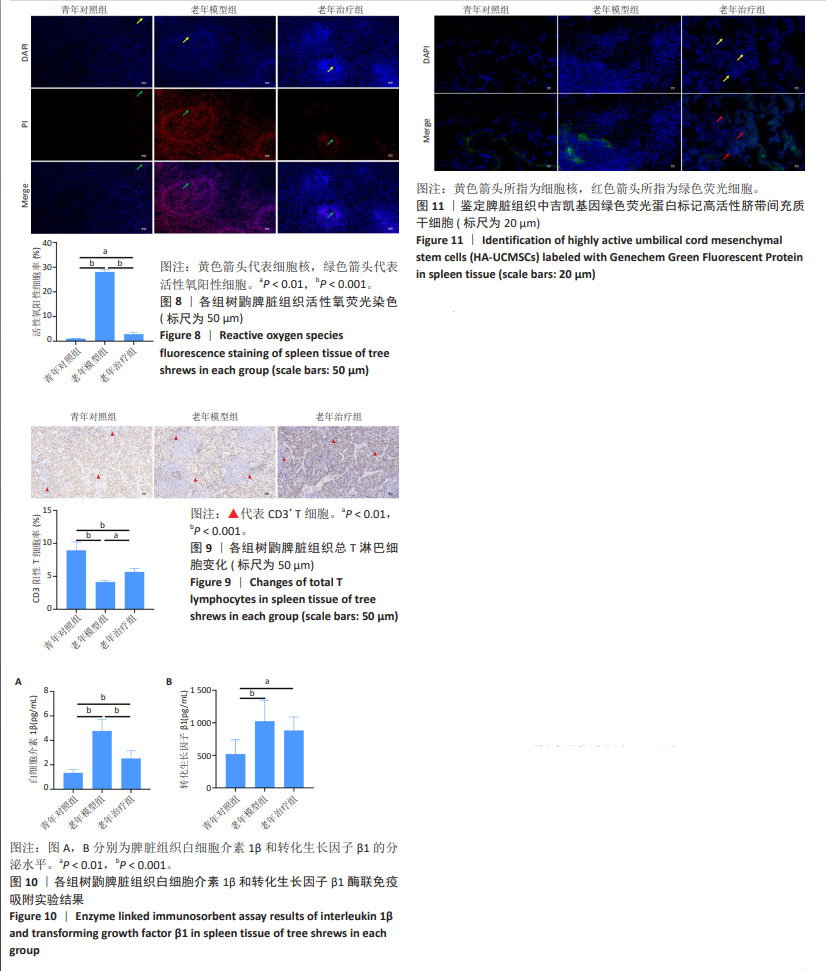
2.7 HA-UCMSCs降低脾脏组织活性氧含量 活性氧等超氧阴离子水平升高是细胞损伤的主要原因,同时也是衰老的重要标志。与青年对照组相比,老年模型组脾脏组织中活性氧含量增加(P < 0.001),与老年模型组相比,HA-UCMSCs治疗显著降低脾脏组织中活性氧含量(P < 0.001),减轻了氧化应激对免疫细胞的损伤,见图8。进一步证实了活性氧过量产生加剧脾脏衰老,与氧化应激性衰老是有关的。 2.8 HA-UCMSCs增加脾脏组织总T淋巴细胞数量 与青年对照组相比,老年模型组树鼩脾脏组织CD3+ T细胞数量减少(P < 0.001),表明树鼩脾脏总T淋巴细胞产生减少,可能影响其细胞免疫功能。与老年模型组相比,HA-UCMSCs治疗后树鼩脾脏组织中CD3+ T细胞升高(P < 0.05),表明HA-UCMSCs治疗具有T细胞相关的作用,见图9。提示HA-UCMSCs促进CD3+ T细胞生成,增加总T淋巴细胞数量和功能,促进T细胞成熟,提高脾脏免疫功能。 2.9 HA-UCMSCs抑制脾脏组织中衰老相关分泌表型因子白细胞介素1β和转化生长因子β1分泌水平 为了证实脾脏组织衰老是否与促炎细胞因子变化一致,通过酶联免疫吸附实验检测了脾脏组织中主要细胞因子的分泌水平。与青年对照组相比,老年模型组脾脏组织中白细胞介素1β和转化生长因子β1分泌水平升高(P < 0.001),说明衰老相关分泌表型因子分泌增多促进脾脏衰老。与老年模型组相比,HA-UCMSCs治疗后白细胞介素1β分泌水平降低(P < 0.001);转化生长因子β1分泌水平降低,但差异无显著性意义(P > 0.05),见图10。提示HA-UCMSCs抑制促炎细胞因子分泌,降低衰老相关分泌表型分子分泌水平,影响脾脏组织衰老变化。 2.10 吉凯基因绿色荧光蛋白标记树鼩HA-UCMSCs在脾脏组织的分布情况 与青年对照组和老年模型组相比,老年治疗组脾脏组织中可见散在分布的绿色荧光细胞,而青年对照组、老年模型组未发现吉凯基因绿色荧光蛋白标记的细胞,见图11,表明尾静脉输注的HA-UCMSCs随血液循环后迁移至脾脏组织,修复衰老组织损伤。"
| [1] BORGONI S, KUDRYASHOVA KS, BURKA K, et al. Targeting immune dysfunction in aging. Ageing Res Rev. 2021;70:101410. [2] LOUREIRO SALGADO C, MENDÉZ COREA AF, COVRE LP, et al. Ageing impairs protective immunity and promotes susceptibility to murine visceral leishmaniasis. Parasitology. 2022;149(9):1249-1256. [3] GUO H, WANG Y, CUI H, et al. Copper Induces Spleen Damage Through Modulation of Oxidative Stress, Apoptosis, DNA Damage, and Inflammation. Biol Trace Elem Res. 2022;200(2):669-677. [4] LEE JY, PAIK IY, KIM JY. Voluntary exercise reverses immune aging induced by oxidative stress in aging mice. Exp Gerontol. 2019;115: 148-154. [5] XIONG H, MANCINI M, GOBERT M, et al. Spleen plays a major role in DLL4-driven acute T-cell lymphoblastic leukemia. Theranostics. 2021;11(4):1594-1608. [6] KIM H, KWON HJ, HAN YB, et al. Increased CD3+ T cells with a low FOXP3+/CD8+ T cell ratio can predict anti-PD-1 therapeutic response in non-small cell lung cancer patients. Mod Pathol. 2019;32(3):367-375. [7] BELGHALI MY, ADMOU B, BRAHIMI M, et al. Immune tumoral microenvironment in gliomas: focus on CD3+ T cells, Vδ1+ T cells, and microglia/macrophages. Immunol Res. 2022;70(2):224-239. [8] CHEN L, CHEN L, NI H, et al. Prediction of CD3 T cells and CD8 T cells expression levels in non-small cell lung cancer based on radiomic features of CT images. Front Oncol. 2023;13:1104316. [9] ZHAO N, ZHANG T, ZHAO Y, et al. CD3+T, CD4+T, CD8+T, and CD4+T/CD8+T Ratio and Quantity of γδT Cells in Peripheral Blood of HIV-Infected/AIDS Patients and Its Clinical Significance. Comput Math Methods Med. 2021;2021:8746264. [10] WEI L, ZHAO J, WU W, et al. Decreased absolute numbers of CD3+ T cells and CD8+ T cells during aging in herpes zoster patients. Sci Rep. 2017;7(1):15039. [11] MITTELBRUNN M, KROEMER G. Hallmarks of T cell aging. Nat Immunol. 2021;22(6):687-698. [12] CISNEROS B, GARCÍA-AGUIRRE I, UNZUETA J, et al. Immune system modulation in aging: Molecular mechanisms and therapeutic targets. Front Immunol. 2022;13:1059173. [13] DENKINGER MD, LEINS H, SCHIRMBECK R, et al. HSC Aging and Senescent Immune Remodeling. Trends Immunol. 2015;36(12): 815-824. [14] ZHU W, GE M, LI X, et al. Hyperoside Attenuates Zearalenone-induced spleen injury by suppressing oxidative stress and inhibiting apoptosis in mice. Int Immunopharmacol. 2022;102:108408. [15] DU K, WANG L, WANG Z, et al. Angelica Sinensis polysaccharide antagonizes 5-Fluorouracil-induced spleen injury and dysfunction by suppressing oxidative stress and apoptosis. Biomed Pharmacother. 2023;162:114602. [16] LI W, WANG X, DONG Y, et al. Nicotinamide riboside intervention alleviates hematopoietic system injury of ionizing radiation-induced premature aging mice. Aging Cell. 2023;22(11):e13976. [17] LI Z, ZHANG Z, REN Y, et al. Aging and age-related diseases: from mechanisms to therapeutic strategies. Biogerontology. 2021;22(2): 165-187. [18] LAI P, WENG J, GUO L, et al. Novel insights into MSC-EVs therapy for immune diseases. Biomark Res. 2019;7:6. [19] XIE M, LI C, SHE Z, et al. Human umbilical cord mesenchymal stem cells derived extracellular vesicles regulate acquired immune response of lupus mouse in vitro. Sci Rep. 2022;12(1):13101. [20] WANG YY, CHANG L, ZHU GH, et al. Mechanism of Thymus Rejuvenation in Elderly Macaques. Rejuvenation Res. 2022;25(5):223-232. [21] ZHOU B, ZHAO Z, ZHANG X, et al. Effect of Allogenic Bone Marrow Mesenchymal Stem Cell Transplantation on T Cells of Old Mice. Cell Reprogram. 2020;22(1):30-35. [22] ANTHONY S, CABANTAN D, MONSOUR M, et al. Neuroinflammation, Stem Cells, and Stroke. Stroke. 2022;53(5):1460-1472. [23] YEO GEC, NG MH, NORDIN FB, et al. Potential of Mesenchymal Stem Cells in the Rejuvenation of the Aging Immune System. Int J Mol Sci. 2021;22(11):5749. [24] WANG K, YAO X, LIN SQ, et al. Cellular and molecular mechanisms of highly active mesenchymal stem cells in the treatment of senescence of rhesus monkey ovary. Stem Cell Res Ther. 2024;15(1):14. [25] FAN Y, LUO R, SU LY, et al. Does the Genetic Feature of the Chinese Tree Shrew (Tupaia belangeri chinensis) Support Its Potential as a Viable Model for Alzheimer’s Disease Research? J Alzheimers Dis. 2018;61(3):1015-1028. [26] FERNANDES SG, DSOUZA R, KHATTAR E. External environmental agents influence telomere length and telomerase activity by modulating internal cellular processes: Implications in human aging. Environ Toxicol Pharmacol. 2021;85:103633. [27] KIBLER A, BUDEUS B, HOMP E, et al. Systematic memory B cell archiving and random display shape the human splenic marginal zone throughout life. J Exp Med. 2021;218(4):e20201952. [28] WANG H, ZHANG Y, WANG Z, et al. Deciphering Nucleic Acid Binding Proteome of Mouse Immune Organs Reveals Hub Proteins for Aging. Mol Cell Proteomics. 2023;22(8):100611. [29] ARAÚJO NC, SUASSUNA JHR. The spleen size in patients undergoing hemodialysis. J Bras Nefrol. 2021;43(1):61-67. [30] MASTERS AR, JELLISON ER, PUDDINGTON L, et al. Attrition of T Cell Zone Fibroblastic Reticular Cell Number and Function in Aged Spleens. Immunohorizons. 2018;2(5):155-163. [31] WANG Z, LIN Y, JIN S, et al. Bone marrow mesenchymal stem cells improve thymus and spleen function of aging rats through affecting P21/PCNA and suppressing oxidative stress. Aging (Albany NY). 2020; 12(12):11386-11397. [32] LI X, LI C, ZHANG W, et al. Inflammation and aging: signaling pathways and intervention therapies. Signal Transduct Target Ther. 2023;8(1):239. [33] SUN T, ZHANG L, FENG J, et al. Characterization of cellular senescence in doxorubicin-induced aging mice. Exp Gerontol. 2022;163:111800. [34] LIU Z, LIANG Q, REN Y, et al. Immunosenescence: molecular mechanisms and diseases. Signal Transduct Target Ther. 2023; 8(1):200. [35] FU M, LIANG X, ZHANG X, et al. Astaxanthin delays brain aging in senescence-accelerated mouse prone 10: inducing autophagy as a potential mechanism. Nutr Neurosci. 2023;26(5):445-455. [36] ZHENG J, HU S, WANG J, et al. Icariin improves brain function decline in aging rats by enhancing neuronal autophagy through the AMPK/mTOR/ULK1 pathway. Pharm Biol. 2021;59(1):183-191. [37] YE L, HUANG J, XIANG X, et al. 17β-Estradiol alleviates cardiac aging induced by d-galactose by downregulating the methylation of autophagy-related genes. Steroids. 2021;170:108829. [38] KAARNIRANTA K, BLASIAK J, LITON P, et al. Autophagy in age-related macular degeneration. Autophagy. 2023;19(2):388-400. [39] LIU R, CUI J, SUN Y, et al. Autophagy deficiency promotes M1 macrophage polarization to exacerbate acute liver injury via ATG5 repression during aging. Cell Death Discov. 2021;7(1):397. |
| [1] | Li Jiagen, Chen Yueping, Huang Keqi, Chen Shangtong, Huang Chuanhong. The construction and validation of a prediction model based on multiple machine learning algorithms and the immunomodulatory analysis of rheumatoid arthritis from the perspective of mitophagy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-15. |
| [2] | Miao Jiahang, Ma Sheng, Li Qupeng, Yu Huilin, Hu Tianyu, Gao Xiao, Feng Hu. Cervical lordosis ratio can be used as a decision-making indicator for selection of posterior surgical approach for multi-level cervical spondylotic myelopathy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1796-1802. |
| [3] | Li Huayuan, Li Chun, Liu Junwei, Wang Ting, Li Long, Wu Yongli. Effect of warm acupuncture on PINK1/Parkin pathway in the skeletal muscle of rats with chronic fatigue syndrome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1618-1625. |
| [4] | Zhou Panpan, Cui Yinglin, Zhang Wentao, Wang Shurui, Chen Jiahui, Yang Tong . Role of cellular autophagy in cerebral ischemic injury and the regulatory mechanism of traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1650-1658. |
| [5] | Zhu Hanmin, Wang Song, Xiao Wenlin, Zhang Wenjing, Zhou Xi, He Ye, Li Wei, . Mitophagy regulates bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1676-1683. |
| [6] | Lyu Liting, Yu Xia, Zhang Jinmei, Gao Qiaojing, Liu Renfan, Li Meng, Wang Lu. Bibliometric analysis of research process and current situation of brain aging and exosomes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1457-1465. |
| [7] | Li Jialin, Zhang Yaodong, Lou Yanru, Yu Yang, Yang Rui. Molecular mechanisms underlying role of mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1512-1522. |
| [8] | Zheng Rongfa, Mo Weibin, Huang Peng, Chen Junji, Liang Ting, Zi Fangyu, Li Guofeng. Effects of electroacupuncture on the expression of metabolic enzymes and autophagy genes in gastrocnemius muscle tissues of exercising rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1127-1136. |
| [9] | Chen Yuning, Jiang Ying, Liao Xiangyu, Chen Qiongjun, Xiong Liang, Liu Yue, Liu Tong. Buqi Huoxue Compounds intervene with the expression of related factors and autophagy related proteins in a rat model of cerebral ischemia/reperfusion [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1152-1158. |
| [10] | Chen Yilin, Jiang Xiaobo, Qu Honglin, Liu Ruilian. General pattern of GSK3/Nrf2-regulated biological rhythms in organismal aging [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1257-1264. |
| [11] | Liu Lingyun, He Guixin, Qin Weibin, Song Hui, Zhang Liwen, Tang Weizhi, Yang Feifei, Zhu Ziyi, Ou Yangbin . Improvement of myocardial injury by traditional Chinese medicine: mitochondrial calcium homeostasis mediates macrophage autophagy and pyroptosis pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1276-1284. |
| [12] | Xu Tianjie, Fan Jiaxin, Guo Xiaoling, Jia Xiang, Zhao Xingwang, Liu kainan, Wang Qian. Metformin exerts a protective effect on articular cartilage in osteoarthritis rats by inhibiting the PI3K/AKT/mTOR pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1003-1012. |
| [13] | Wang Yuru, Li Siyuan, Xu Ye, Zhang Yumeng, Liu Yang, Hao Huiqin. Effects of wogonin on joint inflammation in collagen-induced arthritis rats via the endoplasmic reticulum stress pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1026-1035. |
| [14] | Wen Zixing, Xu Xin, Zhu Shengqun. Correlations between gastrocnemius morphology parameters and physical activity capacity in elderly females under high-frequency ultrasound [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1058-1063. |
| [15] | Wu Guangtao, Qin Gang, He Kaiyi, Fan Yidong, Li Weicai, Zhu Baogang, Cao Ying . Causal relationship between immune cells and knee osteoarthritis: a two-sample bi-directional Mendelian randomization analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1081-1090. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||