Chinese Journal of Tissue Engineering Research ›› 2021, Vol. 25 ›› Issue (4): 638-643.doi: 10.3969/j.issn.2095-4344.2379
Previous Articles Next Articles
Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release
Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia
- School of Life Sciences, Zhengzhou University, Zhengzhou 450001, Henan Province, China
-
Received:2020-04-27Revised:2020-04-28Accepted:2020-05-22Online:2021-02-08Published:2020-11-25 -
Contact:Ma Shanshan, Associate professor, School of Life Sciences, Zhengzhou University, Zhengzhou 450001, Henan Province, China Guan Fangxia, Professor, School of Life Sciences, Zhengzhou University, Zhengzhou 450001, Henan Province, China -
About author:Zhang Zhenkun, Master candidate, School of Life Sciences, Zhengzhou University, Zhengzhou 450001, Henan Province, China -
Supported by:the Key Research and Development and Promotion Projects in Henan Province, No. 202102310211; the Central Plains Thousand People Plan of Henan Province, No. 204200510013
CLC Number:
Cite this article
Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
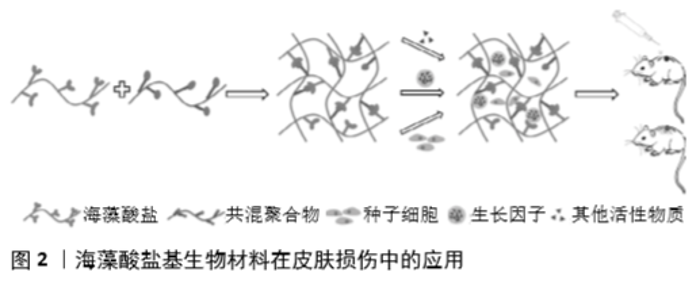
2.1 海藻酸盐生物高聚物 海藻酸盐是由(1→4)-β-交联的D-甘露糖醛酸和(1→4)-α-交联的古洛糖醛酸组成的线性长链阴离子聚合物,是一种亲水生物聚合物,具有无毒、生物相容性好、可降解、生物稳定性好的特点。据报道,水溶性海藻酸盐(海藻酸钠)具有显著的流变性能,是一种很好的胶凝材料,具有良好的锁水性。 另外,海藻酸盐可以通过静电、离子相互作用、共价结合、氧化还原反应和配位等方式与多种金属离子结合,例如:钙离子可与古鲁酸基团上的多糖链发生交联反应形成固体凝胶[7]。目前,这种交联藻酸盐的结合方式已被广泛应用于创面愈合中[8-9]。虽然单一的海藻酸盐水凝胶可用于制备具有环境湿润、止血等功能的创面敷料,但其力学性能较差,没有抗菌性,也没有细胞活性位点,进而限制了藻酸盐水凝胶的应用[10]。不过,海藻酸盐的官能团能够与其他生物聚合物混合形成交联网络结构,这一结构可以增加伤口敷料的物理稳定性,并提供一个湿润的伤口环境[11]。因此,研究人员创新性地将海藻酸盐改性或与其他高分子材料联合,以提高海藻酸盐基水凝胶在皮肤损伤中的适用性。 生物降解性是评价材料优劣的重要因素之一,当植入/注射的水凝胶或支架进入人体后,材料如果永久留在体内没有良好的降解性能,可能会对人体造成长期的生理和心理损伤[12]。虽然海藻酸盐本身的降解速度非常慢,且其凝胶降解后的高分子质量海藻酸聚合物也不容易从体内除去,但海藻酸盐凝胶的降解动力学却可以通过改变藻酸盐的分子质量、化学结构、共价交联及酶降解进行调控[13-14]。ASHTON等[14]制备了一种具有可调节降解率的海藻酸盐水凝胶并将其用作干细胞的支架,结果证明了海藻酸盐凝胶不仅可以通过可控和可调的方式进行酶降解,而且可以显著刺激神经祖细胞的增殖。这种包封干细胞的可降解水凝胶在开发新的组织再生疗法方面具有广泛的应用前景。 2.2 海藻酸盐基生物材料的应用 海藻酸盐水凝胶/敷料目前已被广泛应用于伤口护理。早在2000年,THOMAS等[15]发现一些海藻酸盐敷料可以激活慢性伤口部位的巨噬细胞,从而产生促炎信号,并通过启动创面愈合特性炎症因子的分解达到调控免疫炎症反应的目的。位晓娟等[16]基于体内和体外生物学评价,通过对比海藻酸盐无纺布组和对照组血管内皮生长因子、成纤维细胞生长因子2、转化生长因子β、Ⅰ型胶原等的表达水平,证明海藻酸盐无纺布可以通过改善创面血管生成、细胞增殖、胶原分泌等途径促进糖尿病创面的愈合与修复。这得益于海藻酸盐基医用敷料可为伤口提供封闭润湿微环境,促进细胞增殖分化、具有良好的止血和自清创等功能[3]。 虽然海藻酸盐基水凝胶已被广泛用于各种临床治疗植入物和载体,但由于海藻酸盐本身具有生物惰性,提高其生物活性仍是一个挑战。另外研究表明,超过21%的pO2高氧张力(定义为高氧)可以促进细胞增殖与招募、伤口重建,减少炎症反应,从而加速伤口愈合过程[17-18]。2019年,KANG等[19]报道的产氧藻酸盐水凝胶既提高其生物活性又使其能够精确控制氧的释放,作为生物活性脱细胞基质促进小鼠全层伤口的创面愈合,为海藻酸盐在皮肤损伤修复中的应用打下了坚实的基础。 随着研究者们对天然生物材料的改进及共混聚合物的出现,新一代水凝胶/敷料的应用得到飞速发展。AFJOUL等[20]将海藻酸盐和明胶混合,经冷冻干燥制备海藻凝集素多孔水凝胶,增强了支架材料的机械性能和生物降解性,促进大鼠创面愈合。为了提高创面敷料的抗菌性能,ZHAO等[21]制备了海藻酸钙-壳聚糖敷料,研究表明该敷料具有良好的保湿、抗菌性能,无细胞毒性,通过降低白细胞介素6抑制炎症反应、增加血管内皮生长因子促进血管生成等加速了创面愈合。 此外,为了提高创面愈合效率,创面的湿润情况和敷料的黏附性能对于创面愈合至关重要。LIANG等[22]通过添加丙二醇以提高水凝胶的保水性能,开发了一种可自我调节/抗黏附的海藻酸盐水凝胶,这些抗黏附水凝胶具有良好的透气性,可自我调节创面愈合所需的湿度水平,促进深度二级烧伤创面的愈合。CHEN等[23]通过接枝多巴胺的氧化海藻酸钠和聚丙烯酰胺链之间的氢键与希夫碱交联,制备了一种具有较强细胞亲和性和组织黏附性的自愈水凝胶,提高凝胶的机械性能,促进组织再生,加速创面愈合。 2.3 海藻酸盐基生物材料联合生物活性物质的应用 近年来随着组织工程技术的发展,研究人员利用组织工程技术以海藻酸盐水凝胶为支架,负载种子细胞、生长因子或其他生物活性物质以改善海藻酸盐水凝胶的促创面愈合能力(图2),取得了较好的治疗效果[24-26]。 2.3.1 海藻酸盐基生物材料联合种子细胞的应用 干细胞是一类具有自我更新、高度增殖、多向分化潜能和低免疫原性的细胞群体,目前干细胞疗法已经成为再生医学研究的热点和有效策略[27]。然而,损伤部位移植干细胞的低保留率和低存活率限制了干细胞的治疗效果[28]。干细胞可以通过旁分泌相互作用加速伤口愈合,增加血管生成,有利于调节细胞外基质重构,促进皮肤结构和功能的再生[29]。海藻酸盐基水凝胶/敷料则为干细胞提供了可靠的递送平台。HAN等[30]利用海藻酸钠-氯化钡包封技术制备微囊化血管内皮生长因子基因改性的脐带间充质干细胞,结果发现海藻酸盐微囊包载的血管内皮生长因子基因化脐带间充质干细胞能有效改善组织工程真皮的血管化,提高皮肤全损创面的愈合。GUO等[31]利用氧化藻酸盐微球负载骨髓间充质干细胞治疗大鼠皮肤损伤,结果表明该支架促进骨髓间充质干细胞存活和宿主细胞浸润,明显促进皮肤组织的修复和再生。WANG等[32]将负载脐带间充质干细胞的海藻酸钙水凝胶移植治疗皮肤缺损,结果发现凝胶组的脐带间充质干细胞存活数量和血管内皮生长因子表达显著增加,凝胶支架可促进血管生成并提高皮肤愈合速度。 随着研究人员对组织再生领域的不断开拓,人们将细胞嵌入生物墨水中创建了基于细胞的三维结构来促进皮肤组织再生和修复的技术。CHENG等[33]制备了海藻酸盐-明胶与小鼠足底真皮共混的生物墨水,结果表明海藻酸盐-明胶-足底真皮生物墨水可以促进小鼠间充质干细胞的增殖和迁移,提高小鼠间充质干细胞向汗腺细胞的分化效率,利用3D细胞打印技术可以提高干细胞的功能。 虽然负载细胞水凝胶的挤压3D生物打印是再生医学的一项潜在技术,但是在打印前对水凝胶流变性能的评估仍然是3D生物打印技术发展的主要问题。基于以上问题,LIU等[34]系统研究了用于3D生物打印的海藻酸盐/明胶复合水凝胶的流变性能,研究表明海藻酸盐/明胶水凝胶的黏度与温度、剪切变薄有关。他们利用海藻酸盐/明胶复合水凝胶负载人羊膜上皮细胞和沃顿胶间充质干细胞制备仿生双层膜结构,体外培养6 d后仍然观察到较高的细胞存活率(>95%)。综上所述,海藻酸盐基水凝胶负载干细胞等种子细胞可以提高干细胞对皮肤损伤的治疗效果,已成为一种新型皮肤损伤的治疗策略。 2.3.2 海藻酸盐基生物材料联合生长因子的应用 皮肤组织工程的2个重要问题是真皮的血管化和再生[35]。碱性成纤维细胞生长因子、成纤维细胞生长因子等是已知的促进血管生成和加速创面愈合的生长因子,然而,直接将这些生长因子输送到伤口区域会导致其生物活性的丧失。针对这一问题,LIU等[35]制备了负载碱性成纤维细胞生长因子的海藻酸微球,并将其与羧甲基壳聚糖-聚乙烯醇共混形成复合水凝胶,结果发现该复合水凝胶可以持续释放具有生物活性的碱性成纤维细胞生长因子,促进皮肤的再上皮化、真皮再生和血管生成,提高创面的愈合速度。SHI等[36]利用微流控技术制备可持续释放药物且对pH值敏感的海藻酸盐/碳酸钙复合微粒子(约430 μm),同时负载利福霉素与碱性成纤维细胞生长因子用于大鼠全层皮肤创面的治疗,发现与对照组相比,联合治疗组创面愈合明显加快,肉芽组织厚度增加,血管生成能力有显著提高。此外,这种微粒子在体内表现出良好的生物相容性和生物降解性,可较好地满足创面愈合不同阶段的要求,具有良好的敷料应用潜力。 血小板衍生生长因子BB是糖蛋白血小板衍生生长因子的一种二聚体亚型,在血管形成过程中负责周细胞的招募和分化,从而调节血管的形态和功能[37],为创面愈合过程中血管的形成与稳定提供了理论依据。BABAVALIAN 等[38]证明光交联的海藻酸盐凝胶负载血小板衍生生长因子BB因子可在3周内具有持续的缓释能力,并且促进皮肤创面的愈合。表皮生长因子可趋化巨噬细胞、成纤维细胞向创面聚集,可促进胶原蛋白的合成及创面收缩,此外表皮生长因子的浓度还与瘢痕的严重程度息息相关[39]。因此,合理控制表皮生长因子在皮肤损伤部位的释放是创面愈合质量的重要保证。MANDAPALLI等[40]成功利用壳聚糖和海藻酸钠制备同时负载表皮生长因子和转化生长因子β siRNA的叠层(LbL)聚电解质多层膜,结果显示LbL薄膜具有良好的细胞相容性,通过共传递转化生长因子β siRNA和表皮生长因子加速伤口愈合,减少了胶原沉积,从而有望减少瘢痕形成。HU等[41]发现基于海藻酸盐的n-羧甲基壳聚糖水凝胶负载表皮生长因子不仅可以促进细胞增殖,而且加速创面愈合,在创伤护理管理中具有广阔的应用前景。JEONG等[42]利用A型明胶和海藻酸钠制备传递表皮生长因子的复体凝聚层系统,研究表明该系统促进角质形成细胞的体外迁移,加速链脲霉素诱导的糖尿病小鼠伤口处肉芽形成和上皮再生,提高了创面愈合效率。此外,该系统还可以降低白细胞介素1、白细胞介素6、肿瘤坏死因子α等的表达水平。综上,海藻酸盐基生物材料可以缓释生长因子,提高生长因子的活性和利用度,增强其在皮肤愈合中的应用价值。 2.3.3 海藻酸盐基生物材料联合其他生物活性物质的应用 虽然基于生长因子的海藻酸盐伤口敷料可以促进皮肤修复,但是生长因子价格昂贵,限制了其大量使用。因此在添加其他生物活性物质的情况下,制备具有止血控制功能且机械性能稳定的水凝胶,成为新的皮肤组织工程的潜在治疗手段。ZHAI等[43]利用细胞黏附肽结合物与海藻酸盐共组装成纳米材料,结果发现该超分子材料可以快速止血、促进成纤维细胞NIH3T3的黏附和迁移,并在小鼠全层皮肤缺损模型中加速了创面愈合。DEL GAUDIOA等[44]利用海藻酸钠和果胶共混物负载膜联蛋白A1来源的衍生肽Ac2-26,结果显示载Ac2-26的亚微米级原位水凝胶提高了Ac2-26的包封率、稳定性和缓释效率,并能够显著加速伤口愈合,且在24 h内完全封闭伤口模型,是一个非常有前途的伤口敷料。 然而,对于一些慢性伤口的愈合来说可能有2个关键不利因素:局部炎症的发生和血管生成受损[45],因此需要进一步设计和发展有效、安全和负担得起的战略来处理大的或慢性创伤。皮肤创面愈合过程中常伴有炎症的发生,而局部炎症发生往往是由细菌感染引起不同程度的细胞变性、坏死或组织缺损[46]。局部使用抗生素或者传递其他抗炎药物似乎是一种有效的治疗方式。NUUTILA等[47]制备包含高浓度抗生素的海藻酸盐水凝胶用于烧伤的即时护理,结果表明该材料可显著减少受损皮肤组织中的细菌数量,减轻组织坏死程度。DINIZ等[48]发现海藻酸钠、明胶和银纳米粒子制备的水凝胶具有显著的杀菌活性且无细胞毒性,体内研究证实载银纳米粒子的海藻酸盐水凝胶可促进创面的愈合、提高期肉芽组织的发育和成熟,具有良好的临床应用前景。无效的血管修复可能是慢性创面难愈合的另一个重要因素,这种作用与伤口新生血管的受损有关[49]。WANG等[45]发现鱼精蛋白纳米颗粒介导的复合海藻酸钙水凝胶具有较强的杀菌作用,可减轻创面部位的慢性炎症,还可以促进皮肤伤口的血管生成,加速伤口愈合过程。局部给予抗菌药物可以降低伤口中的细菌感染,但不适当的延长使用可能会阻碍愈合[50]。 此外,创面愈合可能还涉及到真皮和/或表皮细胞对修复性刺激的反应能力[51],因此改善损伤部位细胞微环境对于皮肤愈合尤其重要。LI等[52]发现海藻酸钠和阿拉伯树胶负载Mitsugumin 53蛋白可以持续快速的释放Mitsugumin 53来改善创面微环境,提高细胞的修复能力,进而促进小鼠皮肤创面的愈合。这为开发生物相容性和可调节的生物材料,以满足生物活动的需要提供了一条新的途径。 近年来,天然植物提取物所具备的特殊功效慢慢的被研究人员所发掘。某些天然材料显示出广泛的药理和生物学活性[53-54],如橙皮苷、姜黄素等是存在于植物提取物中的化合物,具有抗炎、抗氧化、促进受损组织修复的功能[55-56]。BAGHER等[57]发现海藻酸盐和壳聚糖凝胶负载橙皮苷可以促进皮肤细胞的增殖,加速创面的闭合。KARRI等[11]制备的包载姜黄素的壳聚糖/胶原/海藻酸盐纳米复合支架具有较好的吸水性、生物相容性和持续的药物利用度,在体内还可以促进皮肤的上皮化及肉芽组织生成,具有较好的创面愈合能力。同样,维生素E也是一类具有明显活性的抗氧化剂之一,它可以通过诱导各种信号转导途径的激活,保护细胞膜和多不饱和脂质免受活性氧的攻击[58]。EHTERAMI等[59]证明负载维生素E的多孔交联海藻酸盐/壳聚糖水凝胶可以促进伤口愈合和皮肤再生,有望应用于临床的皮肤损伤治疗。 蜂蜜也是一种古老的天然创面修复剂,因其具有多种生物活性而被重新引入现代临床伤口护理中。TANG等[60]采用静电纺丝法制备了一种新型的蜂蜜/海藻酸盐/聚乙烯醇纳米纤维膜,结果显示随着蜂蜜含量的增加,纳米纤维膜表现出更强的抗氧化活性和抗菌性,这一研究为新型创面敷料的开发奠定了基础。 "

| [1] KORTING HC, SCHOLLMANN C, WHITE RJ. Management of minor acute cutaneous wounds: importance of wound healing in a moist environment.J Eur Acad Dermatol Venereol.2011;25(2):130. [2] GUO S, DIPIETRO LA. Factors affecting wound healing.J Dent Res. 2010; 89(3):219. [3] 张小林,王兰兰,翁林,等.海藻酸盐医用材料的制备技术及应用现状[J].棉纺织技术,2019,47(4):75. [4] 廖盛涛,张开元,杨莉琴,等.不同伤口敷料在糖尿病足溃疡中的应用[J].山东医药,2014,54(13):95. [5] 石松松,张磊,张兵,等.藻酸衍生物在生物医药领域的研究进展[J].中国海洋药物,2019,38(1):67. [6] GUPTA A, KOWALCZUK M, HEASELGRAVE W, et al. The production and application of hydrogels for wound management:A review.Eur Polym J. 2019;111:134. [7] YEUNG RA, KENNEDY RA. A comparison of selected physico-chemical properties of calcium alginate fibers produced using two different types of sodium alginate.J Mech Behav Biomed Mater.2019;90:155. [8] FRANCO P, PESSOLANO E, BELVEDERE R, et al. Supercritical impregnation of mesoglycan into calcium alginate aerogel for wound healing. J Supercrit Fluid.2020;157:104711. [9] ZHANG M, CHEN S, ZHONG L, et al. Zn(2+)-loaded TOBC nanofiber-reinforced biomimetic calcium alginate hydrogel for antibacterial wound dressing.Int J Biol Macromol.2020;143:235. [10] STUBBE B, MIGNON A, DECLERCQ H, et al. Development of gelatin-alginate hydrogels for burn wound treatment.Macromol Biosci. 2019; 19(8):e1900123. [11] KARRI VV, KUPPUSAMY G, TALLURI SV, et al. Curcumin loaded chitosan nanoparticles impregnated into collagen-alginate scaffolds for diabetic wound healing.Int J Biol Macromol.2016;93(PtB):1519. [12] TSOU YH, KHONEISSER J, HUANG PC, et al. Hydrogel as a bioactive material to regulate stem cell fate.Bioact Mater.2016;1(1):39. [13] BOUHADIR KH, LEE KY, ALSBERG E, et al. Degradation of partially oxidized alginate and its potential application for tissue engineering.Biotechnol Prog.2001;17(5):945. [14] ASHTON RS, BANERJEE A, PUNYANI S, et al. Scaffolds based on degradable alginate hydrogels and poly(lactide-co-glycolide) microspheres for stem cell culture.Biomaterials.2007;28(36):5518 [15] THOMAS A, HARDING KG, MOORE K. Alginates from wound dressings activate human macrophages to secrete tumour necrosis factor-alpha.Biomaterials.2000;21(17):1797. [16] 位晓娟,顾其胜,王南平,等.海藻酸盐无纺布促进糖尿病溃疡创伤修复的功能及机理研究[G].全国第二届海洋与陆地多糖多肽及天然创新药物研发学术会议;中国黑龙江漠河.中国生物化学与分子生物学会海洋生物化学与分子生物学分会(Marine B,Molecular Biology Society CSfB,Molecular B中上中,2015. [17] ZHANG Q, CHANG Q, COX RA, et al. Hyperbaric oxygen attenuates apoptosis and decreases inflammation in an ischemic wound model.J Invest Dermatol.2008;128(8):2102. [18] THOM SR, MILOVANOVA TN, YANG M, et al. Vasculogenic stem cell mobilization and wound recruitment in diabetic patients:increased cell number and intracellular regulatory protein content associated with hyperbaric oxygen therapy.Wound Repair Regen.2011;19(2):149. [19] KANG JI, PARK KM, PARK KD. Oxygen-generating alginate hydrogels as a bioactive acellular matrix for facilitating wound healing.Journal of Industrial and Engineering Chemistry.2019;69:397. [20] AFJOUL H, SHAMLOO A, KAMALI A. Freeze-gelled alginate/gelatin scaffolds for wound healing applications:An in vitro,in vivo study.Mater Sci Eng C.2020;110957. [21] ZHAO WY, FANG QQ, WANG XF, et al. Chitosan-calcium alginate dressing promotes wound healing:A preliminary study.Wound Repair Regen.2020;28(3):326. [22] LIANG M, CHEN Z, WANG F, et al. Preparation of self-regulating/anti-adhesive hydrogels and their ability to promote healing in burn wounds.J Biomed Mater Res B Appl Biomater.2019;107(5):1471. [23] CHEN T, CHEN Y, REHMAN HU, et al. Ultratough,Self-Healing,and Tissue-Adhesive Hydrogel for Wound Dressing.ACS Appl Mater Interfaces. 2018;10(39):33523. [24] GUO J, HU H, GORECKA J, et al. Adipose-derived mesenchymal stem cells accelerate diabetic wound healing in a similar fashion as bone marrow-derived cells.Am J Physiol Cell Physiol.2018;315(6):C885. [25] SCHNEIDER RK, ANRATHS J, KRAMANN R, et al. The role of biomaterials in the direction of mesenchymal stem cell properties and extracellular matrix remodelling in dermal tissue engineering.Biomaterials.2010;31(31):7948. [26] JEE JP, PANGENI R, JHA SK, et al. Preparation and in vivo evaluation of a topical hydrogel system incorporating highly skin-permeable growth factors,quercetin,and oxygen carriers for enhanced diabetic wound-healing therapy.Int J Nanomedicine.2019;14:5449. [27] LI T, XIA M, GAO Y, et al. Human umbilical cord mesenchymal stem cells: an overview of their potential in cell-based therapy.Expert Opin Biol Ther.2015;15(9):1293. [28] HUANG P, WANG L, LI Q, et al. Combinatorial treatment of acute myocardial infarction using stem cells and their derived exosomes resulted in improved heart performance.Stem Cell Res Ther. 2019; 10(1):300. [29] LEE DE, AYOUB N, AGRAWAL DK. Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy.Stem Cell Res Ther.2016;7:37. [30] HAN Y, TAO R, HAN Y, et al. Microencapsulated VEGF gene-modified umbilical cord mesenchymal stromal cells promote the vascularization of tissue-engineered dermis: an experimental study.Cytotherapy.2014;16(2):160. [31] GUO R, WARD CL, DAVIDSON JM, et al. A transient cell-shielding method for viable MSC delivery within hydrophobic scaffolds polymerized in situ. Biomaterials.2015;54:21. [32] WANG S, YANG H, TANG Z, et al. Wound Dressing model of human umbilical cord mesenchymal stem cells-alginates complex promotes skin wound healing by paracrine signaling.Stem Cells Int. 2016; 2016:3269267. [33] CHENG L, YAO B, HU T, et al. Properties of an alginate-gelatin-based bioink and its potential impact on cell migration, proliferation, and differentiation.Int J Biol Macromol.2019;135:1107. [34] LIU P, SHEN H, ZHI Y, et al. 3D bioprinting and in vitro study of bilayered membranous construct with human cells-laden alginate/gelatin composite hydrogels.Colloids Surf B Biointerfaces.2019;181:1026. [35] LIU Q, HUANG Y, LAN Y, et al. Acceleration of skin regeneration in full-thickness burns by incorporation of bFGF-loaded alginate microspheres into a CMCS-PVA hydrogel.J Tissue Eng Regen Med.2017;11(5):1562. [36] SHI M, ZHANG H, SONG T, et al. Sustainable dual release of antibiotic and growth factor from pH-responsive uniform alginate composite microparticles to enhance wound healing.ACS Appl Mater Interfaces.2019;11(25):22730. [37] HELLBERG C, OSTMAN A, HELDIN CH. PDGF and vessel maturation.Recent Results Cancer Res.2010;180:103. [38] BABAVALIAN H, TEBYANIAN H, LATIFI AM, et al. The effect of synthetic alginate sulfate hydrogels with recombinant PDGF-BB on Wound healing. Bratisl Lek Listy.2018;119(6):391. [39] 谭倩,赵鑫,陈贝,等.生长因子在创面愈合中的作用研究进展[J].山东医药,2019,59(4):106. [40] MANDAPALLI PK, LABALA S, JOSE A, et al. Layer-by-Layer thin films for co-delivery of TGF-β siRNA and epidermal growth factor to improve excisional wound healing.AAPS Pharm Sci Tech.2017;18(3):809. [41] HU Y, ZHANG Z, LI Y, et al. Dual-crosslinked amorphous polysaccharide hydrogels based on chitosan/alginate for wound healing applications.Macromol Rapid Commun.2018;39(20):1800069. [42] JEONG S, KIM B, PARK M, et al. Improved diabetic wound healing by EGF encapsulation in gelatin-alginate coacervates.Pharmaceutics. 2020;12(4):E334. [43] ZHAI Z, XU K, MEI L, et al. Co-assembled supramolecular hydrogels of cell adhesive peptide and alginate for rapid hemostasis and efficacious wound healing.Soft Matter.2019;15(42):8603. [44] DEL GAUDIO P, AMANTE C, CIVALE R, et al. In situ gelling alginate-pectin blend particles loaded with Ac2-26:A new weapon to improve wound care armamentarium.Carbohydr Polym.2020;227:115305 [45] WANG T, ZHENG Y, SHI Y, et al. pH-responsive calcium alginate hydrogel laden with protamine nanoparticles and hyaluronan oligosaccharide promotes diabetic wound healing by enhancing angiogenesis and antibacterial activity.Drug Deliv Transl Res.2019;9(1):227. [46] CHEN H, XING X, TAN H, et al. Covalently antibacterial alginate-chitosan hydrogel dressing integrated gelatin microspheres containing tetracycline hydrochloride for wound healing.Mater Sci Eng C Mater Biol Appl.2017;70(Pt1):287. [47] NUUTILA K, GROLMAN J, YANG L, et al. Immediate treatment of burn wounds with high concentrations of topical antibiotics in an alginate hydrogel using a platform wound device.Adv Wound Care(New Rochelle). 2020;9(2):48. [48] DINIZ FR, MAIA RCAP, RANNIER ANDRADE L, et al. Silver Nanoparticles-Composing Alginate/Gelatine Hydrogel Improves Wound Healing In Vivo. Nanomaterials.2020;10(2):390 [49] ICLI B, NABZDYK CS, LUJAN-HERNANDEZ J, et al. Regulation of impaired angiogenesis in diabetic dermal wound healing by microRNA-26a.J Mol Cell Cardiol.2016;91:151. [50] FRYKBERG RG, BANKS J. Challenges in the treatment of chronic wounds.Adv Wound Care(New Rochelle).2015;4(9):560. [51] DEMIDOVA-RICE TN, SALOMATINA EV, YAROSLAVSKY AN, et al. Low-level light stimulates excisional wound healing in mice.Lasers Surg Med. 2007;39(9):706. [52] LI M, LI H, LI X, et al. A bioinspired alginate-gum arabic hydrogel with micro-/nanoscale structures for controlled drug release in chronic wound healing.ACS Appl Mater Interfaces.2017;9(27):22160. [53] BENAVENTE-GARCÍA O, CASTILLO J. Update on uses and properties of citrus flavonoids:new findings in anticancer,cardiovascular,and anti-inflammatory activity.J Agric Food Chem.2008;56(15):6185. [54] YU J, WANG L, WALZEM RL, et al. Antioxidant activity of citrus limonoids,flavonoids,and coumarins.J Agric Food Chem.2005; 53(6): 2009. [55] MANEESAI P, BUNBUPHA S, POTUE P, et al. Hesperidin prevents nitric oxide deficiency-Induced cardiovascular remodeling in rats via suppressing TGF-β1 and MMPs protein expression.Nutrients. 2018; 10(10):E1549. [56] HADDADI GH, REZAEYAN A, MOSLEH-SHIRAZI MA, et al. Hesperidin as radioprotector against radiation-induced lung damage in rat:A histopathological study.J Med Phys.2017;42(1):25. [57] BAGHER Z, EHTERAMI A, SAFDEL MH, et al. Wound healing with alginate/chitosan hydrogel containing hesperidin in rat model.J Drug Deliv Sci Technol.2020;55:101379. [58] BIESALSKI HK. Polyphenols and inflammation:basic interactions.Curr Opin Clin Nutr Metab Care.2007;10(6):724. [59] EHTERAMI A, SALEHI M, FARZAMFAR S, et al. Chitosan/alginate hydrogels containing Alpha-tocopherol for wound healing in rat model.J Drug Deliv Sci Technol.2019;51:204. [60] TANG Y, LAN X, LIANG C, et al. Honey loaded alginate/PVA nanofibrous membrane as potential bioactive wound dressing.Carbohydr Polym. 2019;219:113. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Jiang Hongying, Zhu Liang, Yu Xi, Huang Jing, Xiang Xiaona, Lan Zhengyan, He Hongchen. Effect of platelet-rich plasma on pressure ulcers after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1149-1153. |
| [3] | Wu Xun, Meng Juanhong, Zhang Jianyun, Wang Liang. Concentrated growth factors in the repair of a full-thickness condylar cartilage defect in a rabbit [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1166-1171. |
| [4] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [5] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [6] | Li Cai, Zhao Ting, Tan Ge, Zheng Yulin, Zhang Ruonan, Wu Yan, Tang Junming. Platelet-derived growth factor-BB promotes proliferation, differentiation and migration of skeletal muscle myoblast [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1050-1055. |
| [7] | He Xiangzhong, Chen Haiyun, Liu Jun, Lü Yang, Pan Jianke, Yang Wenbin, He Jingwen, Huang Junhan. Platelet-rich plasma combined with microfracture versus microfracture in the treatment of knee cartilage lesions: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 964-969. |
| [8] | Zhang Bin, Sun Lihua, Zhang Junhua, Liu Yusan, Cui Caiyun. A modified flap immediate implant is beneficial to soft tissue reconstruction in maxillary aesthetic area [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 707-712. |
| [9] | Luo Xuanxiang, Jing Li, Pan Bin, Feng Hu. Effect of mecobalamine combined with mouse nerve growth factor on nerve function recovery after cervical spondylotic myelopathy surgery [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 719-722. |
| [10] | Nie Huijuan, Huang Zhichun. The role of Hedgehog signaling pathway in transforming growth factor beta1-induced myofibroblast transdifferentiation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 754-760. |
| [11] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [12] | Fu Shuanhu, Qin Kai, Lu Dahan, Qin Haibiao, Gu Jin, Chen Yongxi, Qin Haoran, Wei Jiading, Wu Liang, Song Quansheng. Lumbar spinal tuberculosis implanted with artificial bone with streptomycin sulfate and percutaneous pedicle screw under transforaminal endoscopy [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 493-498. |
| [13] | Li Wenjing, Li Haobo, Liu Congna, Cheng Dongmei, Chen Huizhen, Zhang Zhiyong. Comparison of different bioactive scaffolds in the treatment of regenerative pulp of young permanent teeth [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 499-503. |
| [14] | Wang Yujiao, Liu Dan, Sun Song, Sun Yong. Biphasic calcium phosphate loaded with advanced platelet rich fibrin can promote the activity of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 504-509. |
| [15] | Chen Junyi, Wang Ning, Peng Chengfei, Zhu Lunjing, Duan Jiangtao, Wang Ye, Bei Chaoyong. Decalcified bone matrix and lentivirus-mediated silencing of P75 neurotrophin receptor transfected bone marrow mesenchymal stem cells to construct tissue-engineered bone [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 510-515. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||