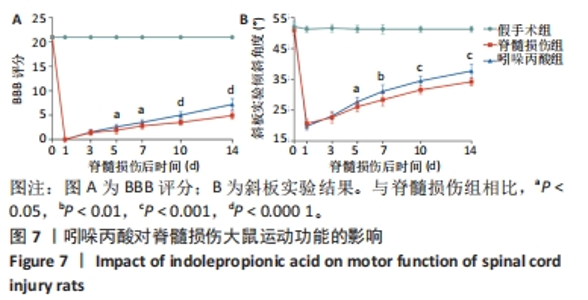
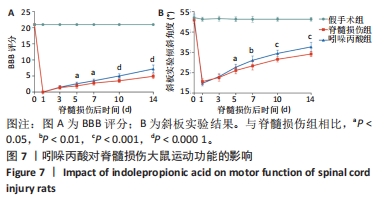
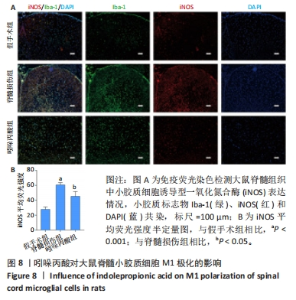
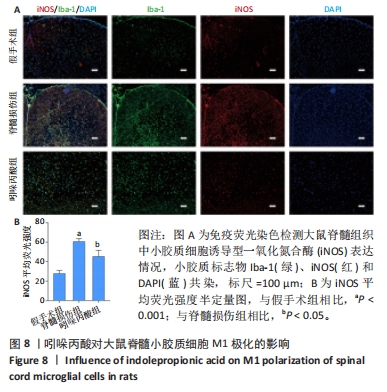
[1] MILICH LM, RYAN CB, LEE JK. The origin, fate, and contribution of macrophages to spinal cord injury pathology. Acta Neuropathol. 2019;137(5):785-797.
[2] AHUJA CS, WILSON JR, NORI S, et al. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3:17018.
[3] PARK J, DECKER JT, MARGUL DJ, et al. Local Immunomodulation with Anti-inflammatory Cytokine-Encoding Lentivirus Enhances Functional Recovery after Spinal Cord Injury. Mol Ther. 2018;26(7):1756-1770.
[4] LAGINHA I, KOPP MA, DRUSCHEL C, et al. Natural Killer (NK) Cell Functionality after human Spinal Cord Injury (SCI): protocol of a prospective, longitudinal study. BMC Neurol. 2016;16(1):170.
[5] NARANG A, QIAO F, ATKINSON C, et al. Natural IgM antibodies that bind neoepitopes exposed as a result of spinal cord injury, drive secondary injury by activating complement. J Neuroinflammation. 2017;14(1):120.
[6] SALTER MW, STEVENS B.Microglia emerge as central players in brain disease. Nat Med. 2017;23(9):1018-1027.
[7] WALSH CM, WYCHOWANIEC JK, BROUGHAM DF, et al. Functional hydrogels as therapeutic tools for spinal cord injury: New perspectives on immunopharmacological interventions. Pharmacol Ther. 2022;234:108043.
[8] LENG F, EDISON P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol. 2021;17(3):157-172.
[9] FANG YP, QIN ZH, ZHANG Y, et al. Implications of microglial heterogeneity in spinal cord injury progression and therapy. Exp Neurol. 2023;359:114239.
[10] PEREZ JC, POULEN G, CARDOSO M, et al. CSF1R inhibition at chronic stage after spinal cord injury modulates microglia proliferation. Glia. 2023. doi: 10.1002/glia.24451.
[11] XU S, WANG J, ZHONG J, et al. CD73 alleviates GSDMD-mediated microglia pyroptosis in spinal cord injury through PI3K/AKT/Foxo1 signaling. Clin Transl Med. 2021;11(1):e269.
[12] ORR MB, GENSEL JC. Spinal Cord Injury Scarring and Inflammation: Therapies Targeting Glial and Inflammatory Responses. Neurotherapeutics. 2018;15(3):541-553.
[13] LI J, ZHANG L, WU T, et al. Indole-3-propionic Acid Improved the Intestinal Barrier by Enhancing Epithelial Barrier and Mucus Barrier. J Agric Food Chem. 2021;69(5):1487-1495.
[14] SERGER E, LUENGO-GUTIERREZ L, CHADWICK JS, et al. The gut metabolite indole-3 propionate promotes nerve regeneration and repair. Nature. 2022;607(7919):585-592.
[15] XUE S, CAO ZX, WANG JN, et al. Receptor-Interacting Protein Kinase 3 Inhibition Relieves Mechanical Allodynia and Suppresses NLRP3 Inflammasome and NF-κB in a Rat Model of Spinal Cord Injury. Front Mol Neurosci. 2022;15:861312.
[16] FENG Y, PENG Y, JIE J, et al.The immune microenvironment and tissue engineering strategies for spinal cord regeneration. Front Cell Neurosci. 2022;16:969002.
[17] FREYERMUTH-TRUJILLO X, SEGURA-URIBE JJ, SALGADO-CEBALLOS H, et al. Inflammation: A Target for Treatment in Spinal Cord Injury. Cells. 2022;11(17):2692.
[18] SONG Y, XUE H, LIU TT, et al. Rapamycin plays a neuroprotective effect after spinal cord injury via anti-inflammatory effects. J Biochem Mol Toxicol. 2015;29(1):29-34.
[19] JIA Z, ZHU H, LI J, et al. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord. 2012;50(4):264-274.
[20] ROTHHAMMER V, MASCANFRONI ID, BUNSE L, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22(6):586-597.
[21] JING Y, YU Y, BAI F, et al. Effect of fecal microbiota transplantation on neurological restoration in a spinal cord injury mouse model: involvement of brain-gut axis. Microbiome. 2021;9(1):59.
[22] PASCOAL TA, BENEDET AL, ASHTON NJ, et al. Microglial activation and tau propagate jointly across Braak stages. Nat Med. 2021;27(9):1592-1599.
[23] LI C, WU Z, ZHOU L, et al. Temporal and spatial cellular and molecular pathological alterations with single-cell resolution in the adult spinal cord after injury. Signal Transduct Target Ther. 2022;7(1):65.
[24] GE X, TANG P, RONG Y, et al. Exosomal miR-155 from M1-polarized macrophages promotes EndoMT and impairs mitochondrial function via activating NF-κB signaling pathway in vascular endothelial cells after traumatic spinal cord injury. Redox Biol. 2021;41:101932.
[25] 赵书杰,陈建,凡进,等.创伤性脊髓损伤后脊髓微环境失衡的研究进展[J].中国脊柱脊髓杂志,2020,30(10):942-947.
[26] FU SP, CHEN SY, PANG QM, et al. Advances in the research of the role of macrophage/microglia polarization-mediated inflammatory response in spinal cord injury. Front Immunol. 2022;13:1014013.
[27] WANG J, JIANG P, DENG W, et al. Grafted human ESC-derived astroglia repair spinal cord injury via activation of host anti-inflammatory microglia in the lesion area. Theranostics. 2022;12(9):4288-4309.
[28] ZHAO H, WU L, YAN G, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021;6(1):263.
[29] CHRISTIAN F, SMITH EL, CARMODY RJ. The Regulation of NF-κB Subunits by Phosphorylation. Cells. 2016;5(1):12.
[30] YU SS, LI ZY, XU XZ, et al. M1-type microglia can induce astrocytes to deposit chondroitin sulfate proteoglycan after spinal cord injury. Neural Regen Res. 2022;17(5):1072-1079.
[31] IP WKE, HOSHI N, SHOUVAL DS, et al. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science. 2017;356(6337):513-519.
[32] HU C, XUAN Y, ZHANG X, et al. Immune cell metabolism and metabolic reprogramming. Mol Biol Rep. 2022;49(10):9783-9795.
[33] TANNAHILL GM, CURTIS AM, ADAMIK J, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496(7444):238-242.
[34] BAIK SH, KANG S, LEE W, et al. A Breakdown in Metabolic Reprogramming Causes Microglia Dysfunction in Alzheimer’s Disease. Cell Metab. 2019;30(3):493-507.e6.
[35] FAIRLEY LH, WONG JH, BARRON AM. Mitochondrial Regulation of Microglial Immunometabolism in Alzheimer’s Disease. Front Immunol. 2021;12:624538.
[36] HERST PM, GRASSO C, BERRIDGE MV. Metabolic reprogramming of mitochondrial respiration in metastatic cancer. Cancer Metastasis Rev. 2018;37(4):643-653.
[37] GAUDET AD, FONKEN LK. Glial Cells Shape Pathology and Repair After Spinal Cord Injury. Neurotherapeutics. 2018;15(3):554-577.
|