Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (11): 2347-2356.doi: 10.12307/2025.338
Previous Articles Next Articles
Mining and verification of inflammation-related genes in skeletal muscle of exhaustive exercise rats undergoing cannabidiol intervention
Zhu Wenning1, Sun Lili2, Peng Lina2, Si Juncheng1, Zang Wanli1, Yin Weidong1, Li Mengqi1
- 1Graduate School, 2School of Human Sports Science, Harbin Sport University, Harbin 150008, Heilongjiang Province, China
-
Received:2024-03-11Accepted:2024-04-28Online:2025-04-18Published:2024-08-12 -
Contact:Sun Lili, PhD, Associate professor, Master’s supervisor, School of Human Sports Science, Harbin Sport University, Harbin 150008, Heilongjiang Province, China -
About author:Zhu Wenning, Master candidate, Graduate School, Harbin Sport University, Harbin 150008, Heilongjiang Province, China -
Supported by:Heilongjiang Provincial Undergraduate Colleges and Universities Basic Research Operating Expenses for Outstanding Youth Innovation Team Project, No. 2023KYYWF-TD04 (to SLL); Introduced Talents Scientific Research Initiation Fee of Harbin Sport University, No. RC20-202107 (to PLN)
CLC Number:
Cite this article
Zhu Wenning, Sun Lili, Peng Lina, Si Juncheng, Zang Wanli, Yin Weidong, Li Mengqi . Mining and verification of inflammation-related genes in skeletal muscle of exhaustive exercise rats undergoing cannabidiol intervention[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(11): 2347-2356.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
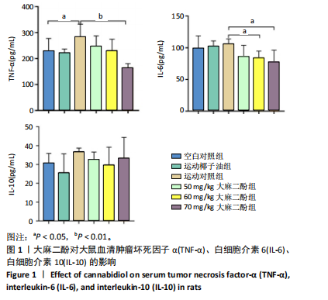
2.1 实验动物数量分析 实验过程中大鼠均无死亡,36只大鼠全部进入结果分析。 2.2 大麻二酚对力竭运动大鼠炎症相关因子的影响 见图1。 2.2.1 肿瘤坏死因子α 相比于空白对照组,运动椰子油组中的肿瘤坏死因子α质量浓度无显著变化,运动对照组显著升高(P < 0.05);大麻二酚干预的各组肿瘤坏死因子α质量浓度低于运动对照组,并随浓度增加呈现依次递减趋势,70 mg/kg大麻二酚组中肿瘤坏死因子α质量浓度最低,与运动对照组比较差异有显著性意义(P < 0.01),说明70 mg/kg大麻二酚药物的灌胃效果最好。 2.2.2 白细胞介素6 与空白对照组相比,运动椰子油组和运动对照组中白细胞介素6的质量浓度呈现升高趋势;大麻二酚干预的各组白细胞介素6质量浓度随大麻二酚"
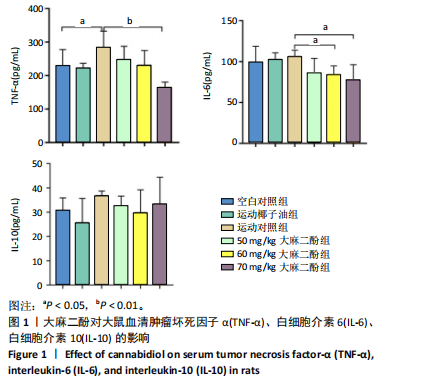

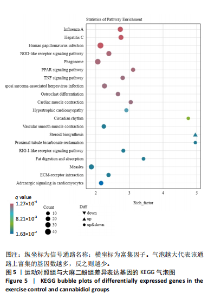
的浓度增加呈现依次递减趋势,70 mg/kg大麻二酚组中白细胞介素6质量浓度最低,其中60 mg/kg大麻二酚组和70 mg/kg大麻二酚组显著低于运动对照组(P < 0.05)。 2.2.3 白细胞介素10因子 与空白对照组相比,运动椰子油组中白细胞介素10质量浓度降低,运动对照组中白细胞介素10质量浓度升高,大麻二酚干预的各组白细胞介素10呈现下降趋势,70 mg/kg大麻二酚组白细胞介素10质量浓度接近空白对照组,各组之间比较差异无显著性意义。 综合以上ELISA结果可知,70 mg/kg大麻二酚药物灌胃后抗炎效果最好,因此,后续实验选取空白对照组、运动对照组、70 mg/kg大麻二酚组大鼠骨骼肌进行转录组学测序分析。 2.3 差异表达基因的火山图分析 为了能够更加直观地展示3组之间差异基因的表达水平,绘制了火山图,见图2。比较空白对照组和运动对照组,有588个基因上调,487个基因下调;比较空白对照组和大麻二酚组,有626个基因上调,747个基因下调;比较运动对照组和大麻二酚组,有522个基因上调,758个基因下调。 2.4 差异表达基因的KEGG富集分析 KEGG数据库整合了基因组化学和系统功能信息,为了鉴定差异表达基因所涉及的通路,将3组差异表达基因映射到KEGG数据库所记录的通路中进行绘图。在图3-5中显示了3组KEGG气泡图的类别。 2.4.1 空白对照组与运动对照组KEGG通路 如图3所示,空白对照组与运动对照组的KEGG通路主要涉及人瘤病毒感染、ECM-受体相互作用、肥厚性心肌病、扩张型心肌病、丙型肝炎、NOD样受体信号通路、甲型流感、癌症通路、病毒性致癌、P13-Akt等信号通路。根据显著性水平对比,"
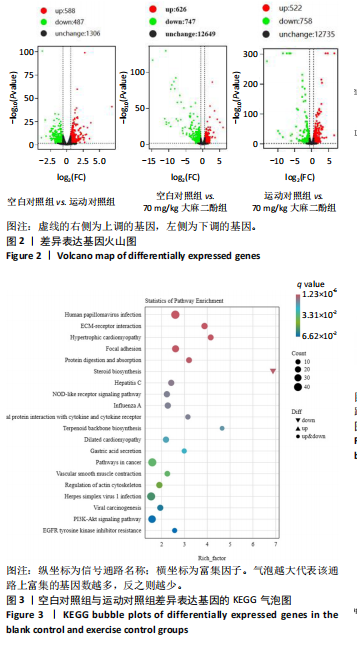
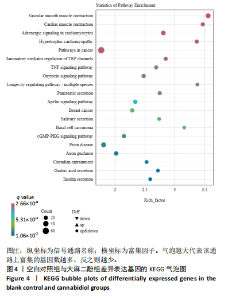
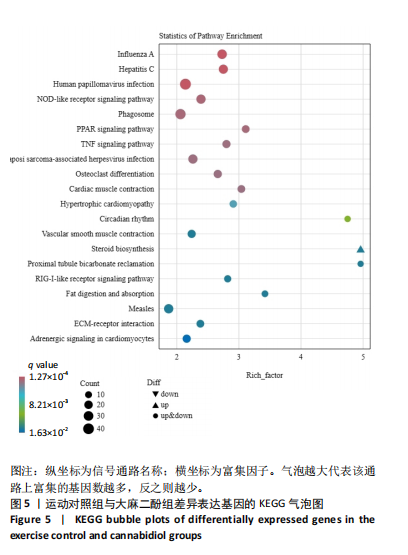
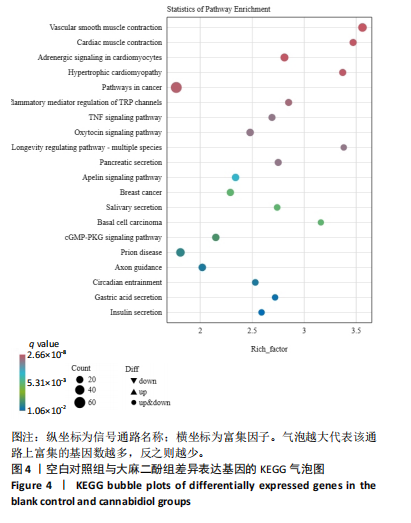
推测力竭运动大鼠可能与扩张型心肌病、丙型肝炎、NOD样受体信号通路、癌症通路等相关。 2.4.2 空白对照组与大麻二酚组KEGG通路 如图4所示,空白对照组与大麻二酚组KEGG通路主要涉及癌症通路、TRP通道的炎症递质调节、肿瘤坏死因子(TNF)通路、乳腺癌、基底细胞癌、cGMP-PKG通路、胰岛素分泌等通路。 2.4.3 运动对照组与大麻二酚组KEGG通路 如图5所示,运动对照组与大麻二酚组KEGG通路主要涉及甲型流感、丙型肝炎、NOD样受体信号通路、PPAR信号通路、肿瘤坏死因子信号通路、肥厚性心肌病、RIG-I样受体信号通路、ECM-受体相互作用等通路。根据显著性水平对比,推测大麻二酚干预后骨骼肌疲劳状态得到缓解的作用机制可能与NOD样受体信号通路、PPAR信号通路、丙型肝炎、肿瘤坏死因子信号通路等有关。"
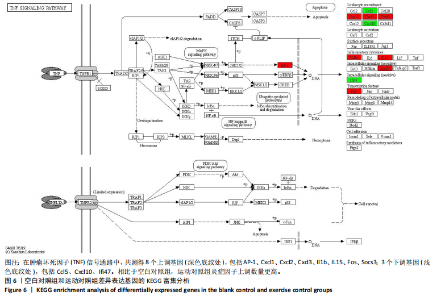
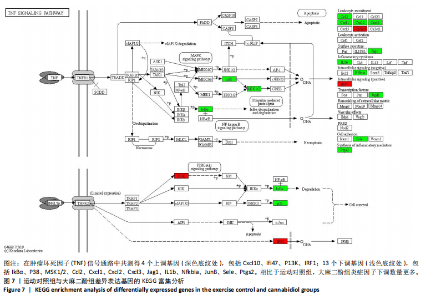
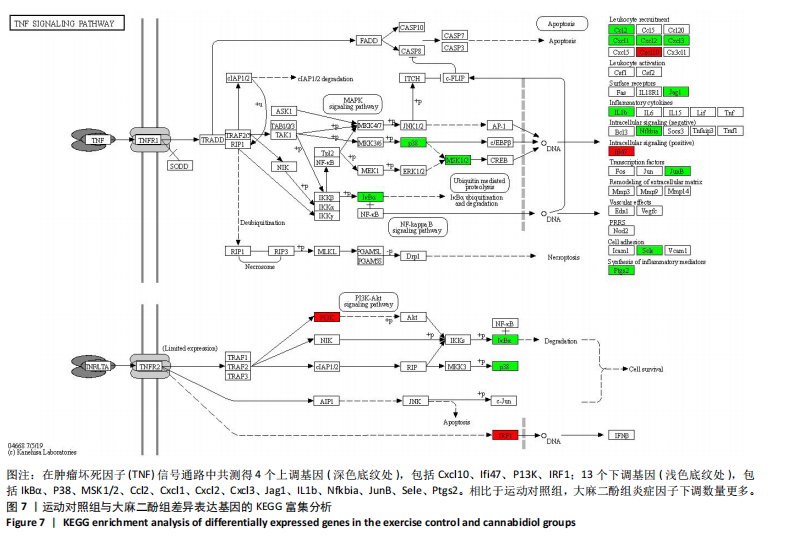
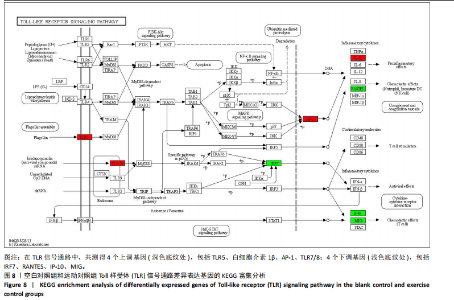
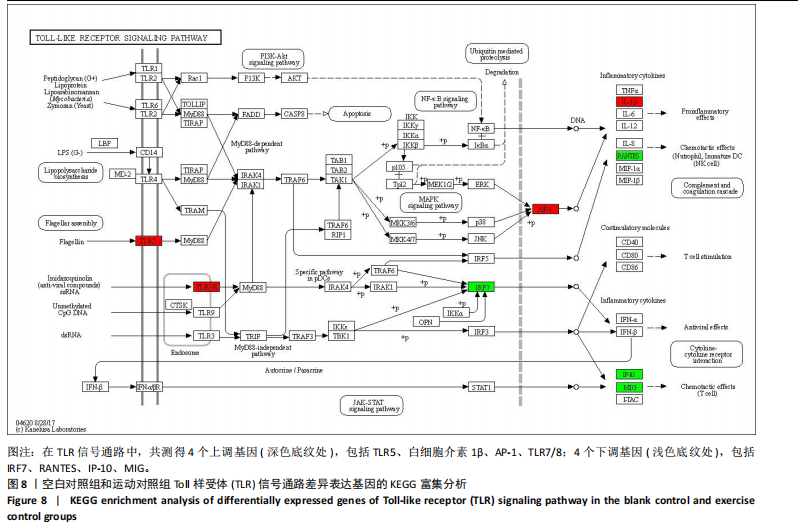
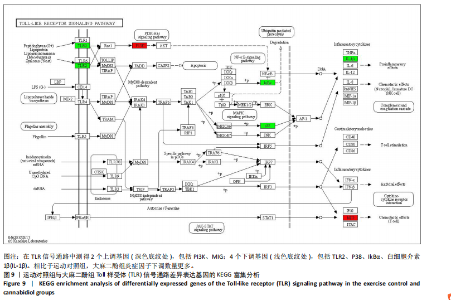
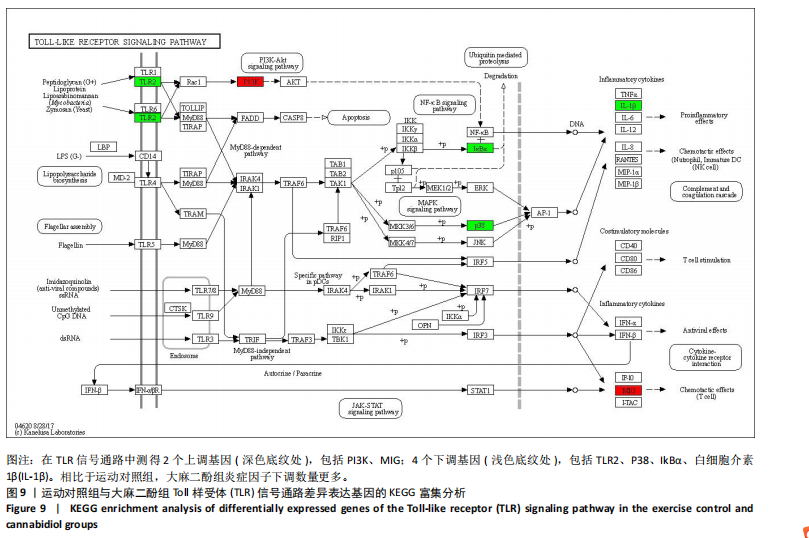
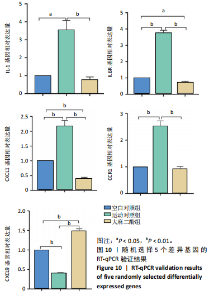
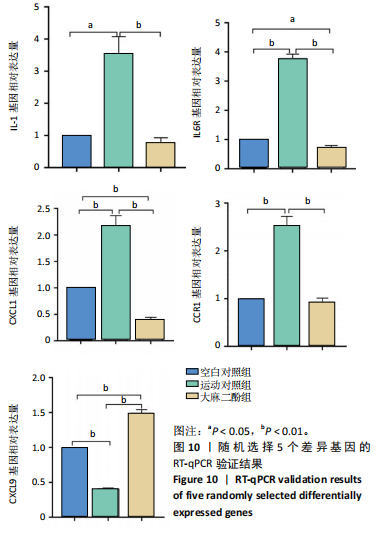
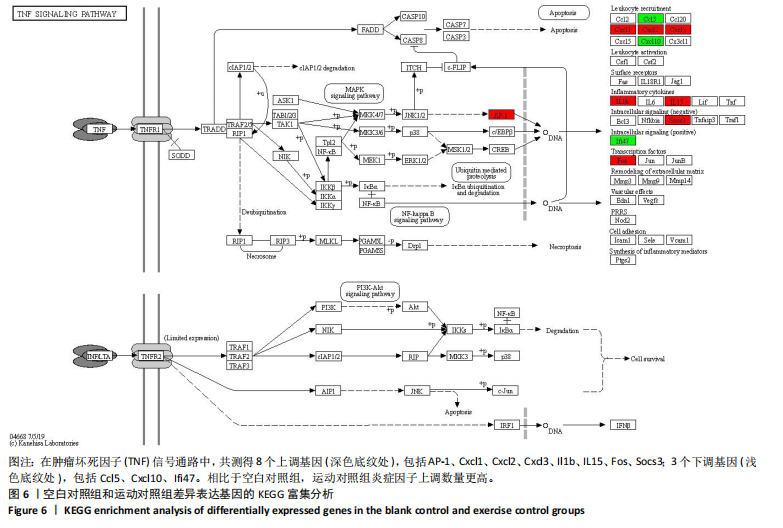
2.5 肿瘤坏死因子信号通路的KEGG富集分析 肿瘤坏死因子信号通路上的基因调控机体的炎症反应与免疫应答,使用KEGG的富集通路进行分析,发现该通路上的基因表达变化情况。 2.5.1 空白对照组和运动对照组比较 如图6所示。在肿瘤坏死因子信号通路中,共测得8个上调基因,包括AP-1、Cxcl1、Cxcl2、Cxcl3、Il1b、IL15、Fos、Socs3;3个下调基因,包括Ccl5、Cxcl10、Ifi47。相比于空白对照组,运动对照组炎症因子上调数量更高,这在一定程度说明了剧烈运动后机体产生了免疫炎症反应。 2.5.2 运动对照组和大麻二酚组比较 如图7所示,在肿瘤坏死因子信号通路中共测得4个上调基因,包括Cxcl10、Ifi47、P13K、IRF1;13个下调基因,包括IkBα、P38、MSK1/2、Ccl2、Cxcl1、Cxcl2、Cxcl3、Jag1、IL1b、Nfkbia、JunB、Sele、Ptgs2。相比于运动对照组,大麻二酚组炎症因子下调数量更多,这从侧面证明,大麻二酚能够改善力竭运动大鼠自身的炎症状态。 2.6 TLR信号通路的KEGG富集分析 TLR信号通路上的基因参与机体的炎症反应与免疫应答,使用KEGG的富集通路进行分析,发现该通路上的基因表达变化情况。 2.6.1 空白对照组与运动对照组比较 如图8所示,在TLR信号通路中共测得4个上调基因,包括TLR5、白细胞介素1β、AP-1、TLR7/8,4个下调基因,包括IRF7、RANTES、IP-10、MIG。 2.6.2 运动对照组和大麻二酚组比较 如图9所示,在TLR信号通路中测得2个上调基因,包括PI3K、MIG,4个下调基因,包括TLR2、P38、IkBα、白细胞介素1β。相比于运动对照组,大麻二酚组炎症因子下调数量更多,这从侧面证明大麻二酚能够改善力竭运动大鼠自身的炎症状态。 2.7 RT-qPCR验证结果 如图10所示,5个基因的变化趋势与转录组测序结果相一致,进一步证实了转录组数据具有较高的可信度。"
| [1] DA ROCHA AL, PINTO AP, KOHAMA EB, et al. The proinflammatory effects of chronic excessive exercise. Cytokine. 2019;119:57-61. [2] CERQUEIRA E, MARINHO DA, NEIVA HP, et al. Inflammatory Effects of High and Moderate Intensity Exercise-A Systematic Review. Front Physiol. 2020;10:1550. [3] MCCARTNEY D, BENSON MJ, DESBROW B, et al. Cannabidiol and Sports Performance: a Narrative Review of Relevant Evidence and Recommendations for Future Research. Sports Med Open. 2020;6(1):27. [4] BURSTEIN S. Cannabidiol (CBD) and its analogs: a review of their effects on inflammation. Bioorg Med Chem. 2015;23(7):1377-1385. [5] ZUARDI AW, RODRIGUES JA, CUNHA JM. Effects of cannabidiol in animal-models predictive of antipsychotic activity. Psychopharmacology (Berl). 1991;104(2):260-264. [6] LEWEKE FM, SCHNEIDER U, RADWAN M, et al. Different effects of nabilone and cannabidiol on binocular depth inversion in man. Pharmacol Biochem Behav. 2000;66(1):175-181. [7] DEVINSKY O, MARSH E, FRIEDMAN D, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15(3):270-278. [8] BLESSING EM, STEENKAMP MM, MANZANARES J, et al. Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics. 2015;12(4): 825-836. [9] BORRELLI F, AVIELLO G, ROMANO B, et al. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J Mol Med (Berl). 2009;87(11):1111-1121. [10] WEISS L, ZEIRA M, REICH S, et al. Cannabidiol arrests onset of autoimmune diabetes in NOD mice. Neuropharmacology.2008;54(1):244-249. [11] ARRUZA L, PAZOS MR, MOHAMMED N, et al. Cannabidiol reduces lung injury induced by hypoxic-ischemic brain damage in newborn piglets. Pediatr Res. 2017;82(1):79-86. [12] DE FILIPPIS D, ESPOSITO G, CIRILLO C, et al. Cannabidiol Reduces Intestinal Inflammation through the Control of Neuroimmune Axis. PLoS One. 2011; 6(12):e28159. [13] LI HB, KONG WM, CHAMBERS CR, et al. The non-psychoactive phytocannabinoid cannabidiol (CBD) attenuates pro-inflammatory mediators, T cell infiltration, and thermal sensitivity following spinal cord injury in mice. Cell Immunol. 2018;329:1-9. [14] DOPKINS N, MIRANDA K, WILSON K, et al. Effects of Orally Administered Cannabidiol on Neuroinflammation and Intestinal Inflammation in the Attenuation of Experimental Autoimmune Encephalomyelitis. J Neuroimmune Pharmacol. 2022;17(1-2):15-32. [15] ZHANG J, LUO ZH, ZHANG Z, et al. Protective effect and mechanism of cannabidiol on myocardial injury in exhaustive exercise training mice. Chem Biol Interact. 2022;365:110079. [16] 侯莉娟, 刘晓莉, 乔德才. 大鼠游泳运动疲劳模型建立的研究[J]. 实验动物科学与管理,2005(1):1-3. [17] QIAO X, ZHANG W, ZHAO W. Role of CXCL10 in Spinal Cord Injury. Int J Med Sci. 2022;19(14):2058-2070. [18] ARIAS AY, KOLISHETTI N, VASHIST A, et al. Anti-inflammatory effects of CBD in human microglial cell line infected with HIV-1.Sci Rep. 2023;13(1):7376. [19] KURET T, KREFT M E, ROMIH R, et al. Cannabidiol as a Promising Therapeutic Option in IC/BPS: In Vitro Evaluation of Its Protective Effects against Inflammation and Oxidative Stress. Int J Mol Sci. 2023;24(5):5055. [20] WANG Y, CHE M, XIN J, et al. The role of IL-1 beta and TNF-alpha in intervertebral disc degeneration. Biomed Pharmacother. 2020;131:110660. [21] GARCIA-MORALES L, CASTILLO AM, RAMIREZ JT, et al. CBD Reverts the Mesenchymal Invasive Phenotype of Breast Cancer Cells Induced by the Inflammatory Cytokine IL-1 beta. Int J Mol Sci. 2020;21(7):2429. [22] LEWIS ND, MUTHUKUMARANA A, FOGAL SE, et al. CCR1 Plays a Critical Role in Modulating Pain through Hematopoietic and Non-Hematopoietic Cells.PLoS One. 2014;9(8):e105883. [23] CHEN YY, LIU SY, WU LL, et al. Epigenetic regulation of chemokine (CC-motif) ligand 2 in inflammatory diseases. Cell Prolif. 2023;56(7):e13428. [24] MA H, XU F, LIU C, et al. A Network Pharmacology Approach to Identify Potential Molecular Targets for Cannabidiol’s Anti-Inflammatory Activity. Cannabis Cannabinoid Res. 2021;6(4):288-299. [25] SEDGER LM, MCDERMOTT MF. TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants - past, present and future. Cytokine Growth Factor Rev. 2014;25(4):453-472. [26] JANG DI, LEE AH, SHIN HY, et al. The Role of Tumor Necrosis Factor Alpha (TNF-alpha) in Autoimmune Disease and Current TNF-alpha Inhibitors in Therapeutics. Int J Mol Sci. 2021;22(5):2719. [27] CHEN CY, HUNG YF, TSAI CY, et al. Transcriptomic Analysis and C-Terminal Epitope Tagging Reveal Differential Processing and Signaling of Endogenous TLR3 and TLR7. Front Immunol. 2021;12:686060. [28] Rinaldi B, Cuzzocrea S, Donniacuo M,et al. Hyperbaric oxygen therapy reduces the toll-like receptor signaling pathway in multiple organ failures. Intensive Care Med. 2011;37(7):1110-1119. [29] MASUDA S, TANAKA M, INOUE T, et al. Chemokine (C-X-C motif) ligand 1 is a myokine induced by palmitate and is required for myogenesis in mouse satellite cells. Acta Physiol (Oxf). 2018;222(3). doi: 10.1111/apha.12975. [30] HOGAN KA, CHO DS, ARNESON PC, et al. Tumor-derived cytokines impair myogenesis and alter the skeletal muscle immune microenvironment. Cytokine. 2018;107:9-17. [31] COMPTON T, POELLINGER N, STRUVE J, et al. Non-thermal Infrared Light Treatment of Ischemia/Reperfusion Injury and Subsequent Analysis of Macrophage Differentiation. J Vis Exp. 2021;(178). doi: 10.3791/62908. [32] MACHADO TR, MACHADO TR, PASCUTTI PG. The p38 MAPK Inhibitors and Their Role in Inflammatory Diseases. Chemistryselect. 2021;6(23):5729-5742. [33] UENO M, MAESHIGE N, HIRAYAMA Y, et al. Pulsed ultrasound prevents lipopolysaccharide-induced muscle atrophy through inhibiting p38 MAPK phosphorylation in C2C12 myotubes. Biochem Biophys Res Commun. 2021;570:184-190. [34] MACIEJEWSKA-SKRENDO A, TARNOWSKI M, KOPYTKO P, et al. CCL2 Gene Expression and Protein Level Changes Observed in Response to Wingate Anaerobic Test in High-Trained Athletes and Non-Trained Controls.Int J Environ Res Public Health. 2022;19(16):9947. [35] ZHAO LL, LIU XG, ZHANG J, et al. Hydrogen Sulfide Alleviates Skeletal Muscle Fibrosis via Attenuating Inflammation and Oxidative Stress. Front Physiol. 2020;11:533690. [36] PATEL H, YONG C, NAVI A, et al. Toll-like receptors 2 and 6 mediate apoptosis and inflammation in ischemic skeletal myotubes. Vasc Med. 2019;24(4): 295-305. [37] KIM JW, SHIN SK, KWON EY. Luteolin Protects Against Obese Sarcopenia in Mice with High-Fat Diet-Induced Obesity by Ameliorating Inflammation and Protein Degradation in Muscles. Mol Nutr Food Res. 2023;67(6):e2200729. |
| [1] | Zhang Yibo, Lu Jianqi, Mao Meiling, Pang Yan, Dong Li, Yang Shangbing, Xiao Xiang. Exploring the causal relationship between rheumatoid arthritis and coronary atherosclerosis: a Mendel randomized study involving serum metabolites and inflammatory factors [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [2] | Chi Wenxin, Zhang Cunxin, Gao Kai, Lyu Chaoliang, Zhang Kefeng. Mechanism by which nobiletin inhibits inflammatory response of BV2 microglia [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1321-1327. |
| [3] | Yu Ting, Lyu Dongmei, Deng Hao, Sun Tao, Cheng Qian. Icariin pretreatment enhances effect of human periodontal stem cells on M1-type macrophages [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1328-1335. |
| [4] | De Ji, Suo Langda, Wei Yuchen, Wang Bin, Awangcuoji, Renqingcuomu, Cui Jiuzeng, Zhang Lei, Ba Gui. Comprehensive analysis of genes related to endometrial receptivity and alternative splicing events in northwest Tibetan cashmere goats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1429-1436. |
| [5] | Wang Rongrong, Huang Yushan, Li Xiangmiao, Bai Jinzhu. Prostaglandin E1 regulates vascular-related factors and protects microcirculatory function during the acute phase of traumatic spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 958-967. |
| [6] | Zhao Zengbo, Li Chenxi, Dou Chenlei, Ma Na, Zhou Guanjun. Anti-inflammatory and osteogenic effects of chitosan/sodium glycerophosphate/sodium alginate/leonurine hydrogel [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 678-685. |
| [7] | Wang Tong, Zheng Yu, Jia Chengming, Yang Hu, Zhang Guangfei, Ji Yaoyao. Action mechanism of Gegenmaqi prescription in treatment of periarthritis of shoulder combined with type 2 diabetes based on TCMSP database [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(35): 7669-7678. |
| [8] | Xu Biao, Dong Yuzhen, Lu Tan. Effect of dihydroquercetin on the expression of inflammatory response markers in rats with spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6843-6850. |
| [9] | Wang Ziheng, Wu Shuang. Oxidative stress-related genes and molecular mechanisms after spinal cord injury: data analysis and verification based on GEO database [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6893-6904. |
| [10] | Hu Shujuan, Liu Dang, Ding Yiting, Liu Xuan, Xia Ruohan, Wang Xianwang. Ameliorative effect of walnut oil and peanut oil on atherosclerosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6482-6488. |
| [11] | Zhu Xuekun, Liu Heng, Feng Hui, Gao Yunlong, Wen Lei, Cai Xiaosong, Zhao Ben, Zhong Min. Identification of core genes of osteoarthritis by bioinformatics [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(3): 637-644. |
| [12] | Si Juncheng, Peng Lina, Sun Lili, Wang Yu, Shi Lei, Shen Wenhui, Li Mengqi, Zang Wanli. Transcriptome sequencing analysis of the mechanism by which cold water swimming regulates inflammatory response in rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6205-6211. |
| [13] | Geng Longyu, Sheng Li, Bai Shuo, Gao Beiyao, Ge Ruidong, Jiang Shan . Role and molecular mechanism of pyroptosis in motor system diseases [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5695-5703. |
| [14] | Ye Xing, Liu Renyi. Effects of voluntary exercise on molecular expression profiles in the hippocampus of mice: a gene expression profile analysis based on the GEO database [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(24): 5237-5244. |
| [15] | Zhang Yibo, Lu Jianqi, Mao Meiling, Pang Yan, Dong Li, Yang Shangbing, Xiao Xiang. Rheumatoid arthritis and coronary atherosclerosis: data analysis of serum metabolite and inflammatory factor in the European population [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(24): 5263-5271. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||