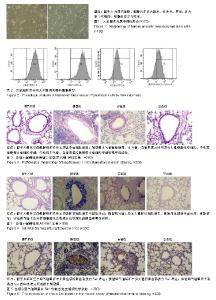
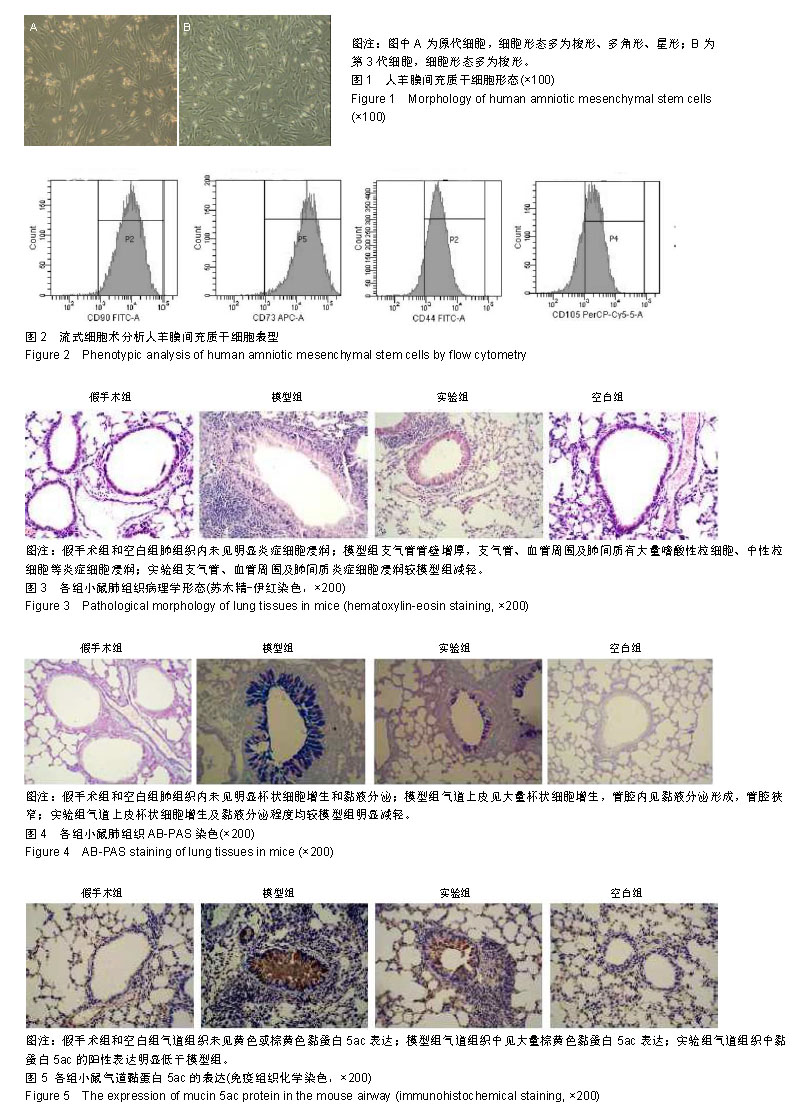
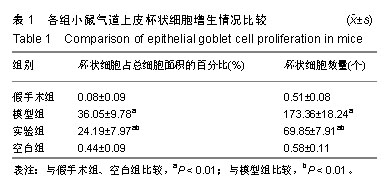
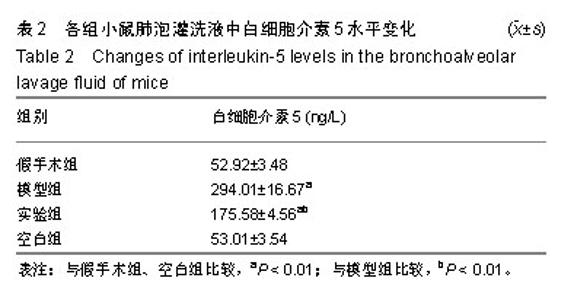
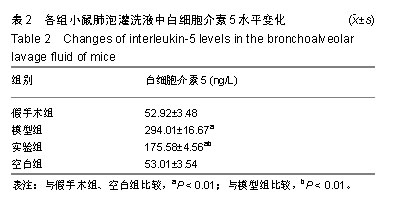
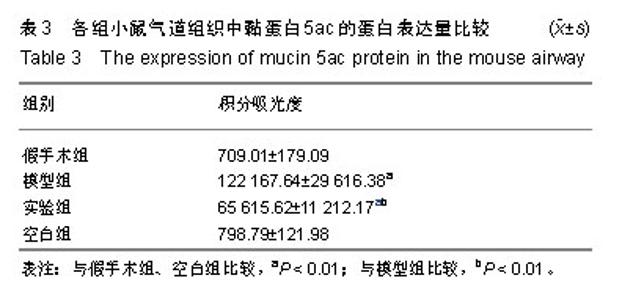
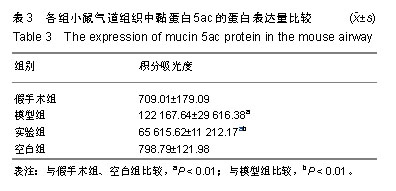
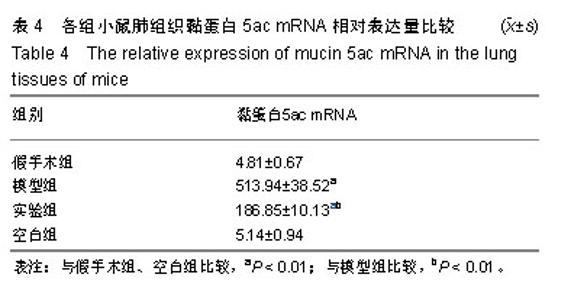
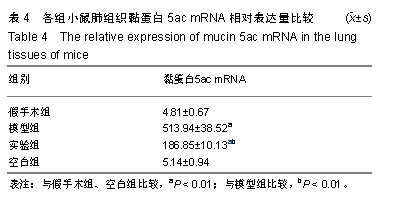
| [1]Becker AB, Abrams EM. Asthma guidelines: the Global Initiative for Asthma in relation to national guidelines. Curr Opin Allergy Clin Immunol. 2017;17(2):99-103.[2]Kesimer M, Ford AA, Ceppe A, et al. Airway Mucin Concentration as a Marker of Chronic Bronchitis. N Engl J Med. 2017;377(10):911-922. [3]慢性气道炎症性疾病气道黏液高分泌管理中国专家共识编.慢性气道炎症性疾病气道黏液高分泌管理中国专家共识[J].中华结核和呼吸杂志,2015,38(10):723-729. [4]王立宾,祝贺,郝捷, 等.干细胞与再生医学研究进展[J].生物工程学报,2015,31(6):871-879.[5]欧阳文,廖正权,夏增飞,等.羊膜间充质干细胞的免疫调节功能研究进展[J].中华细胞与干细胞杂志(电子版), 2017,7(5):313-316.[6]宋洁,高雅,卓伟彬,等.人羊膜间充质干细胞与骨髓间充质干细胞对外周血淋巴细胞的免疫调节作用比较[J].南方医科大学学报, 2017, 37(6): 780-785.[7]Wang Z, Wang Y, Wang Z, et al. Engineered mesenchymal stem cells with enhanced tropism and paracrine secretion of cytokines and growth factors to treat traumatic brain injury. Stem Cells. 2015;33(2): 456-467.[8]Cui P, Xin H, Yao Y, et al. Human amnion-derived mesenchymal stem cells alleviate lung injury induced by white smoke inhalation in rats. Stem Cell Res Ther. 2018;9(1):101. [9]Chen Z, Chen L, Zeng C, et al. Functionally Improved Mesenchymal Stem Cells to Better Treat Myocardial Infarction. Stem Cells Int. 2018;2018:7045245. [10]Zhang B, Zhang J, Zhu D, et al. Mesenchymal stem cells rejuvenate cardiac muscle after ischemic injury. Aging (Albany NY). 2019;11(1):63-72.[11]石清红.人羊膜间充质干细胞静脉移植对哮喘小鼠气道炎症及气道重塑的影响[D].遵义:遵义医学院, 2016.[12]Lachowicz-Scroggins ME, Yuan S, Kerr SC, et al. Abnormalities in MUC5AC and MUC5B Protein in Airway Mucus in Asthma. Am J Respir Crit Care Med. 2016;194(10): 1296-1299.[13]陈勇,方宁,陈代雄,等.人羊膜间充质干细胞移植治疗帕金森病模型大鼠的疗效评价[J].中风与神经疾病杂志, 2015,32(5): 406-410.[14]Zuhdi Alimam M, Piazza FM, Selby DM, et al. Muc-5/5ac mucin messenger RNA and protein expression is a marker of goblet cell metaplasia in murine airways. Am J Respir Cell Mol Biol. 2000;22(3): 253-260.[15]蔡霜,邹文静,王婷,等. Brg1通过STAT6促进哮喘气道黏液高分泌[J].南方医科大学学报,2018,38(1):42-47.[16]杜丽君,王荣丽.黏蛋白MUC5AC与肺部疾病的研究进展[J].国际呼吸杂志,2014,34(4):315-318.[17]Pantano F, Baldi A, Santini D, et al. MUC2 but not MUC5 expression correlates with prognosis in radically resected pancreatic cancer patients. Cancer Biol Ther. 2009;8(11): 996-999.[18]刘仁慧,王秀娟,许利平,等.培本中药及其拆方调节哮喘大鼠Th1/Th2平衡的实验研究[J].北京中医药大学学报, 2014,37(3): 165-170,183,后插2. [19]Duffy MM, Ritter T, Ceredig R, et al. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res Ther. 2011; 2(4):34. [20]Lin YD, Fan XL, Zhang H, et al. The genes involved in asthma with the treatment of human embryonic stem cell-derived mesenchymal stem cells. Mol Immunol. 2018;95:47-55.[21]Chan CK, Lin TC, Huang YA, et al. The modulation of Th2 immune pathway in the immunosuppressive effect of human umbilical cord mesenchymal stem cells in a murine asthmatic model. Inflamm Res. 2016;65(10):795-801.[22]Dai R, Liu J, Cai S, et al. Delivery of adipose-derived mesenchymal stem cells attenuates airway responsiveness and inflammation in a mouse model of ovalbumin-induced asthma. Am J Transl Res. 2017;9(5):2421-2428. [23]任妮娜,陈鸿杰,陈巧林,等.人脐带间充质干细胞移植对支气管哮喘小鼠气道炎症的作用及其机制[J].中华医学杂志,2017,97(34): 2697-2702.[24]Mareschi K, Castiglia S, Sanavio F, et al. Immunoregulatory effects on T lymphocytes by human mesenchymal stromal cells isolated from bone marrow, amniotic fluid, and placenta. Exp Hematol. 2016;44(2): 138-150.e1.[25]Mariñas-Pardo L, Mirones I, Amor-Carro O, et al. Mesenchymal stem cells regulate airway contractile tissue remodeling in murine experimental asthma. Allergy. 2014; 69(6):730-740.[26]Braza F, Dirou S, Forest V, et al. Mesenchymal Stem Cells Induce Suppressive Macrophages Through Phagocytosis in a Mouse Model of Asthma. Stem Cells. 2016;34(7):1836-1845.[27]丛姗,宋瑾,张惠娟,等.人羊膜间充质干细胞(hAMSCs)的分离、体外培养及诱导分化[J].农业生物技术学报,2015,23(1):20-31.[28]洪佳琼,高雅,宋洁,等.人羊膜间充质干细胞与骨髓间充质干细胞的生物学特性及免疫抑制作用的比较[J].中国实验血液学杂志, 2016,24(3):858-864. [29]Gunawardena TNA, Rahman MT, Abdullah BJJ, et al. Conditioned media derived from mesenchymal stem cell cultures: The next generation for regenerative medicine. J Tissue Eng Regen Med. 2019;13(4):569-586.[30]姚银,郭洁波,邓学泉,等.间充质干细胞对哮喘模型小鼠的免疫调节作用:局部与全身应用效果不同[J].中国组织工程研究, 2015, 19(28):4478-4484. |