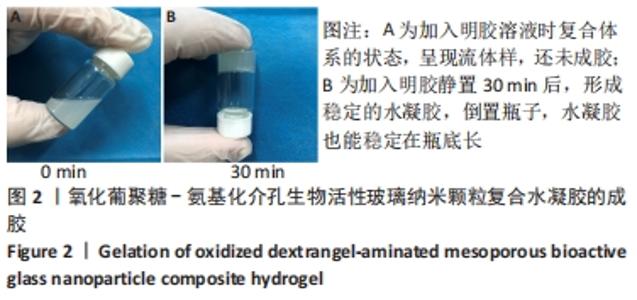
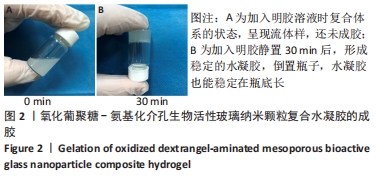
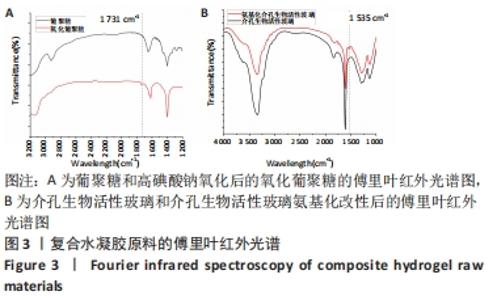
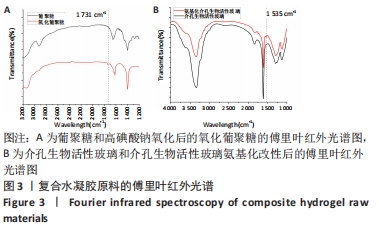
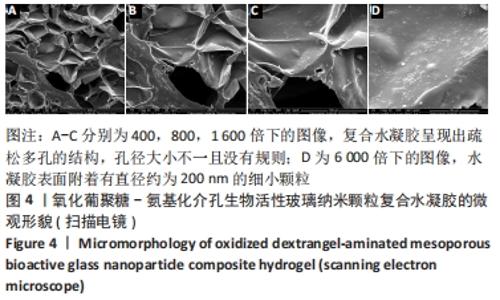
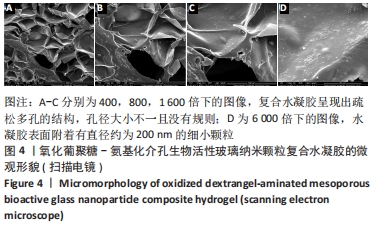
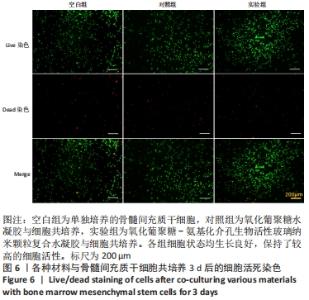
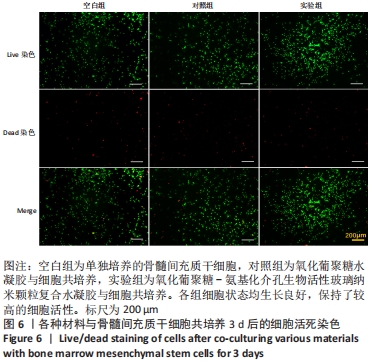
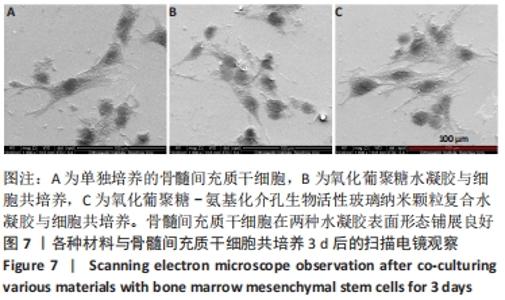
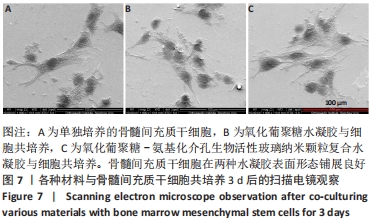
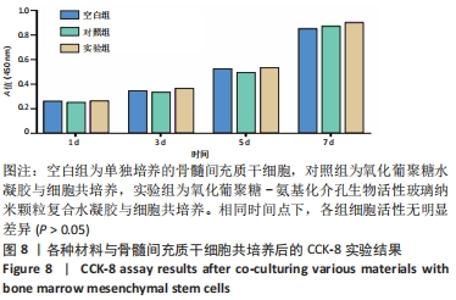
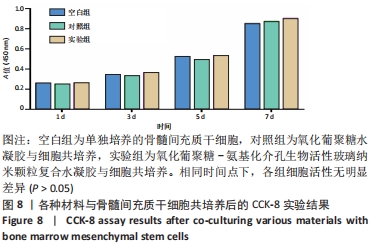
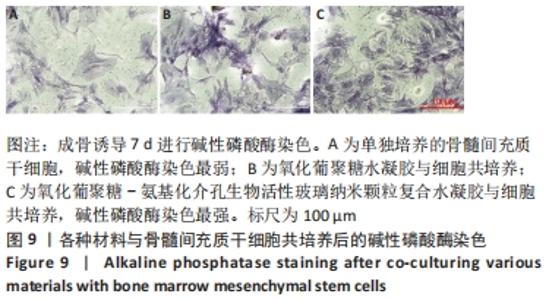
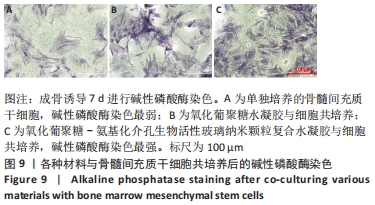
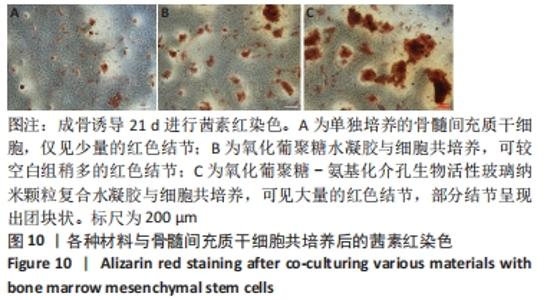
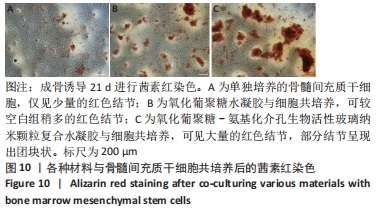
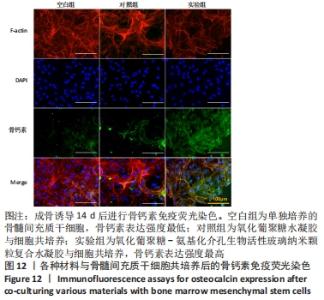
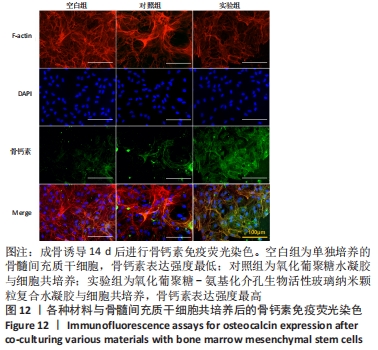
Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (22): 3521-3527.doi: 10.12307/2022.280
Previous Articles Next Articles
Preparation of modified dextran composite hydrogel with osteogenetic effect and in vitro experiment
Tang Zhenzhou, Gu Yong, Chen Liang
- Department of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215008, Jiangsu Province, China
-
Received:2020-12-07Revised:2021-01-23Accepted:2021-06-27Online:2022-08-08Published:2022-01-11 -
Contact:Chen Liang, Chief physician, Professor, Doctoral supervisor, Department of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215008, Jiangsu Province, China -
About author:Tang Zhenzhou, Master candidate, Department of Orthopedics, First Affiliated Hospital of Soochow University, Suzhou 215008, Jiangsu Province, China -
Supported by:the National Natural Science Foundation of China, No. 81972078, 81772312 (to CL); the National Youth Science Fund, No. 81601891 (to GY); the Special Diagnosis and Treatment Technology for Key Clinical Diseases in Suzhou, No. LCZX201701 (to CL)
CLC Number:
Cite this article
Tang Zhenzhou, Gu Yong, Chen Liang. Preparation of modified dextran composite hydrogel with osteogenetic effect and in vitro experiment[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3521-3527.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
| [1] WANG W, YEUNG KWK. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact Mater. 2017;2(4):224-247. [2] TURNBULL G, CLARKE J, PICARD F, et al. 3D bioactive composite scaffolds for bone tissue engineering. Bioact Mater. 2018;3(3):278-314. [3] 于明琨.自体骨移植修补颅骨缺损的材料与方法 [J].中国临床康复, 2006,10(41):126-129. [4] 张贝利,张伟滨,邓廉夫.同种异体骨移植研究进展[J].国际骨科学杂志,2005,26(3):164-167. [5] BAO X, ZHU L, HUANG X, et al. 3D biomimetic artificial bone scaffolds with dual-cytokines spatiotemporal delivery for large weight-bearing bone defect repair. Sci Rep. 2017;7(1):1-13. [6] RAPOSO-AMARAL CE, BUENO DF, ALMEIDA AB, et al. Is bone transplantation the gold standard for repair of alveolar bone defects? J Tissue Eng. 2014;5:2041731413519352. [7] SHEGARFI H, REIKERAS O. Review article: bone transplantation and immune response. J Orthop Surg (Hong Kong). 2009;17(2):206-211. [8] TOOSI S, BEHRAVAN N, BEHRAVAN J. Nonunion fractures, mesenchymal stem cells and bone tissue engineering. J Biomed Mater Res A. 2018; 106(9):2552-2562. [9] QIAO C, MA X, ZHANG J, et al. Molecular interactions in gelatin/chitosan composite films. Food Chem. 2017;235:45-50. [10] 林艳房,霍丹.葡聚糖基水凝胶的制备及其应用的研究进展[J].林产化学与工业,2015,35(6):148-52. [11] 李丹丹,莫秀梅.基于席夫碱反应的氧化葡聚糖/胺化羧甲基壳聚糖双组分水凝胶粘合剂[J].中国组织工程研究,2018,22(22):3527-3532. [12] KUNDU B, RAJKHOWA R, KUNDU SC, et al. Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliv Rev. 2013;65(4):457-470. [13] 刘冬,秦虎,汪永新,等. 3D打印羟基磷灰石/聚乳酸网状复合物修复颅骨缺损[J].中国组织工程研究,2019,23(6):833-837. [14] XIN T, MAO J, LIU L, et al. Programmed Sustained Release of Recombinant Human Bone Morphogenetic Protein-2 and Inorganic Ion Composite Hydrogel as Artificial Periosteum. ACS Appl Mater Interfaces. 2020;12(6):6840-6851. [15] HUANG K, WU J, GU Z. Black Phosphorus Hydrogel Scaffolds Enhance Bone Regeneration via a Sustained Supply of Calcium-Free Phosphorus. ACS Appl Mater Interfaces. 2019;11(3):2908-2916. [16] XU T, YANG H, YANG D, et al. Polylactic Acid Nanofiber Scaffold Decorated with Chitosan Islandlike Topography for Bone Tissue Engineering. ACS Appl Mater Interfaces. 2017;9(25):21094-21104. [17] SHARIFI F, ATYABI SM, NOROUZIAN D, et al.Polycaprolactone/carboxymethyl chitosan nanofibrous scaffolds for bone tissue engineering application. Int J Biol Macromol. 2018;115:243-248. [18] JAIPAN P, NGUYEN A, NARAYAN RJ. Gelatin-based hydrogels for biomedical applications. MRS Commun. 2017;7(3):416-426. [19] EICHELE DD, KHARBANDA KK. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J Gastroenterol. 2017;23(33):6016. [20] NONSUWAN P, MATSUGAMI A, HAYASHI F, et al. Controlling the degradation of an oxidized dextran-based hydrogel independent of the mechanical properties. Carbohydr Polym. 2019;204:131-141. [21] HENCH LL. The story of Bioglass. J Mater Sci Mater Med. 2006;17(11): 967-978. [22] VALLET‐REGÍ M, RUIZ‐HERNÁNDEZ E. Bioceramics: from bone regeneration to cancer nanomedicine. Adv Mater. 2011;23(44):5177-5218. [23] ZHENG K, BALASUBRAMANIAN P, PATERSON TE, et al. Ag modified mesoporous bioactive glass nanoparticles for enhanced antibacterial activity in 3D infected skin model. Mater Sci Eng C Mater Biol Appl. 2019;103:109764. [24] XIN T, GU Y, CHENG R, et al. Inorganic Strengthened Hydrogel Membrane as Regenerative Periosteum. ACS Appl Mater Interfaces. 2017;9(47):41168-41180. [25] LENG Y, YANG F, WANG Q, et al. Material-based therapy for bone nonunion. Mater Des.2019;183:108161. [26] WINKLER T, SASS FA, DUDA GN, et al. A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering: The unsolved challenge. Bone Joint Res. 2018; 7(3):232-243. [27] LIU L, LI X, SHI X, et al. Injectable alendronate-functionalized GelMA hydrogels for mineralization and osteogenesis. RSC advances. 2018; 8(40):22764-22776. [28] PAN J, YUAN H, GUO C, et al. One-step cross-linked injectable hydrogels with tunable properties for space-filling scaffolds in tissue engineering. RSC Adv. 2015;5(51):40820-40830. [29] CHEN QZ, THOMPSON ID, BOCCACCINI AR. 45S5 Bioglass®-derived glass–ceramic scaffolds for bone tissue engineering. Biomaterials. 2006;27(11):2414-2425. [30] FRAYSSINET P, TROUILLET JL, ROUQUET N, et al. Osseointegration of macroporous calcium phosphate ceramics having a different chemical composition. Biomaterials. 1993;14(6):423-429. [31] VUKAJLOVIC D, PARKER J, BRETCANU O, et al. Chitosan based polymer/bioglass composites for tissue engineering applications. Mater Sci Eng C Mater Biol Appl. 2019;96:955-967. [32] NARUPHONTJIRAKUL P, PORTER AE, JONES JR. In vitro osteogenesis by intracellular uptake of strontium containing bioactive glass nanoparticles. Acta Biomater. 2018;66:67-80. [33] HAO Z, SONG Z, HUANG J, et al. The scaffold microenvironment for stem cell based bone tissue engineering. Biomater Sci. 2017;5(8): 1382-1392. [34] WEISS S, BAUMGART R, JOCHUM M, et al. Systemic regulation of distraction osteogenesis: a cascade of biochemical factors. J Bone Miner Res. 2002;17(7):1280-1289. [35] LI Y, XIAO Y, LIU C. The Horizon of Materiobiology: A Perspective on Material-Guided Cell Behaviors and Tissue Engineering. Chem Rev. 2017;117(5):4376-4421. [36] ZHU D, SU Y, YOUNG ML, et al. Biological Responses and Mechanisms of Human Bone Marrow Mesenchymal Stem Cells to Zn and Mg Biomaterials. ACS Appl Mater Interfaces. 2017;9(33):27453-27461. [37] WU T, CHENG N, XU C, et al. The effect of mesoporous bioglass on osteogenesis and adipogenesis of osteoporotic BMSCs. J Biomed Mater Res A. 2016;104(12):3004-3014. [38] KAWAGUCHI J, MEE PJ, SMITH AG. Osteogenic and chondrogenic differentiation of embryonic stem cells in response to specific growth factors. Bone. 2005;36(5):758-769. [39] AUBIN JE, LIU F, MALAVAL L, et al. Osteoblast and chondroblast differentiation. Bone. 1995;17(2):S77-S83. [40] ICER MA, GEZMEN-KARADAG M. The multiple functions and mechanisms of osteopontin. Clin Biochem. 2018;59:17-24. |
| [1] | Xue Yadong, Zhou Xinshe, Pei Lijia, Meng Fanyu, Li Jian, Wang Jinzi . Reconstruction of Paprosky III type acetabular defect by autogenous iliac bone block combined with titanium plate: providing a strong initial fixation for the prosthesis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1424-1428. |
| [2] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [3] | Wu Weiyue, Guo Xiaodong, Bao Chongyun. Application of engineered exosomes in bone repair and regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1102-1106. |
| [4] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [5] | Huang Chuanjun, Zou Yu, Zhou Xiaoting, Zhu Yangqing, Qian Wei, Zhang Wei, Liu Xing. Transplantation of umbilical cord mesenchymal stem cells encapsulated in RADA16-BDNF hydrogel promotes neurological recovery in an intracerebral hemorrhage rat model [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 510-515. |
| [6] | Tan Guozhong, Tu Xinran, Guo Liyang, Zhong Jialin, Zhang Yang, Jiang Qianzhou. Biosafety evaluation of three-dimensional printed gelatin/sodium alginate/58S bioactive glass scaffolds for bone defect repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 521-527. |
| [7] | Le Guoping, Zhang Ming, Xi Licheng, Luo Hanwen. Preparation and in vitro evaluation of vancomycin hydrochloride@polylactic acid-glycolic acid copolymer-chitosan-hyaluronic acid composite sustained-release microspheres [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 528-534. |
| [8] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [9] | Han Zhi, Wang Zhimiao, Gaxi Sijia, Lu Qingling, Guo Tao. Tissue engineered cartilage constructed by polyurethane composite chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3455-3459. |
| [10] | Liu Ming, Wang Kai. Theaflavin-3-gallate modified nano-hydroxyapatite/polycaprolactone composite porous scaffold in bone defect repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3480-3486. |
| [11] | Guo Xiaopeng, Liu Yingsong, Shang Hui. Silk fibroin/nano hydroxyapatite composite combined with icariin can promote the proliferation and differentiation of bone marrow mesenchymal stem cells into nucleus pulposus like cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3528-3534. |
| [12] | Wu Shuhan, Zhu Xulong, Bai Lu, Zhang Feifei, Lin Guangshuai, Li Jiang, Du Jia, Li Jianhui. Hydrogel-based adipose tissue engineering strategies for vascularization [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3573-3579. |
| [13] | Lu Haiping, Lang Xuemei, Zhang Cheng, Ju Songli, Zhang Yi, Wang Xin. Application of polycaprolactone modified biological barrier membrane in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3580-3585. |
| [14] | Li Duchenhui, Tian Ai, Tang Zhenglong. Angiogenesis induced by bone bioscaffold materials [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3602-3608. |
| [15] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(16): 2480-2486. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||