Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (7): 1475-1485.doi: 10.12307/2025.026
Previous Articles Next Articles
Research hotspots and frontiers of stem cells for Alzheimer’s disease
Xie Liugang1, 2, Cui Shuke3, Guo Nannan4, Li Aoyu1, 2, Zhang Jingrui1, 2
- 1First Clinical Medical College of Henan University of Chinese Medicine, Zhengzhou 450046, Henan Province, China; 2Fourth Ward, Department of Encephalopathy, First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China; 3Henan University of Chinese Medicine, Zhengzhou 450046, Henan Province, China; 4School of Pediatrics, Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China
-
Received:2023-11-16Accepted:2024-02-03Online:2025-03-08Published:2024-06-28 -
Contact:Cui Shuke, MD, Professor, Henan University of Chinese Medicine, Zhengzhou 450046, Henan Province, China -
About author:Xie Liugang, Master candidate, First Clinical Medical College of Henan University of Chinese Medicine, Zhengzhou 450046, Henan Province, China; Fourth Ward, Department of Encephalopathy, First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China -
Supported by:Chinese Medicine Discipline Construction Project of Characteristic Backbone Discipline in Henan Province, No. STG-ZYX03-202130 (to CSK); Application and Research of Constructing Integrated Practice Teaching System of Simulated Hospital in TCM colleges and Universities in 2021, No. 2021SJGLX175 (to CSK)
CLC Number:
Cite this article
Xie Liugang, Cui Shuke, Guo Nannan, Li Aoyu, Zhang Jingrui. Research hotspots and frontiers of stem cells for Alzheimer’s disease[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1475-1485.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

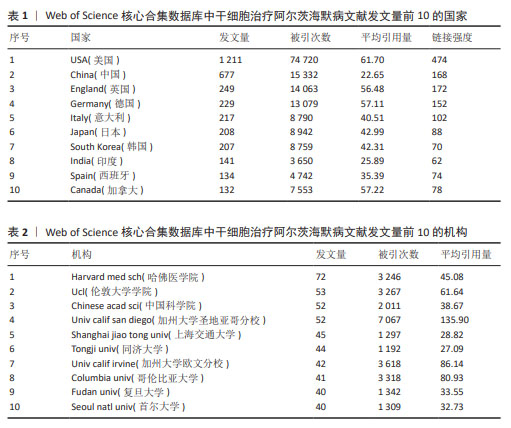
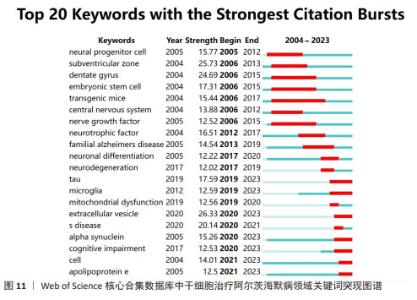
2.1 发文量趋势 在CiteSpace软件分析的数据基础上,使用Excel制作年发文量趋势图(图2)。近20年干细胞治疗阿尔茨海默病领域共发表文献3 521篇,整体来看年发文量呈稳定上升趋势,只有在2006-2007年、2014-2015年出现少量下降,而在2015-2022年增长趋势显著,这表明干细胞治疗阿尔茨海默病的研究已经引起了研究人员的极大兴趣。2020年发文数量急剧增加,是近年来发文量增长率最高的一年,达到了26.1%。2021年、2022年发文量仍分别以10.2%,5.5%的增长率上涨,2022年发文量达到顶峰。由于2023年的文献只统计至10月,所以会出现断崖式下跌的情况,但根据目前的趋势来看2023年的发文量将会继续增加。 2.2 国家/地区合作分析 3 521篇文献分别来自94个国家/地区。表1罗列了近20年发文量最多的前10个国家。其中,美国的发文量(1 211篇,占比34.39%)、文献被引用次数(74 720次)、平均引用量(61.70)和总连接强度(474)均位居第一,这表明美国在该领域的影响力最大。加拿大的发文量位居第10位,但其平均引用量高居第2,这意味着虽然加拿大在该领域的发文量较少但文章质量高,易被其他学者所接受。例如不列颠哥伦比亚大学学者在2009年发表的1篇关于干细胞在神经系统疾病中发挥的作用的综述[16],这篇文献被国内外学者大量引用。对该领域发表文献数量5篇以上的国家/地区进行合作网络分析(图3),反映了该领域内国家/地区的合作关系,图谱共纳入54个国家/地区,合作关系连线495条,总链接线强度1 939,这表明国家/地区间存在一定强度的合作关系。链接强度前5名的国家分别为美国、中国、英国、德国、意大利。 2.3 机构分布和合作分析 通过VOSviewer软件对发文量在20篇以上的机构(59家)进行分析,制作发文量前10的机构排名表(表2)。从表2可以看出,前10位机构的发文量均在40篇以上,被引次数均高于1 100次,平均每篇文献被引次数均在27以上。发文量前3的机构分别是哈佛医学院(72篇,占比2.04%)、伦敦大学学院(53篇,占比1.50%)、中国科学院(52篇,占比1.477%)和加州大学圣地亚哥分校(52篇,占比1.477%),分别位于美国、英国和中国。哈佛医学院发表的文献最多,同时其被引次数也位居前列,这表明哈佛医学院在该领域的研究能被大多数研究者所接受,其主要研究方向之一为神经退行性疾病的基础实验和临床诊断、治疗的研究[17-19]。图4为主要机构的合作网络图,图中圆形节点越大代表发文量越多,节点之间连线越粗代表机构之间联系更为密切,该图表明不同国家的机构间已经形成了一定规模的合作关系,但主要的合作关系还是在国内。 2.4 作者合作分析 根据普赖斯定律计算核心作者最低发文量[20],计算公式为:M≈0.749×√nmax,其中,M为核心作者的最低发文量,nmax为发文量最多作者的文献数量。利用VOSviewer分析得出nmax=24,计算M≈3.67,故发文量≥4篇的作者为核心作者,共463位,发文量2 701篇,占比76.71%,大于普莱斯定律的半数标准,因此可以认为干细胞治疗阿尔茨海默病领域的研究者有各自的研究团体。表3为发文量前10的作者排名,其中美国纽约分子与信号研究中心的Maiese,kenneth教授发文量最多,其在神经退行性疾病和神经再生方面做了大量基础研究[21-22];被引次数最高(2 200次)的是加州大学欧文分校的Blurton-jones,Mathew教授,其均篇引用量高达115.79。 通过VOSviewer 软件可视化分析生成该领域核心专家学者及其研究团队的合作关系图谱(图5),图中颜色深浅代表该领域核心专家学者的研究产力热度,颜色越深代表热度越高;核心专家团队之间的合作程度由其分布密度代表,距离越近代表合作程度越高。可以看到该领域核心带头人显著,并且大部分学者均有自己的研究团队,团队内部合作紧密,不足之处在于不同研究团队之间的合作关系 较少。"

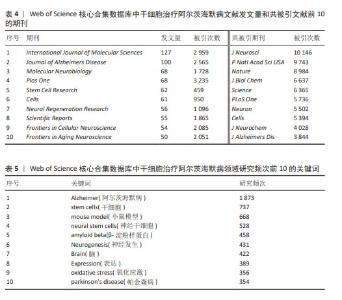
2.5 发文期刊和共被引期刊分析 通过VOSviewer软件分析发现,从2004-01-01/2023-10-31共有812家期刊在干细胞治疗阿尔茨海默病领域发表了3 521篇文章。从表4可以看出,发文量前10的期刊发文量均在50篇以上。其中《International Journal of Molecular Sciences》期刊累计发文量最多(共127篇,占比3.61%),其被引次数2 959次,位居第二,这说明该期刊对干细胞治疗阿尔茨海默病领域比较重视,同时在该领域也具有一定的影响力。被引次数最多的期刊是《Plos One》,共发表了68篇文献,被引次数高达3 235次,平均每篇被引次数约为48次,这表明该期刊在此领域具有较高的学术影响力,发表的研究成果具有一定突破性,更容易被国内外专家所接受,该期刊在此领域的发文方向之一是基于诱导多能干细胞创建阿尔茨海默病三维模型[23-24],如WASEEM K RAJA从诱导多能干细胞中建立了一套有效性更高的阿尔茨海默病病理模型系统,以帮助研究阿尔茨海默病的发病机制。 共被引期刊是指发表于2个不同期刊的文章同时被一篇文章所引用的情况。共被引最多的前10种期刊的被引次数决定了它们的影响力[25]。如表4所示,共被引次数最多的期刊是《Journal of Neuroscience》(10 146次),其次是《Proceedings of The National Academy of Sciences of The United States of America》(9 743次)和《Nature》(8 984次)。图6,7是通过 VOSviewer制作的发文期刊和共被引期刊网络图,其节点不同的颜色代表期刊聚类的研究方向不同,节点的大小代表该领域内期刊文献的被引次数,节点之间的连线代表不同的被引/共被引关联程度,连线的粗细与被引/共被引期刊的关联程度相关,其中《PLoS One》《Cells》《Journal of Alzheimers Disease》三家期刊的发文量和共被引次数均较高,领域内影响力较大。 2.6 关键词分析 2.6.1 共现分析 该文利用VOSviewer和Citespace对关键词进行处理分析,得到如下研究频次表、共现网络图、密度图、聚类图谱、时间线图谱以及突现图谱。表5主要展示了研究频次前10的关键词,其中Alzheimer(阿尔茨海默病)(1 873次)、stem cells(干细胞)(737次)、mouse model(小鼠模型)(668次)位居前3,说明在干细胞治疗阿尔茨海默病领域的研究文献大多与动物实验有关。 关键词作为一篇文章的要点,通过其密度分布图可以了解到该领域内此关键词的探究热度。如图8所示,利用VOSviewer制作关键词密度分布图,颜色越深代表其研究热度越高,关键词的关联程度由关键词之间的距离表示,距离越近关联程度越高。 2.6.2 聚类分析 利用聚类分析对纳入的关键词进行聚类并总结研究主题,有助于研究人员识别热门话题,协助学者们更好地理解当前的科学关注点[25]。利用Citespace对关键词进行知识图谱的聚类分析(图9),聚类显示设置为0-15,进一步预测当前的研究热点方向。根据聚类模块值Q值、S值确定聚类结果的合理性和有效性:Q值> 0.3则表示聚类结构显著,S > 0.5则聚类结果合理,S > 0.7则聚类结果可信度高[26]。结果显示:Q值=0.917 3 > 0.3,S值=0.938 8 > 0.7,说明该文关键词聚类结果具有高度的显著性和合理性。聚类号越小代表该聚类的研究热度越高,对各聚类进一步总结:聚类#3、聚类#4、聚类#5、聚类#7、聚类#15为干细胞具体分类;聚类#2、聚类#6、聚类#9为不同疾病名称;聚类#1、聚类#8、聚类#10、聚类#11、聚类#13、聚类#14侧重于阿尔茨海默病的病理生理;聚类#0、聚类#12为阿尔茨海默病的治疗。"


2.6.3 时间线分析 基于Citespace关键词聚类图谱,进一步利用Citespace软件,设置时间切片为1,生成关键词时间线图谱(图10),在此基础上了解该领域热点主题词时间分布情况,预测未来发展趋势。图中直线线条代表0-15不同的聚类,线段上的圆形节点对应此聚类下的关键词,节点越大则关键词出现的频次越高。聚类#0、聚类#3、聚类#4、聚类#14出现的时间跨度均较长,一直是研究的热点方向。大多数关键词在近20年的时间内均以较高的频次被引用,如小鼠模型(mouse model)、神经发生(neurogenesis)等,但也有部分关键词仅仅短暂出现过几年,如创伤性脑损伤(traumatic brain injury)、记忆缺失(memory deficit)、认知功能(cognitive function)等。如图10所示,近20年来该领域的研究热点一直是成人神经发生(adult neurogenesis)[27-28]、间充质干细胞(mesenchymal stem cells)[29-30]、氧化应激(oxidative stress)等方向[31-32]。 2.6.4 突现分析 关键词突现是指某一时间段内关键词高频出现的现象,反映了不同时期内某一领域的研究热点,并且可进一步预测该领域未来的研究趋势[33]。故为了更加深入了解干细胞治疗阿尔茨海默病领域突现的研究热点,该文进一步利用Citespace软件的“Bursts”功能对关键词进行分析总结,并按时间排列呈现排名前20的关键词,结果如图11所示。从图可以看出,大部分关键词的突现发生在2017-2023年,与此同时,干细胞治疗阿尔茨海默病领域也进入了发文高峰期。 通过对图11的分析总结,可以将干细胞治疗阿尔茨海默病领域关键词突现历程分为2个阶段:第一阶段是2005-2016年,在此期间的关键词主要是神经祖细胞(neural progenitor cell)、胚胎干细胞(embryonic stem cell)、齿状回(dentate gyrus)、亚脑室区(subventricular zone)、转基因鼠(transgenic mice),其中亚脑室区的突现强度最大,表明在这个阶段该领域的研究重点主要是围绕机制和动物实验研究;第二阶段是2017-2023年,此阶段的关键词主要是细胞外囊泡 (extracellular vesicle)、α核蛋白(alpha synuclein)、tau蛋白、线粒体功能障碍(mitochondrial dysfunction),其中突现强度最大的关键词是细胞外囊泡,这也是近期新兴的一个研究热潮。文献证实神经炎症是阿尔茨海默病发病的重要机制之一,Morris Losurdo、Allaura S Cone等发现分泌于间充质干细胞的细胞外囊泡可以减轻阿尔茨海默病发病的炎症反应和氧化应激反应,重塑受损神经通路,改善阿尔茨海默病患者的学习和认知功能[34-35]。"

| [1] DUNCAN T, VALENZUELA M. Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res Ther. 2017;8(1):111. [2] HAQUE RU, LEVEY AI. Alzheimer’s disease: A clinical perspective and future nonhuman primate research opportunities. Proc Natl Acad Sci U S A. 2019;116(52):26224-26229. [3] OZBEN T, OZBEN S. Neuro-inflammation and anti-inflammatory treatment options for Alzheimer’s disease. Clin Biochem. 2019;72: 87-89. [4] HARDY J, SELKOE DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353-356. [5] KNOPMAN DS, AMIEVA H, PETERSEN RC, et al. Alzheimer disease. Nat Rev Dis Primers. 2021;7(1):33. [6] SCHELTENS P, DE STROOPER B, KIVIPELTO M, et al. Alzheimer’s disease. Lancet. 2021;397(10284):1577-1590. [7] WANG ZB, WANG ZT, SUN Y, et al. The future of stem cell therapies of Alzheimer’s disease. Ageing Res Rev. 2022;80:101655. [8] LIVINGSTON G, HUNTLEY J, SOMMERLAD A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248): 413-446. [9] WANG SM, LEE CU, LIM HK. Stem cell therapies for Alzheimer’s disease: is it time? Curr Opin Psychiatry. 2019;32(2):105-116. [10] BOESE AC, HAMBLIN MH, LEE JP. Neural stem cell therapy for neurovascular injury in Alzheimer’s disease. Exp Neurol. 2020;324: 113112. [11] LI M, GUO K, IKEHARA S. Stem cell treatment for Alzheimer’s disease. Int J Mol Sci. 2014;15(10):19226-19238. [12] NINKOV A, FRANK JR, MAGGIO LA. Bibliometrics: Methods for studying academic publishing. Perspect Med Educ. 2022;11(3):173-176. [13] MOSHARI A, ASLANI A, ZOLFAGHARI Z, et al. Forecasting and gap analysis of renewable energy integration in zero energy-carbon buildings: a comprehensive bibliometric and machine learning approach. Environ Sci Pollut Res Int. 2023;30(40):91729-91745. [14] CHEN Y, LIN M, ZHUANG D. Wastewater treatment and emerging contaminants: Bibliometric analysis. Chemosphere. 2022; 297:133932. [15] WEI N, XU Y, LI Y, et al. A bibliometric analysis of T cell and atherosclerosis. Front Immunol. 2022;13:948314. [16] KIM SU, DE VELLIS J. Stem cell-based cell therapy in neurological diseases: a review. J Neurosci Res. 2009;87(10):2183-2200. [17] LEOW JJ, COLE AP, SUN M, et al. Association of Androgen Deprivation Therapy With Alzheimer’s Disease: Unmeasured Confounders. J Clin Oncol. 2016;34(23):2801-2803. [18] ARON L, YANKNER BA. Neurodegenerative disorders: Neural synchronization in Alzheimer’s disease. Nature. 2016; 540(7632):207-208. [19] RENTZ DM, PAPP KV. Commentary on Composite cognitive and functional measures for early stage Alzheimer’s disease trials. Alzheimers Dement (Amst). 2020;12(1):e12012. [20] 姚雪,徐川平,李杰,等.基于普赖斯定律和二八定律及在线投稿系统构建某科技期刊核心作者用户库[J].编辑学报, 2017,29(1):64-66. [21] MAIESE K. The Metabolic Basis for Nervous System Dysfunction in Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease. Curr Neurovasc Res. 2023;20(3):314-333. [22] MAIESE K. A Common Link in Neurovascular Regenerative Pathways: Protein Kinase B (Akt). Curr Neurovasc Res. 2022;19(1):1-4. [23] RAJA WK, MUNGENAST AE, LIN YT, et al. Self-Organizing 3D Human Neural Tissue Derived from Induced Pluripotent Stem Cells Recapitulate Alzheimer’s Disease Phenotypes. PLoS One. 2016;11(9):e161969. [24] LEE HK, VELAZQUEZ SC, CHEN M, et al. Three Dimensional Human Neuro-Spheroid Model of Alzheimer’s Disease Based on Differentiated Induced Pluripotent Stem Cells. PLoS One. 2016;11(9):e163072. [25] ZHANG Q, ZENG Y, ZHENG S, et al. Research hotspots and frotiers of stem cells in stroke: A bibliometric analysis from 2004 to 2022. Front Pharmacol. 2023;14:1111815. [26] 王越晗,黄雨露,夏煜,等.基于文献计量和可视化分析的中国水生态环境治理研究热点与趋势[J]. 长江科学院院报, 2022,39(9):137-143. [27] TAUPIN P. Adult neurogenesis, neural stem cells and Alzheimer’s disease: developments, limitations, problems and promises. Curr Alzheimer Res. 2009;6(6):461-470. [28] NOUREDDINI M, BAGHERI-MOHAMMADI S. Adult Hippocampal Neurogenesis and Alzheimer’s Disease: Novel Application of Mesenchymal Stem Cells and their Role in Hippocampal Neurogenesis. Int J Mol Cell Med. 2021;10(1):1-10. [29] KIM HJ, CHO KR, JANG H, et al. Intracerebroventricular injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: a phase I clinical trial. Alzheimers Res Ther. 2021;13(1):154. [30] ANDRZEJEWSKA A, DABROWSKA S, LUKOMSKA B, et al. Mesenchymal Stem Cells for Neurological Disorders. adv Sci (Weinh). 2021;8(7):2002944. [31] KAHROBA H, RAMEZANI B, MAADI H, et al. The role of Nrf2 in neural stem/progenitors cells: From maintaining stemness and self-renewal to promoting differentiation capability and facilitating therapeutic application in neurodegenerative disease. Ageing Res Rev. 2021;65:101211. [32] TANDON A, SINGH SJ, CHATURVEDI RK. Stem Cells as Potential Targets of Polyphenols in Multiple Sclerosis and Alzheimer’s Disease. Biomed Res Int. 2018;2018:1483791. [33] 孙宇康,宋丽娟,温春丽,等.基于Web of Science近十年干细胞治疗心肌梗死的可视化分析[J].中国组织工程研究, 2024,28(7): 1143-1148. [34] LOSURDO M, PEDRAZZOLI M, D’AGOSTINO C, et al. Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer’s disease. Stem Cells Transl Med. 2020;9(9):1068-1084. [35] CONE AS, YUAN X, SUN L, et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate Alzheimer’s disease-like phenotypes in a preclinical mouse model. Theranostics. 2021;11(17): 8129-8142. [36] ALZHEIMER’S ASSOCIATION .2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12(4):459-509. [37] DE MIRANDA AS, ZHANG CJ, KATSUMOTO A, et al. Hippocampal adult neurogenesis: Does the immune system matter? J Neurol Sci. 2017;372:482-495. [38] MING GL, SONG H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011; 70(4):687-702. [39] DENG W, SAXE MD, GALLINA IS, et al. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009; 29(43):13532-13542. [40] KOZAREVA DA, CRYAN JF, NOLAN YM. Born this way: Hippocampal neurogenesis across the lifespan. Aging Cell. 2019;18(5):e13007. [41] MORENO-JIMENEZ EP, FLOR-GARCIA M, TERREROS-RONCAL J, et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med. 2019;25(4):554-560. [42] 孙世标,潘小龙,魏智慧,等.补肾抗衰类中药联合干细胞疗法治疗阿尔茨海默病的机制研究进展[J]. 中国实验方剂学杂志,2023,29(15):199-211. [43] VASIC V, BARTH K, SCHMIDT M. Neurodegeneration and Neuro-Regeneration-Alzheimer’s Disease and Stem Cell Therapy. Int J Mol Sci. 2019;20(17):4272. [44] JIA Y, CAO N, ZHAI J, et al. HGF Mediates Clinical-Grade Human Umbilical Cord-Derived Mesenchymal Stem Cells Improved Functional Recovery in a Senescence-Accelerated Mouse Model of Alzheimer’s Disease. Adv Sci (Weinh). 2020;7(17): 1903809. [45] WEI Y, XIE Z, BI J, et al. Anti-inflammatory effects of bone marrow mesenchymal stem cells on mice with Alzheimer’s disease. Exp Ther Med. 2018;16(6):5015-5020. [46] 谷青芳,郭敏芳,刘晓琴,等.骨髓间充质干细胞改善APP/PS1模型小鼠认知功能的机制[J].中国组织工程研究,2022, 26(19):2964-2969. [47] QIN C, LU Y, WANG K, et al. Transplantation of bone marrow mesenchymal stem cells improves cognitive deficits and alleviates neuropathology in animal models of Alzheimer’s disease: a meta-analytic review on potential mechanisms. Transl Neurodegener. 2020; 9(1):20. [48] DOSHMANZIARI M, SHIRIAN S, KOUCHAKIAN MR, et al. Mesenchymal stem cells act as stimulators of neurogenesis and synaptic function in a rat model of Alzheimer’s disease. Heliyon. 2021;7(9):e7996. [49] DUBEY S, SINGH E. Antioxidants: an approach for restricting oxidative stress induced neurodegeneration in Alzheimer’s disease. Inflammopharmacology. 2023; 31(2):717-730. [50] BUTTERFIELD DA, BOYD-KIMBALL D. Oxidative Stress, Amyloid-beta Peptide, and Altered Key Molecular Pathways in the Pathogenesis and Progression of Alzheimer’s Disease. J Alzheimers Dis. 2018;62(3): 1345-1367. [51] ISLAM MT. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol Res. 2017;39(1):73-82. [52] 王准,孙谕莹,黄汉昌.氧化应激与阿尔茨海默病的病理关系及干预措施[J].生命科学,2023,35(4):519-528. [53] CUI GH, WU J, MOU FF, et al. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J. 2018; 32(2):654-668. [54] CHEN YA, LU CH, KE CC, et al. Mesenchymal Stem Cell-Derived Exosomes Ameliorate Alzheimer’s Disease Pathology and Improve Cognitive Deficits. Biomedicines. 2021;9(6):594. [55] LI B, LIU J, GU G, et al. Impact of neural stem cell-derived extracellular vesicles on mitochondrial dysfunction, sirtuin 1 level, and synaptic deficits in Alzheimer’s disease. J Neurochem. 2020;154(5): 502-518. [56] DE GODOY MA, SARAIVA LM, DE CARVALHO L, et al. Mesenchymal stem cells and cell-derived extracellular vesicles protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-beta oligomers. J Biol Chem. 2018;293(6):1957-1975. [57] 任巧,张林,刘小慧,等.β淀粉样蛋白1-42寡聚体对人诱导性多能干细胞源性小胶质细胞炎症和氧化应激反应的影响[J].中国药理学与毒理学杂志,2020,34(11): 817-824. [58] CUI Y, MA S, ZHANG C, et al. Human umbilical cord mesenchymal stem cells transplantation improves cognitive function in Alzheimer’s disease mice by decreasing oxidative stress and promoting hippocampal neurogenesis. Behav Brain Res. 2017;320:291-301. [59] HARRELL CR, JANKOVIC MG, FELLABAUM C, et al. Molecular Mechanisms Responsible for Anti-inflammatory and Immunosuppressive Effects of Mesenchymal Stem Cell-Derived Factors. Adv Exp Med Biol. 2019;1084:187-206. [60] ZHU X, BADAWI M, POMEROY S, et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J Extracell Vesicles. 2017;6(1):1324730. [61] ZHU H, WANG Z, YU J, et al. Role and mechanisms of cytokines in the secondary brain injury after intracerebral hemorrhage. Prog Neurobiol. 2019;178:101610. [62] WANG SS, JIA J, WANG Z. Mesenchymal Stem Cell-Derived Extracellular Vesicles Suppresses iNOS Expression and Ameliorates Neural Impairment in Alzheimer’s Disease Mice. J Alzheimers Dis. 2018;61(3):1005-1013. [63] 李震,孙晓,谢永鹏,等.神经元来源细胞外囊泡促进神经干细胞的神经生成[J].中国组织工程研究,2024,28(25):3994-3999. [64] APODACA LA, BADDOUR A, GARCIA CJ, et al. Human neural stem cell-derived extracellular vesicles mitigate hallmarks of Alzheimer’s disease. Alzheimers Res Ther. 2021;13(1):57. [65] GALLART-PALAU X, GUO X, SERRA A, et al. Alzheimer’s disease progression characterized by alterations in the molecular profiles and biogenesis of brain extracellular vesicles. Alzheimers Res Ther. 2020;12(1):54. [66] SU H, RUSTAM YH, MASTERS CL, et al. Characterization of brain-derived extracellular vesicle lipids in Alzheimer’s disease. J Extracell Vesicles. 2021;10(7):e12089. [67] LIU R, LIU J, JI X, et al. Synthetic nucleic acids delivered by exosomes: a potential therapeutic for generelated metabolic brain diseases. Metab Brain Dis. 2013;28(4):551-562. [68] HUBER CC, WANG H. Pathogenic and therapeutic role of exosomes in neurodegenerative disorders. Neural Regen Res. 2024;19(1):75-79. [69] JIANG L, DONG H, CAO H, et al. Exosomes in Pathogenesis, Diagnosis, and Treatment of Alzheimer’s Disease. Med Sci Monit. 2019;25:3329-3335. [70] WANG S, CESCA F, LOERS G, et al. Synapsin I is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia-derived exosomes. J Neurosci. 2011;31(20): 7275-7290. [71] HAO P, LIANG Z, PIAO H, et al. Conditioned medium of human adipose-derived mesenchymal stem cells mediates protection in neurons following glutamate excitotoxicity by regulating energy metabolism and GAP-43 expression. Metab Brain Dis. 2014;29(1):193-205. [72] SALWA, KUMAR L. Engrafted stem cell therapy for Alzheimer’s disease: A promising treatment strategy with clinical outcome. J Control Release. 2021;338: 837-857. [73] KARVELAS N, BENNETT S, POLITIS G, et al. advances in stem cell therapy in Alzheimer’s disease: a comprehensive clinical trial review. Stem Cell Investig. 2022;9:2. [74] DUMA C, KOPYOV O, KOPYOV A, et al. Human intracerebroventricular (ICV) injection of autologous, non-engineered, adipose-derived stromal vascular fraction (ADSVF) for neurodegenerative disorders: results of a 3-year phase 1 study of 113 injections in 31 patients. Mol Biol Rep. 2019;46(5):5257-5272. [75] XIE X, SONG Q, DAI C, et al. Clinical safety and efficacy of allogenic human adipose mesenchymal stromal cells-derived exosomes in patients with mild to moderate Alzheimer’s disease: a phase I/II clinical trial. Gen Psychiatr. 2023;36(5):e101143. [76] KIM HJ, SEO SW, CHANG JW, et al. Stereotactic brain injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: A phase 1 clinical trial. Alzheimers Dement (N Y). 2015;1(2):95-102. [77] ZHANG GL, ZHU ZH, WANG YZ. Neural stem cell transplantation therapy for brain ischemic stroke: Review and perspectives. World J Stem Cells. 2019;11(10):817-830. [78] LADEWIG J, KOCH P, BRUSTLE O. Auto-attraction of neural precursors and their neuronal progeny impairs neuronal migration. Nat Neurosci. 2014;17(1):24-26. [79] DATE I, KAWAMURA K, NAKASHIMA H. Histological signs of immune reactions against allogeneic solid fetal neural grafts in the mouse cerebellum depend on the MHC locus. Exp Brain Res. 1988;73(1):15-22. [80] WANG Q, MATSUMOTO Y, SHINDO T, et al. Neural stem cells transplantation in cortex in a mouse model of Alzheimer’s disease. J Med Invest. 2006;53(1-2):61-69. [81] JUENGST E, FOSSEL M. The ethics of embryonic stem cells--now and forever, cells without end. JAMA. 2000;284(24): 3180-3184. [82] CHAN HJ, YANSHREE, ROY J, et al. Therapeutic Potential of Human Stem Cell Implantation in Alzheimer’s Disease. Int J Mol Sci. 2021;22(18):10151. |
| [1] | Liang Haobo, Wang Zeyu, Ma Wenlong, Liu Hao, Liu Youwen. Hot issues in the field of joint revision: infection, rehabilitation nursing, bone defect, and prosthesis loosening [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1963-1971. |
| [2] | Yuan Weibo, Liu Chan, Yu Limei. Potential application of liver organoids in liver disease models and transplantation therapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1684-1692. |
| [3] | Yu Ting, Lyu Dongmei, Deng Hao, Sun Tao, Cheng Qian. Icariin pretreatment enhances effect of human periodontal stem cells on M1-type macrophages [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1328-1335. |
| [4] | Yang Zhihang, Sun Zuyan, Huang Wenliang, Wan Yu, Chen Shida, Deng Jiang. Nerve growth factor promotes chondrogenic differentiation and inhibits hypertrophic differentiation of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1336-1342. |
| [5] | Hu Taotao, Liu Bing, Chen Cheng, Yin Zongyin, Kan Daohong, Ni Jie, Ye Lingxiao, Zheng Xiangbing, Yan Min, Zou Yong. Human amniotic mesenchymal stem cells overexpressing neuregulin-1 promote skin wound healing in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1343-1349. |
| [6] | Jin Kai, Tang Ting, Li Meile, Xie Yuan. Effects of conditioned medium and exosomes of human umbilical cord mesenchymal stem cells on proliferation, migration, invasion, and apoptosis of hepatocellular carcinoma cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1350-1355. |
| [7] | Li Dijun, Jiu Jingwei, Liu Haifeng, Yan Lei, Li Songyan, Wang Bin. Three-dimensional gelatin microspheres loaded human umbilical cord mesenchymal stem cells for chronic tendinopathy repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1356-1362. |
| [8] | Lou Guo, Zhang Min, Fu Changxi. Exercise preconditioning for eight weeks enhances therapeutic effect of adipose-derived stem cells in rats with myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1363-1370. |
| [9] | Liu Qi, Li Linzhen, Li Yusheng, Jiao Hongzhuo, Yang Cheng, Zhang Juntao. Icariin-containing serum promotes chondrocyte proliferation and chondrogenic differentiation of stem cells in the co-culture system of three kinds of cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1371-1379. |
| [10] | Huang Ting, Zheng Xiaohan, Zhong Yuanji, Wei Yanzhao, Wei Xufang, Cao Xudong, Feng Xiaoli, Zhao Zhenqiang. Effects of macrophage migration inhibitory factor on survival, proliferation, and differentiation of human embryonic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1380-1387. |
| [11] | Aikepaer · Aierken, Chen Xiaotao, Wufanbieke · Baheti. Osteogenesis-induced exosomes derived from human periodontal ligament stem cells promote osteogenic differentiation of human periodontal ligament stem cells in an inflammatory microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1388-1394. |
| [12] | Zhang Zhenyu, Liang Qiujian, Yang Jun, Wei Xiangyu, Jiang Jie, Huang Linke, Tan Zhen. Target of neohesperidin in treatment of osteoporosis and its effect on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1437-1447. |
| [13] | Chang Jinxia, Liu Yufei, Niu Shaohui, Wang Chang, Cao Jianchun. Visualization analysis of macrophage polarization in tissue repair process [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1486-1496. |
| [14] | Li Jialin, Zhang Yaodong, Lou Yanru, Yu Yang, Yang Rui. Molecular mechanisms underlying role of mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1512-1522. |
| [15] | Sun Yuting, Wu Jiayuan, Zhang Jian. Physical factors and action mechanisms affecting osteogenic/odontogenic differentiation of dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1531-1540. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||