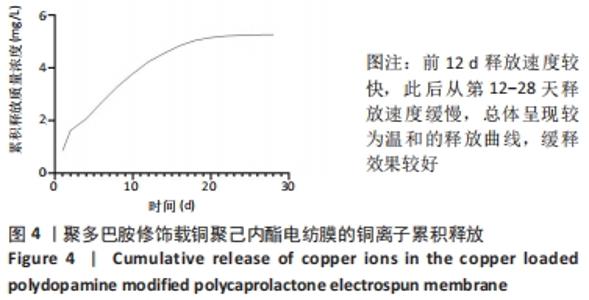
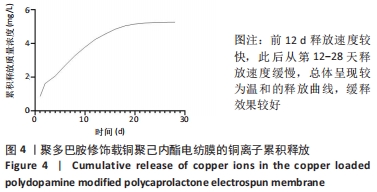
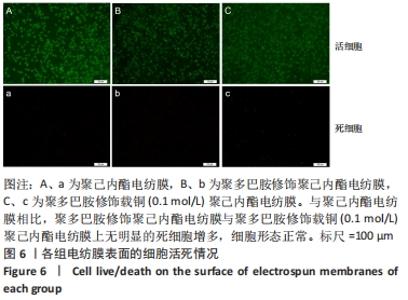
Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (27): 4272-4278.doi: 10.12307/2022.855
Previous Articles Next Articles
Preparation of copper loaded coating on polydopamine-modified polycaprolactone electrospun membrane and antibacterial and cellular properties evaluation
Dong Xiling, Hui Min, Cao Fei, Lin Peng, Zhou Han, Wang Le, Zhang Xiaoming, Liu Tongbin
- Department of Prosthodontics, Affiliated Hospital of Binzhou Medical College, Binzhou 256600, Shandong Province, China
-
Received:2021-02-10Accepted:2021-04-15Online:2022-09-28Published:2022-03-11 -
Contact:Zhang Xiaoming, Master, Chief physician, Master’s supervisor, Department of Prosthodontics, Affiliated Hospital of Binzhou Medical College, Binzhou 256600, Shandong Province, China Liu Tongbin, Master, Department of Prosthodontics, Affiliated Hospital of Binzhou Medical College, Binzhou 256600, Shandong Province, China -
About author:Dong Xiling, Master candidate, Department of Prosthodontics, Affiliated Hospital of Binzhou Medical College, Binzhou 256600, Shandong Province, China -
Supported by:Shandong Medical and Health Science and Technology Development Plan Project, No. 2016WS0121 (to LP, LTB); Binzhou Medical College Science and Technology Plan Project, No. BY2017KJ05 (to LTB)
CLC Number:
Cite this article
Dong Xiling, Hui Min, Cao Fei, Lin Peng, Zhou Han, Wang Le, Zhang Xiaoming, Liu Tongbin. Preparation of copper loaded coating on polydopamine-modified polycaprolactone electrospun membrane and antibacterial and cellular properties evaluation[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(27): 4272-4278.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
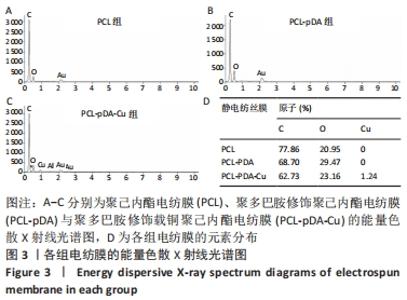
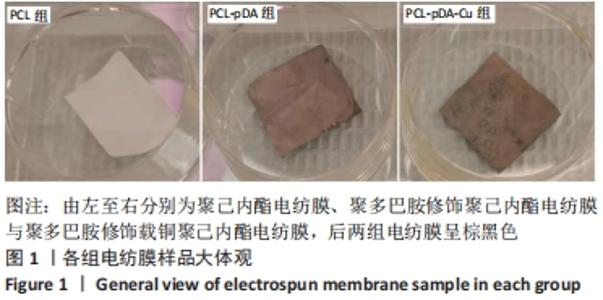
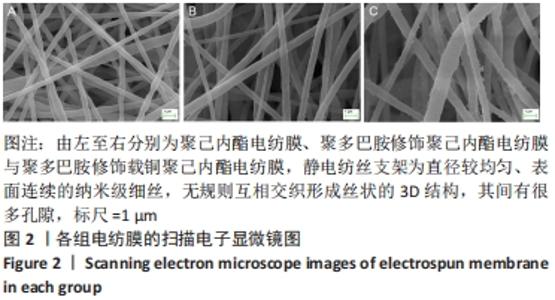
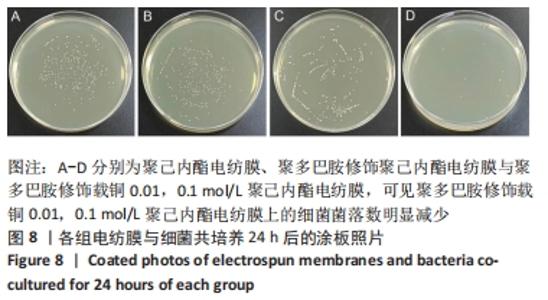
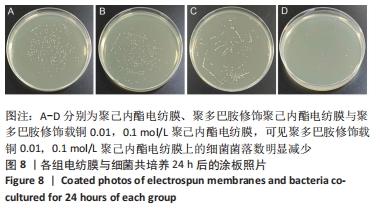
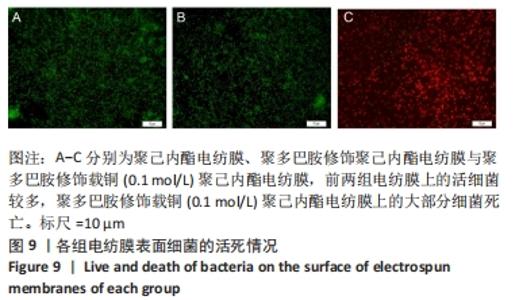
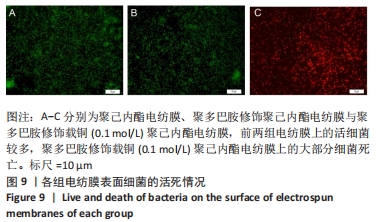
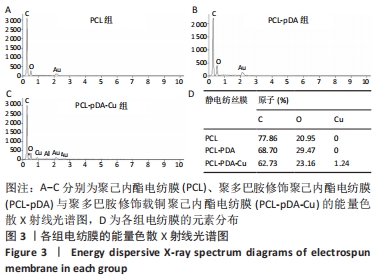
聚己内酯电纺膜、聚多巴胺修饰聚己内酯电纺膜与聚多巴胺修饰载铜聚己内酯电纺膜的平均纳米纤维直径分别为(497±156) ,(559±106),(628±166) nm。聚多巴胺修饰聚己内酯电纺膜、聚多巴胺修饰载铜聚己内酯电纺膜纤维变粗,表面变粗糙,可见颗粒状沉积;同时很大程度上保持了原始的3D支架框架;聚多巴胺修饰载铜聚己内酯电纺膜有更多的颗粒聚集,可能由于铜离子的共沉积。 能量色散X射线光谱结果,如图3所示,聚己内酯电纺膜、聚多巴胺修饰聚己内酯电纺膜表面的元素主要为C和O;聚多巴胺修饰载铜聚己内酯电纺膜表面的元素中出现Cu,铜元素的占比为1.24%,提示铜元素可成功掺入静电纺丝膜表面。聚多巴胺修饰聚己内酯电纺膜、聚多巴胺修饰载铜聚己内酯电纺丝膜经聚多巴胺修饰后C原子占比逐渐降低,可能因为聚己内酯电纺丝膜表面被聚多巴胺和铜离子覆盖。"
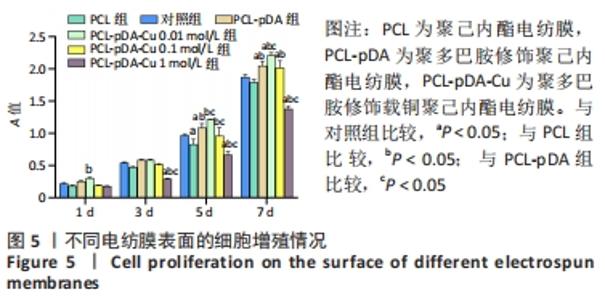
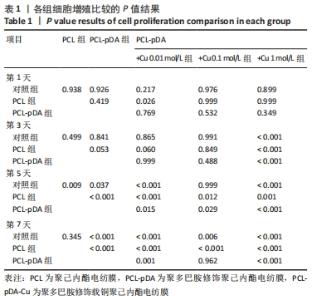
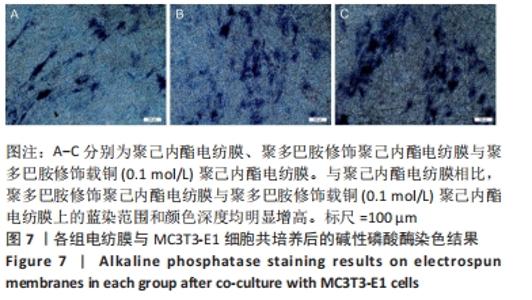
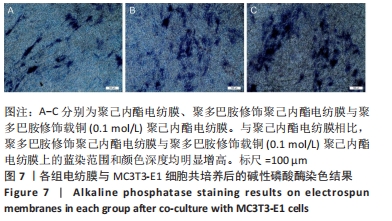
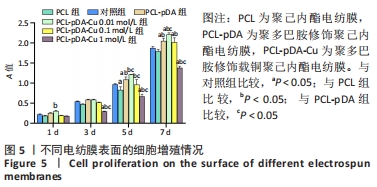
2.3 各组电纺膜的细胞生物学性能评价 2.3.1 细胞增殖 CCK-8分析结果,如图5所示。与空白对照组和聚己内酯电纺膜相比,聚多巴胺修饰聚己内酯电纺膜、聚多巴胺修饰载铜(0.01 mol/L)聚己内酯电纺膜上培养第5,7天的细胞增殖速率更高(P < 0.05),这可能是由于聚多巴胺涂层改性后静电纺丝膜的亲水性和生物相容性提高,增强细胞黏附增殖和Cu2+缓释浓度低所致;与聚多巴胺修饰聚己内酯电纺膜相比,聚多巴胺修饰载铜(0.01 mol/L)聚己内酯电纺膜上培养第5,7天的细胞增殖能力提高(P < 0.05),聚多巴胺修饰载铜(0.1 mol/L)聚己内酯电纺膜上第1,3,7天的细胞增殖速率无明显变化(P > 0.05);与对照组、聚己内酯电纺膜、聚多巴胺修饰聚己内酯电纺膜相比,聚多巴胺修饰载铜(1 mol/L)聚己内酯电纺膜上培养第3,5,7天的细胞增殖速率降低(P < 0.05),证明此铜离子浓度过高产生毒性作用,抑制了细胞增殖活力。"
| [1] BOTTINO MC, THOMAS V, SCHMIDT G, et al. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent Mater. 2012;28(7):703-721. [2] 宋菊青.静电纺丝聚己内酯三维复合纳米纤维及其细胞生物学性能研究[D].广州:华南理工大学,2016. [3] XUE J, WU T, DAI Y, et al. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem Rev. 2019;119(8):5298-5415. [4] DAELEMANS L, STEYAERT I, SCHOOLAERT E, et al. Nanostructured Hydrogels by Blend Electrospinning of Polycaprolactone/Gelatin Nanofibers. Nanomaterials. 2018;8(7):551. [5] 李珍妮,邓字巍.仿贻贝黏附性多巴胺的研究与应用进展[J].高分子材料科学与工程,2015,31(1):185-190. [6] CHEN X, WANG X, WANG S, et al. Mussel-inspired polydopamine-assisted bromelain immobilization onto electrospun fibrous membrane for potential application as wound dressing. Mat Sci Eng C-mater. 2020;110:110624. [7] JIN A, WANG Y, LIN K, et al. Nanoparticles modified by polydopamine: Working as “drug” carriers. Bioact Mater. 2020;5(3):522-541. [8] AGNIHOTRI R, GAUR S, ALBIN S. Nanometals in Dentistry: Applications and Toxicological Implications-a Systematic Review. Biol Trace Elem Res. 2020;197(1):70-88. [9] FAN Y, PHAM MT, HUANG C. Development of Antimicrobial and Antifouling Universal Coating via Rapid Deposition of Polydopamine and Zwitterionization. Langmuir. 2018;35(5):1642-1651. [10] CHANG T, ZHOU X, LIANG J. Synthesis and characterization of Ag-Cu alloy nanoparticles for antimicrobial applications: A polydopamine chemistry application. Mat Sci Eng C-mater. 2019;98:675-684. [11] QIAN Y, ZHOU X, ZHANG F, et al. Triple PLGA/PCL scaffold modification including silver-impregnation, collagen-coating, and electrospinning significantly improve biocompatibility, antimicrobial, and osteogenic properties for oro-facial tissue regeneration. ACS Appl Mater Interfaces. 2019;11(41):37381-37396. [12] KAWEETEERAWAT C, CHANG CH, ROY KR, et al. Cu Nanoparticles Have Different Impacts in Escherichia coli and Lactobacillus brevis than Their Microsized and Ionic Analogues. ACS Nano. 2015;9(7):7215-7225. [13] NING C, WANG X, LI L, et al. Concentration Ranges of Antibacterial Cations for Showing the Highest Antibacterial Efficacy but the Least Cytotoxicity against Mammalian Cells: Implications for a New Antibacterial Mechanism. Chem Res Toxicol. 2015;28(9):1815-1822. [14] LI K, XIA C, QIAO Y, et al. Dose-response relationships between copper and its biocompatibility/antibacterial activities. J Trace Elem Med Biol. 2019;55:127-135. [15] 王乐,惠敏,董西玲,等.缓释阿托伐他汀钙纳米纤维支架对细胞黏附增殖的影响[J].中国组织工程研究,2020,24(28):4492-4497. [16] CHENG G, YIN C, TU H, et al. Controlled Co-delivery of Growth Factors through Layer-by-Layer Assembly of Core–Shell Nanofibers for Improving Bone Regeneration. ACS Nano. 2019;13(6):6372-6382. [17] CHEN X, WANG X, WANG S, et al. Mussel-inspired polydopamine-assisted bromelain immobilization onto electrospun fibrous membrane for potential application as wound dressing. Mat Sci Eng C-mater. 2020;110:110624. [18] 陈媛,刘延辉,张佩华.胆管支架静电纺覆膜工艺及性能[J].东华大学学报(自然科学版),2016,42(6):800-808. [19] FENG B, TU H, YUAN H, et al. Acetic-Acid-Mediated Miscibility toward Electrospinning Homogeneous Composite Nanofibers of GT/PCL. Biomacromolecules. 2012;13(12):3917-3925. [20] GRITSCH L, LOVELL C, GOLDMANN WH, et al. Fabrication and characterization of copper(II)-chitosan complexes as antibiotic-free antibacterial biomaterial. Carbohydr Polym. 2018;179:370-378. [21] KASRAEI S, SAMI L, HENDI S, et al. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus. Restor Dent Endod. 2014; 39(2):109-114. [22] RAMAZANZADEH B, JAHANBIN A, YAGHOUBI M, et al. Comparison of Antibacterial Effects of ZnO and CuO Nanoparticles Coated Brackets against Streptococcus Mutans. J Dent (Shiraz). 2015;16(3):200-205. [23] ZHAO L, WANG H, HUO K, et al. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials. 2011; 32(24):5706-5716. [24] HEO DN, KO WK, LEE HR, et al. Titanium dental implants surface-immobilized with gold nanoparticles as osteoinductive agents for rapid osseointegration. J Colloid Interface Sci. 2016;469:129-137. [25] KANIKIREDDY V, VARAPRASAD K, JAYARAMUDU T, et al. Carboxymethyl cellulose-based materials for infection control and wound healing: A review. Int J Biol Macromol. 2020;164:963-975. [26] LIN CC, FU SJ. Osteogenesis of human adipose-derived stem cells on poly(dopamine)-coated electrospun poly(lactic acid) fiber mats. Mater Sci Eng C Mater Biol Appl. 2016;58:254-263. [27] HARDES J, AHRENS H, GEBERT C, et al. Lack of toxicological side-effects in silver-coated megaprostheses in humans. Biomaterials. 2007;28(18): 2869-2875. [28] GIOVANNI M, YUE J, ZHANG L, et al. Pro-inflammatory responses of RAW264.7 macrophages when treated with ultralow concentrations of silver, titanium dioxide, and zinc oxide nanoparticles. J Hazard Mater. 2015;297:146-152. [29] JIN A, WANG Y, LIN K, et al. Nanoparticles modified by polydopamine: Working as “drug” carriers. Bioact Mater. 2020;5(3):522-541. [30] BURGHARDT I, LUTHEN F, PRINZ C, et al. A dual function of copper in designing regenerative implants. Biomaterials. 2015;44:36-44. [31] GAO X, SONG J, JI P, et al. Polydopamine-Templated Hydroxyapatite Reinforced Polycaprolactone Composite Nanofibers with Enhanced Cytocompatibility and Osteogenesis for Bone Tissue Engineering. ACS Appl Mater Interfaces. 2016;8(5):3499-3515. [32] 李伯超.生物医用聚多巴胺功能涂层及纳米微粒的研究[D].杭州:浙江大学,2018. [33] KUSUMBE AP, RAMASAMY SK, ADAMS RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014; 507(7492):323-328. [34] JIMÉNEZ-HOLGUÍN J, SÁNCHEZ-SALCEDO S, VALLET-REGÍ M, et al. Development and evaluation of copper-containing mesoporous bioactive glasses for bone defects therapy. Microporous Mesoporous Mater. 2020;308:110454. [35] LIN R, DENG C, LI X, et al. Copper-incorporated bioactive glass-ceramics inducing anti-inflammatory phenotype and regeneration of cartilage/bone interface. Theranostics. 2019;9(21):6300-6313. [36] MILAN PB, KHAMSEH S, ZARRINTAJ P, et al. Copper-enriched diamond-like carbon coatings promote regeneration at the bone-implant interface. Heliyon. 2020;6(4):e3798. [37] LIN Z, CAO Y, ZOU J, et al. Improved osteogenesis and angiogenesis of a novel copper ions doped calcium phosphate cement. Mater Sci Eng C Mater Biol Appl. 2020;114:111032. |
| [1] | Wang Hailong, Li Long, Maihemuti·Yakufu, Chen Hongtao, Liu Xu, Yilihamu·Tuoheti. Finite element analysis of stress distribution of acetabular prosthesis in the Lewinnek safety zone [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 843-847. |
| [2] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [3] | Tan Guozhong, Tu Xinran, Guo Liyang, Zhong Jialin, Zhang Yang, Jiang Qianzhou. Biosafety evaluation of three-dimensional printed gelatin/sodium alginate/58S bioactive glass scaffolds for bone defect repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 521-527. |
| [4] | Hu Qiuyu, Yang Long, Yang Yong, Song Shenchao. Aligned poly(butylene adipate-co-terephthalate)/type I collagen fibers promote tendon-bone healing after anterior cruciate ligament rupture [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(27): 4314-4319. |
| [5] | Wang Yuying, Yu Limei. Key problems of preparation and quality control of human amniotic mesenchymal stem cells pharmaceutics [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(25): 4016-4021. |
| [6] | Xu Xinzhi, Zhang Yue, Jin Ying, Jin Chunxiang. Preparation and imaging experiment of a new type of safe near-infrared luminescent nanoparticles [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3450-3454. |
| [7] | Liu Ming, Wang Kai. Theaflavin-3-gallate modified nano-hydroxyapatite/polycaprolactone composite porous scaffold in bone defect repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3480-3486. |
| [8] | Liu Liang, Hu Gaoquan, Wei Zhao, Chen Lin, Hong Feng. Potential of bacterial nanocellulose/polydopamine composite tubes as small-diameter artificial blood vessel [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3535-3542. |
| [9] | Guo Yangyan, Yu Zhengwen, Zhang Jian. Research hotspots of magnesium alloy biomaterials in an in vivo animal [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3556-3565. |
| [10] | Lu Haiping, Lang Xuemei, Zhang Cheng, Ju Songli, Zhang Yi, Wang Xin. Application of polycaprolactone modified biological barrier membrane in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3580-3585. |
| [11] | Feng Le, Qiu Peng, Liu Min, Zhou Hui. Characterization of chitosan-modified polyetheretherketone and its effect on MC3T3-E1 cell adhesion and proliferation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(21): 3351-3356. |
| [12] | Shan Zhengming, Tao Shuchun, Hu Chunmei, Zhang Zhiyuan, Ding Yinan, He Mengcheng, Tang Qiusha. Extraction, identification and proteomic analysis of exosomes derived from human umbilical cord mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(19): 3036-3042. |
| [13] | Liu Jiali, Suo Hairui, Yang Han, Wang Ling, Xu Mingen. Influence of lay-down angles on mechanical properties of three-dimensional printed polycaprolactone scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(16): 2492-2497. |
| [14] | Dong Zhiheng, Gao Yan, Liu Zhen, Han Xiaoqian. Construction of icariin/polycaprolactone nanofiber membrane and its osteogenic ability in vitro [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1510-1514. |
| [15] | Liang Pengchen, Shi Junfeng, Sun Miaomiao, Liang Dongyu, Sha Shuang, Yi Qingqing, Chang Qing. Polydopamine assisted salidroside to improve bone formation on micro-arc oxidation of pure titanium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1522-1529. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||