Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (6): 1359-1367.doi: 10.12307/2026.568
Previous Articles Next Articles
Irisin inhibits ferroptosis in human articular chondrocytes: roles and mechanisms
Lyu Guoqing, Aizimaitijiang·Rouzi, Xiong Daohai
- The Fifth Affiliated Hospital of Xinjiang Medical University, Urumqi 830000, Xinjiang Uygur Autonomous Region, China
-
Received:2024-11-21Accepted:2025-01-14Online:2026-02-28Published:2025-07-14 -
Contact:Xiong Haidao, MS, Chief physician, The Fifth Affiliated Hospital of Xinjiang Medical University, Urumqi 830000, Xinjiang Uygur Autonomous Region, China -
About author:Lyu Guoqing, MS, Associate chief physician, The Fifth Affiliated Hospital of Xinjiang Medical University, Urumqi 830000, Xinjiang Uygur Autonomous Region, China -
Supported by:the Natural Science Foundation of Xinjiang Uygur Autonomous Region, No. 2022D01C313 (to LGQ)
CLC Number:
Cite this article
Lyu Guoqing, Aizimaitijiang·Rouzi, Xiong Daohai. Irisin inhibits ferroptosis in human articular chondrocytes: roles and mechanisms[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1359-1367.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
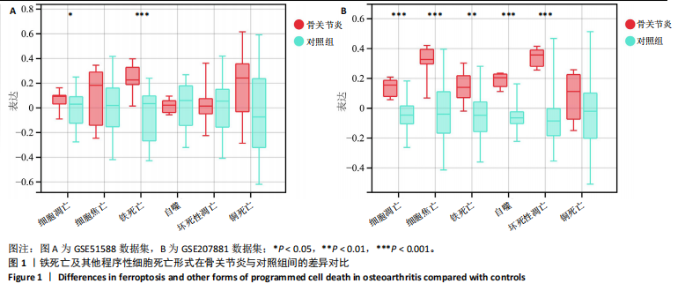
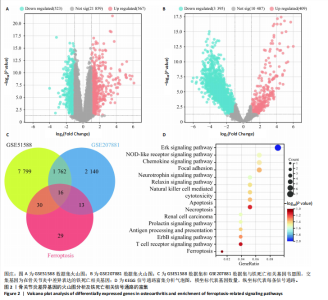
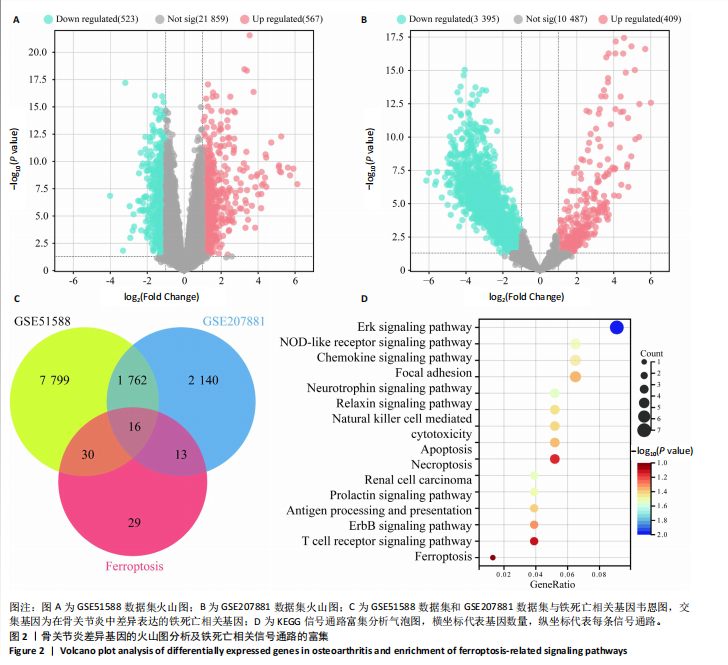
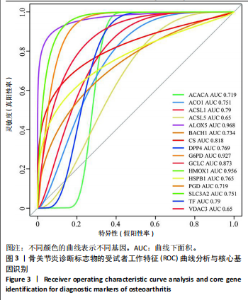
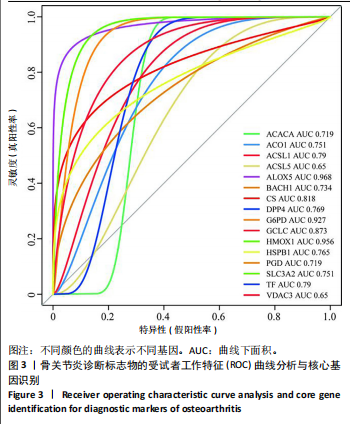
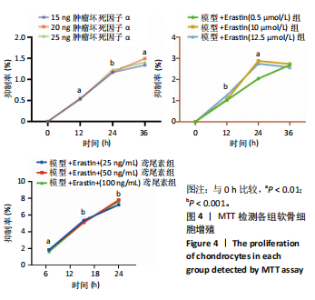
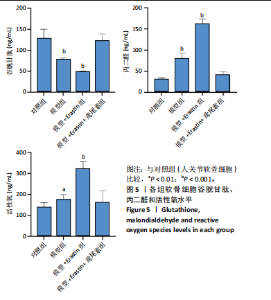
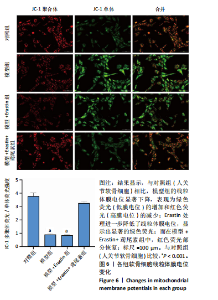
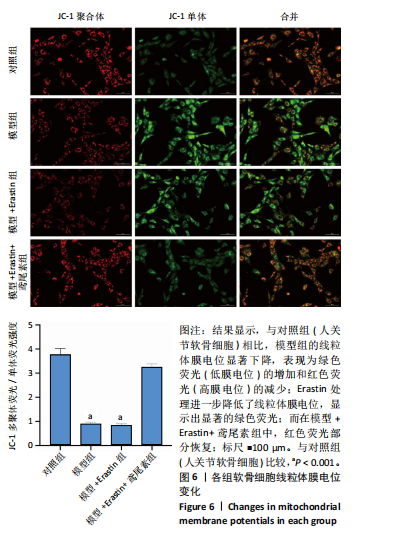
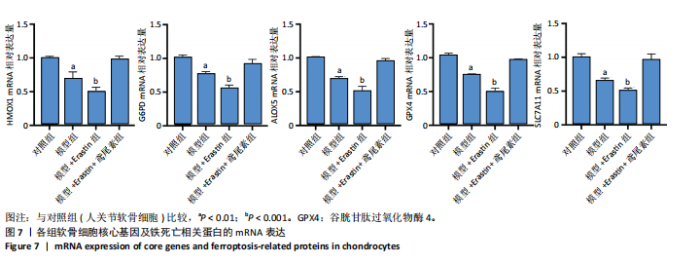
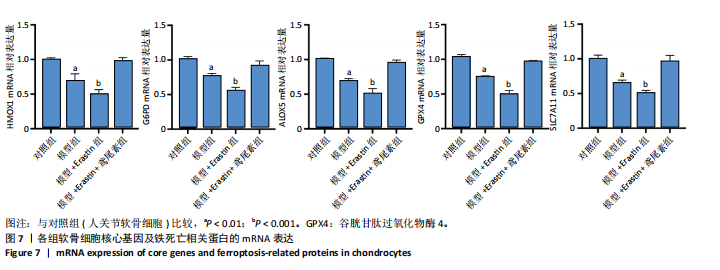
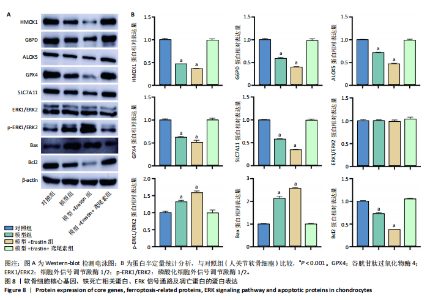
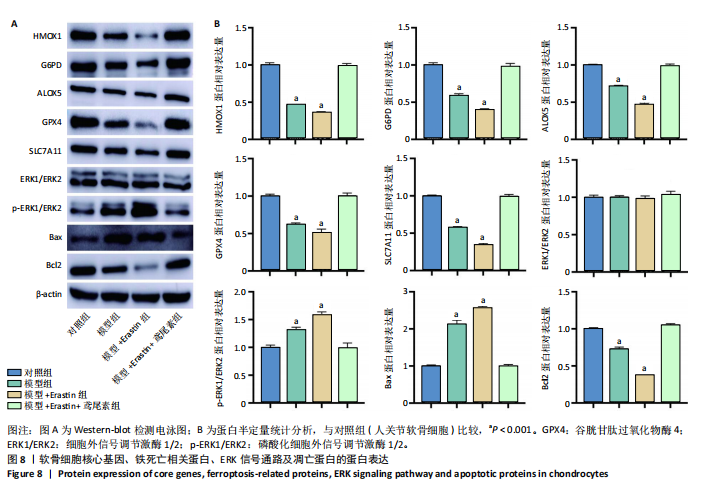
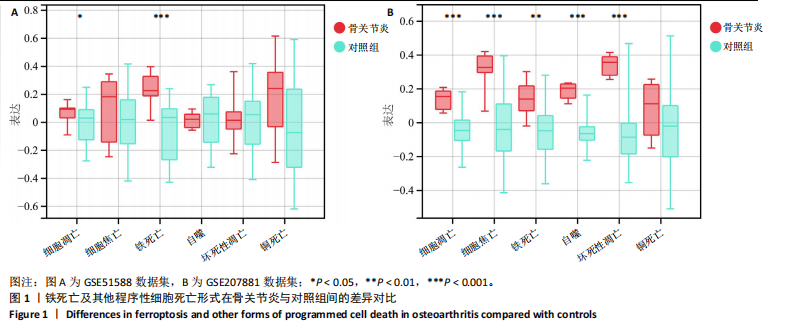
2.1 铁死亡及其他程序性细胞死亡形式在骨关节炎与对照组间的差异对比 在骨关节炎软骨组织中,不同类型的程序性细胞死亡形式表现出差异化的表达模式。GSE51588数据集中,与健康对照组相比,细胞凋亡和铁死亡在骨关节炎组中显著激活(P < 0.001);在GSE207881数据集中,除细胞凋亡和铁死亡外,自噬、坏死性凋亡和细胞焦亡也表现出显著上调(P < 0.001),见图1。 2.2 骨关节炎差异基因及富集分析 在GSE51588数据集中,共鉴定到567个显著上调基因和523个显著下调基因(图2A),而在GSE207881数据集中,显著上调基因为409个,下调基因为3 395个(图2B)。进一步通过韦恩图分析(图2C),共发现1 762个基因在两个数据集中同时存在,而与铁死亡相关的差异基因中,有16个基因(PGD、ALOX5、HMOX1、G6PD、GCLC、DPP4、SLC3A2、ACSL1、CS、ACACA、TF、HSPB1、ACSL5、VDAC3、ACO1、BACH1)在GSE51588、GSE207881和铁死亡相关基因中均有交集,提示这些基因可能与骨关节炎中的铁死亡机制密切相关。信号通路富集分析(图2D)显示,差异基因显著富集于Erk信号通路、焦亡、坏死性凋亡及铁死亡等程序性细胞死亡相关通路,这表明这些信号通路可能在骨关节炎的发病机制中起到重要作用。 2.3 骨关节炎诊断标志物的ROC曲线分析与核心基因识别 结果显示,ALOX5、HMOX1和G6PD的AUC值分别为0.968,0.956和0.927,显示出较高的诊断效能,提示这些基因可能作为区分骨关节炎患者与健康个体的核心诊断标志物,见图3。 2.4 MTT检测结果 结果显示,肿瘤坏死因子α的最佳剂量为20 ng/mL,Erastin的最佳剂量为10 μmol/L,鸢尾素的最佳剂量为100 ng/mL,见图4。后续实验各组按照上述最佳剂量进行。 2.5 软骨细胞谷胱甘肽、丙二醛和活性氧水平 ELISA法检测结果表明,在人关节软骨细胞的炎症模型中,Erastin处理显著降低了抗氧化物谷胱甘肽的水平,并显著增加了氧化应激标志物丙二醛和活性氧的水平(P < 0.001)。然而,加入鸢尾素后,谷胱甘肽水平显著回升,丙二醛和活性氧的水平显著降低,表明鸢尾素具有显著的抗氧化作用,能够有效缓解Erastin诱导的氧化应激反应,详见图5。 2.6 线粒体膜电位变化 JC-1检测结果显示,与对照组相比,模型组的线粒体膜电位显著下降,表现为绿色荧光(低膜电位)的增加和红色荧光(高膜电位)的减少。Erastin处理进一步降低了线粒体膜电位,显示出显著的绿色荧光。而在Erastin+鸢尾素组中,红色荧光部分恢复,说明鸢尾素能够显著改善Erastin诱导的线粒体功能障碍,恢复线粒体膜电位,详见图6。 2.7 软骨细胞核心基因及铁死亡相关蛋白的mRNA表达 RT-qPCR检测结果表明,在骨关节炎炎症模型中,HMOX1、G6PD、ALOX5、谷胱甘肽过氧化物酶4和SLC7A11的mRNA表达水平均显著下调(均P <"
| [1] HU S, LI Y, ZHANG X, et al. Increasing burden of osteoarthritis in China: trends and projections from the Global Burden of Disease Study 2019. Med Sci Monit. 2024;30:e942626. [2] HOLYOAK DT, TIAN YF, VAN DER MEULEN MC, et al. Osteoarthritis: Pathology, Mouse Models, and Nanoparticle Injectable Systems for Targeted Treatment. Ann Biomed Eng. 2016;44(6):2062-2075. [3] EKMAN B, NERO H, LOHMANDER LS, et al. Costing analysis of a digital first-line treatment platform for patients with knee and hip osteoarthritis in Sweden. Plos one.2020;15(8):e0236342. [4] XU Y, YANG Z, DAI T, et al. Characteristics and time points to inhibit ferroptosis in human osteoarthritis. Sci Rep. 2023;13(1):21592. [5] CHANG S, TANG M, ZHANG B, et al. Ferroptosis in inflammatory arthritis: a promising future. Front Immunol. 2022;13:955069. [6] HU Y, WANG Y, LIU S, et al. The Potential Roles of Ferroptosis in Pathophysiology and Treatment of Musculoskeletal Diseases-Opportunities, Challenges, and Perspectives. J Clin Med. 2023; 12(6):2125. [7] ZHAO R, CHEN Y, WANG D, et al. Role of irisin in bone diseases. Front Endocrinol (Lausanne). 2023;14:1212892. [8] ROGGIO F, PETRIGNA L, TROVATO B, et al. The role of lubricin, irisin and exercise in the prevention and treatment of osteoarthritis. Int J Mol Sci. 2023;24(6):5126. [9] AL-HETTY HRAK, ABDULAMEER SJ, ALGHAZALI MW, et al. The role of ferroptosis in the pathogenesis of osteoarthritis. J Membr Biol. 2023;256(3):223-228. [10] YAO X, SUN K, YU S, et al. Chondrocyte ferroptosis contribute to the progression of osteoarthritis. J Orthop Translat. 2020;27:33-43. [11] GUO Z, LIN J, SUN K, et al. Deferoxamine alleviates osteoarthritis by inhibiting chondrocyte ferroptosis and activating the Nrf2 pathway.Front Pharmacol. 2022;13:791376. [12] ZHANG S, XU J, SI H, et al. The role played by ferroptosis in osteoarthritis: evidence based on iron dyshomeostasis and lipid peroxidation. Antioxidants (Basel). 2022;11(9):1668. [13] LIU Y, ZHANG Z, FANG Y, et al. Ferroptosis in osteoarthritis: current understanding. J Inflamm Res. 2024;17:8471-8486. [14] TAO L, WANG J, WANG K, et al. Exerkine FNDC5/irisin‐enriched exosomes promote proliferation and inhibit ferroptosis of osteoblasts through interaction with Caveolin‐1. Aging Cell. 2024;23(8):e14181. [15] ASKARI H, RAJANI SF, POOREBRAHIM M, et al. A glance at the therapeutic potential of irisin against diseases involving inflammation, oxidative stress, and apoptosis: an introductory review. Pharmacol Res. 2018;129:44-55. [16] TIAN L, LIU Q, WANG X, et al. Fighting ferroptosis: Protective effects of dexmedetomidine on vital organ injuries. Life Sci. 2024;354:122949. [17] RAYEGO-MATEOS S, MARQUEZ-EXPOSITO L, BASANTES P, et al. CCN2 activates RIPK3, NLRP3 inflammasome, and NRF2/oxidative pathways linked to kidney inflammation. Antioxidants (Basel). 2023;12(8):1541. [18] KONG K, YANG Y, CHANG Y, et al. A Ubiquitin‐Competitive Strategy Based on the Element Microenvironment to Treat Osteoarthritis. Adv Funct Mater. 2024;34(51):2409707. [19] HAN J, ZHAN L, HUANG Y, et al. Moderate mechanical stress suppresses chondrocyte ferroptosis in osteoarthritis by regulating NF-κB p65/GPX4 signaling pathway. Sci Rep. 2024;14(1):5078. [20] LI X, ZHU X, WU H, et al. Roles and mechanisms of irisin in attenuating pathological features of osteoarthritis. Front Cell Dev Biol. 2021;9: 703670. [21] JIA S, YANG Y, BAI Y, et al. Mechanical stimulation protects against chondrocyte pyroptosis through irisin-induced suppression of PI3K/Akt/NF-κB signal pathway in osteoarthritis. Front Cell Dev Biol. 2022; 10:797855. [22] LI X, LIU Y, LIU Q, et al. Recombinant human irisin regulated collagen II, matrix metalloproteinase‑13 and the Wnt/β‑catenin and NF‑κB signaling pathways in interleukin‑1β‑induced human SW1353 cells. Exp Ther Med. 2020;19(4):2879-2886. [23] JIA S, YU Z, BAI L. Exerkines and osteoarthritis. Front Physiol. 2023;14: 1302769. [24] POSA F, ZERLOTIN R, ARIANO A, et al. Irisin role in chondrocyte 3D culture differentiation and its possible applications. Pharmaceutics. 2023;15(2):585. [25] LIU Y, HOU M, PAN Z, et al. Arctiin-reinforced antioxidant microcarrier antagonizes osteoarthritis progression. J Nanobiotechnology. 2022; 20(1):303. [26] AMIRKHIZI F, HAMEDI-SHAHRAKI S, RAHIMLOU M. Dietary total antioxidant capacity is associated with lower disease severity and inflammatory and oxidative stress biomarkers in patients with knee osteoarthritis. J Health Popul Nutr. 2023;42(1):104. [27] NING K, WANG Z, ZHANG X. Exercise-induced modulation of myokine irisin in bone and cartilage tissue—Positive effects on osteoarthritis: A narrative review. Front Aging Neurosci. 2022;14:934406. [28] ROGGIO F, PETRIGNA L, TROVATO B, et al. The role of lubricin, irisin and exercise in the prevention and treatment of osteoarthritis. Int J Mol Sci. 2023;24(6):5126. [29] LV J, ZHANG H, CHEN N. Exercise Mimetic Pills for Chronic Diseases Based on Autophagy. Exercise, Autophagy and Chronic Diseases. 2021: 247-260. [30] GUBERT C, HANNAN A J. Exercise mimetics: harnessing the therapeutic effects of physical activity. Nat Rev Drug Discov. 2021;20(11):862-879. |
| [1] | Zhang Qian, Huang Dongfeng. Weighted gene co-expression network analysis combined with machine learning to screen and validate biomarkers for osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1096-1105. |
| [2] | Wang Zhengye, Liu Wanlin, Zhao Zhenqun. Advance in the mechanisms underlying miRNAs in steroid-induced osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1207-1214. |
| [3] | Bu Yangyang, Ning Xinli, Zhao Chen. Intra-articular injections for the treatment of osteoarthritis of the temporomandibular joint: different drugs with multiple combined treatment options [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1215-1224. |
| [4] | Zou Rongji, Yu Fangfang, Wang Maolin, Jia Zhuopeng. Triptolide inhibits ferroptosis and improves cerebral ischemia-reperfusion injury in a rat model of cerebral artery occlusion/reperfusion [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 873-881. |
| [5] | Yang Xiao, Bai Yuehui, Zhao Tiantian, Wang Donghao, Zhao Chen, Yuan Shuo. Cartilage degeneration in temporomandibular joint osteoarthritis: mechanisms and regenerative challenges [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 926-935. |
| [6] | Qi Xiang, Cao Shan, Chen Jian, Zhang Yijia, Liu Keke, Xu Zifu, Liu Wang, Fu Xiaoxiao, Yin Xiaolei. Screening of genes related to mitochondrial dysfunction and ferroptosis in atherosclerosis and target prediction of regulatory traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(10): 2641-2652. |
| [7] | Xu Fanping, Li Qinchun, Tang Dongfang. Targeting diverse chimeric antigen receptor T cell-related targets in treatment of B-cell hematological malignancies: a review of long-term follow-up data [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 248-259. |
| [8] | Yin Lu, Jiang Chuanfeng, Chen Junjie, Yi Ming, Wang Zihe, Shi Houyin, Wang Guoyou, Shen Huarui. Effect of Complanatoside A on the apoptosis of articular chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1541-1547. |
| [9] | Zhao Nannan, Li Yanjie, Qin Hewei, Zhu Bochao, Ding Huimin, Xu Zhenhua. Changes in ferroptosis in hippocampal neurons of vascular dementia model rats treated with Tongmai Kaiqiao Pill [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1401-1407. |
| [10] | Zhang Mingyang, Yang Xinling. Verbascoside inhibits Erastin-induced ferroptosis of dopaminergic nerve cell line MN9D cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1408-1413. |
| [11] | Wang Mi, Ma Shujie, Liu Yang, Qi Rui. Identification and validation of characterized gene NFE2L2 for ferroptosis in ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1466-1474. |
| [12] | Zhao Ruihua, Chen Sixian, Guo Yang, Shi Lei, Wu Chengjie, Wu Mao, Yang Guanglu, Zhang Haoheng, Ma Yong. Wen-Shen-Tong-Du Decoction promoting spinal cord injury repair in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1118-1126. |
| [13] | Gao Yang, Qin Hewei, Liu Dandan. ACSL4 mediates ferroptosis and its potential role in atherosclerotic cardiovascular disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1239-1247. |
| [14] | Xu Tianjie, Fan Jiaxin, Guo Xiaoling, Jia Xiang, Zhao Xingwang, Liu kainan, Wang Qian. Metformin exerts a protective effect on articular cartilage in osteoarthritis rats by inhibiting the PI3K/AKT/mTOR pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1003-1012. |
| [15] | Lang Mecuo, Zhang Yilin, Wang Li. MiR-338-3p affects proliferation and apoptosis of alveolar bone osteoblasts by targeting receptor activator of nuclear factor-kappaB ligand [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 899-907. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||