Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (22): 5876-5885.doi: 10.12307/2026.255
Previous Articles Next Articles
Shared genetic basis and causal relationship between nutrition, nutritional status and inflammatory bowel disease
Liao Guibin1, Wu Yixuan2, Tang Jing3, Huang Jinke1, Wang Jun1, Yan Ziqi2, Liu Shujun1, Zhang Haiyan1, 4
- 1The Second Affiliated Hospital of Guangzhou University of Chinese Medicine (Guangdong Provincial Hospital of Chinese Medicine), Guangzhou 510006, Guangdong Province, China; 2The First Clinical Medical College, Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; 3The Second Clinical Medical College, Guangzhou University of Chinese Medicine, Guangzhou 510405, Guangdong Province, China; 4Guangdong Provincial Key Laboratory of Clinical Research on Traditional Chinese Medicine Syndrome, Guangzhou 510120, Guangdong Province, China
-
Received:2025-09-17Accepted:2025-11-22Online:2026-08-08Published:2025-12-29 -
Contact:Zhang Haiyan, MD, Associate chief physician, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine (Guangdong Provincial Hospital of Chinese Medicine), Guangzhou 510006, Guangdong Province, China; Guangdong Provincial Key Laboratory of Clinical Research on Traditional Chinese Medicine Syndrome, Guangzhou 510120, Guangdong Province, China -
About author:Liao Guibin, MS, Physician, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine (Guangdong Provincial Hospital of Chinese Medicine), Guangzhou 510006, Guangdong Province, China -
Supported by:Guangdong Provincial Key Laboratory of Clinical Research on Syndromes in Traditional Chinese Medicine, No. 2023KT15486 (to ZHY); State Administration of Traditional Chinese Medicine Project, No. ZDYN-2024-A-079 (to ZHY); National Natural Science Foundation of China, No. 82400635 (to WJ)
CLC Number:
Cite this article
Liao Guibin, Wu Yixuan, Tang Jing, Huang Jinke, Wang Jun, Yan Ziqi, Liu Shujun, Zhang Haiyan. Shared genetic basis and causal relationship between nutrition, nutritional status and inflammatory bowel disease[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(22): 5876-5885.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
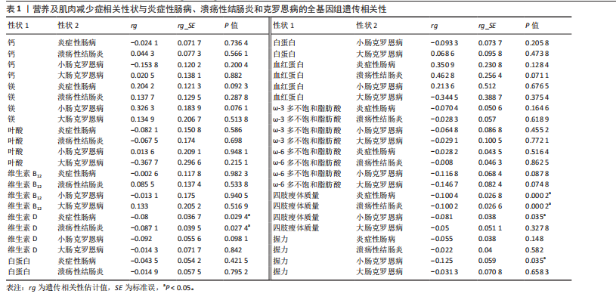
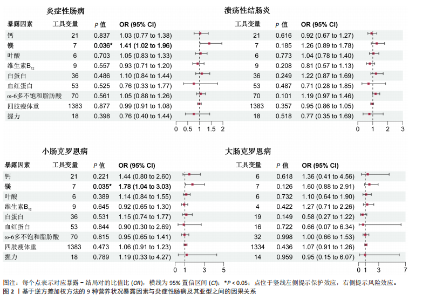
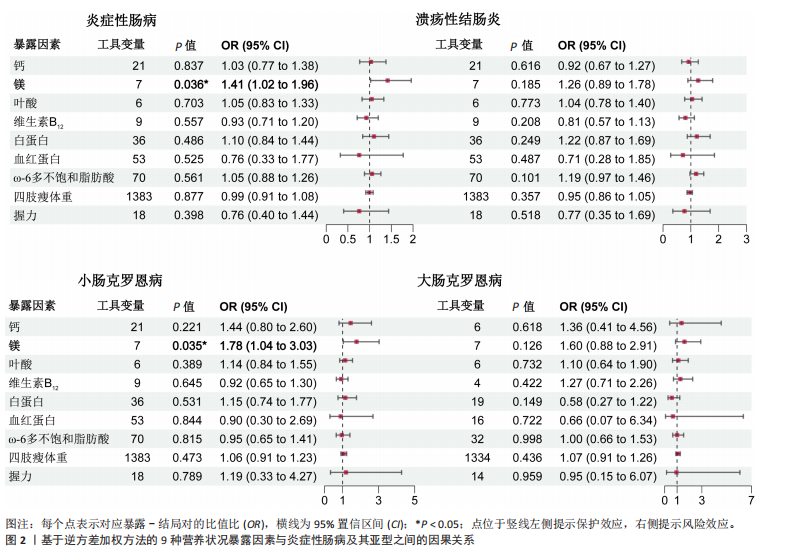
2.1 营养状况相关性状与炎症性肠病的连锁不平衡评分回归分析 为探讨营养状态与炎症性肠病及其亚型之间的遗传相关性,采用连锁不平衡评分回归方法进行评估。结果显示,维生素D与炎症性肠病之间存在显著负相关(rg=-0.080,P=0.0294),同时维生素D与溃疡性结肠炎亦呈负相关(rg=-0.0871,P=0.0274)。此外,四肢瘦体质量与炎症性肠病(rg=-0.1004,P=0.000 2)、四肢瘦体质量与溃疡性结肠炎(rg=-0.1002,P=0.000 2)、四肢瘦体质量与小肠型克罗恩病(rg=-0.081,P=0.035)、握力与小肠型克罗恩病(rg=-0.125,P=0.035)之间均存在一定程度的负向遗传相关。值得注意的是,对于部分微量营养素(如铁),由于样本量有限、单核苷酸多态性覆盖不足或原始数据质量欠佳,连锁不平衡评分回归模型难以可靠估算遗传力,遗传力与相关性结果无法计算。相关结果详见表1。 2.2 营养状况相关性状与炎症性肠病的跨表型关联分析 为识别同时影响营养状态与炎症性肠病的关键单核苷酸多态性,进行了跨表型关联分析,结果发现,维生素D与炎症性肠病之间最显著的共享单核苷酸多态性为rs9273321 (PCAPSSOC=3.348×10-10),位于HLA-DQB1基因区域内。白蛋白与炎症性肠病的显著共享单核苷酸多态性为rs4656309(PCAPSSOC=1.288×10-8),位于FCGR2A基因区域,该基因与营养不良及自身免疫疾病密切相关[34-35]。在血红蛋白与溃疡性结肠炎之间共鉴定出5个共享单核苷酸多态性,其中最显著的为rs9266237 (PCAPSSOC=1.195×10-12),位于HLA-B区域,该基因参与蛋白质代谢[36]。rs9274685为血红蛋白与炎症性肠病(PCAPSSOC=8.288×10-9)及小肠型克罗恩病(PCAPSSOC=1.599×10-9)间显著的共享单核苷酸多态性,该位点所在的区域包含HLA-DQB1,此前已被报道与初发诊断克罗恩病时具有较低血红蛋白水平以及发展为重症相关[37]。在ω-3多不饱和脂肪酸与炎症性肠病中,显著共享单核苷酸多态性为rs1979033 (PCAPSSOC=1.850×10-8),位于CCNT2基因区域;而ω-3多不饱和脂肪酸与溃疡性结肠炎之间最显著的单核苷酸多态性为rs3846730(PCAPSSOC=4.466×10-9),其位于IRF1基因区域,IRF1被认为在应对脂毒性损伤中发挥关键调控作用[38-39]。四肢瘦体质量与溃疡性结肠炎之间较为显著的共享位点为rs10060626(PCAPSSOC=4.736×10-14),位于 CARINH基因区域内,被认为参与肠道稳态调节失衡的遗传易感机制[40]。 四肢瘦体质量与小肠型克罗恩病之间的关键共享单核苷酸多态性为rs206018(PCAPSSOC=2.886×10-12),位于NOTCH4基因区域。握力与多个炎症性肠病亚型之间也发现显著共享单核苷酸多态性,其中rs2073643 (PCAPSSOC=1.920×10-11)与炎症性肠病显著相关;rs274555(PCAPSSOC=1.784×10-9)与大肠型克罗恩病相关;rs2523578 (PCAPSSOC=3.712×10-11)与溃疡性结肠炎相关;rs17799110(PCAPSSOC=4.237×10-11)则与小肠型克罗恩病相关。这些位点分别定位于SLC22A5、HLA-B与NCOA1基因区域,这些基因与骨骼肌的代谢功能密切相关[41-42]。 值得注意的是,在跨表型关联分析中共识别出702个显著单核苷酸多态性,其中164个位点尚未明确对应于已知基因。此外,未在铁、镁、叶酸、维生素B12与炎症性肠病之间,或在ω-3多不饱和脂肪酸与小肠型克罗恩病之间发现独立位点。对于大肠型克罗恩病,仅在血红蛋白、四肢瘦体质量及握力中发现具有独立关联性的单核苷酸多态性。所有纳入的单核苷酸多态性均满足以下筛选标准:单性状P < 1×10-3,跨表型关联联合分析P < 5×10-8。 2.3 营养状况相关性状与炎症性肠病的孟德尔随机化分析 双向两样本孟德尔随机化分析探讨了营养相关性状与炎症性肠病之间的潜在因果关系。工具变量的F统计量范围20.84-473.50,均值为38.25,表明研究结果不太容易受到弱工具偏倚的影响。正向孟德尔随机化逆方差加权法分析发现,每标准差(SD)镁水平的升高显著增加炎症性肠病风险(OR=1.41,95%CI:1.02-1.96,P=0.036),见图2。加权中位数法分析结果亦呈相同趋势(OR=1.289,95%CI:0.874-1.900,P=0.200),尽管结果未显示统计学显著。此外,逆方差加权法分析还发现镁与小肠型克罗恩病风险之间存在显著正向因果关联(OR=1.78,95%CI:1.04-3.03,P=0.035),加权中位数法亦支持该结果(OR=2.112,95%CI:1.022-4.365,P=0.004 4),而MR-Egger分析未显示统计学显著性(OR=2.835,95%CI:0.587-13.699,"


P=0.251)。 除镁外,其他营养或肌肉减少症相关性状与炎症性肠病及其亚型之间未观察到显著因果关系,其中铁、维生素D、ω-3多不饱和脂肪酸因缺乏工具变量无法进行正向孟德尔随机化分析。逆方差加权法与MR-Egger模型的Q统计量P值提示结果无异质性,MR-Egger截距项检验未见水平多效性(P > 0.05),Leave-one-out分析亦未发现单一位点对总体估计造成过大影响。 在反向孟德尔随机化分析中,发现多种炎症性肠病表型与白蛋白水平之间存在显著因果关联,包括炎症性肠病(Beta=-0.017,95%CI:-0.028至-0.005,P=0.006)、溃疡性结肠炎(Beta=-0.014,95%CI:-0.025至-0.004,P=0.008)、小肠型克罗恩病(Beta= -0.014,95%CI:-0.025至-0.002,P=0.031)及大肠型克罗恩病(Beta=-0.012,95%CI:-0.022至-0.001,P=0.017)。维生素D也显示出对炎症性肠病(Beta=-0.005,95%CI:-0.010至-0.000 4,P=0.034)及大肠型克罗恩病(Beta=-0.008,95%CI:-0.012至-0.004,P=0.000 3)具有保护性因果作用。此外,溃疡性结肠炎与握力之间亦观察到可信的负向因果关系(逆方差加权法:Beta=-0.014,95%CI:-0.023至-0.005,P=0.002)。分析中未检出显著的异质性与多效性,Leave-one-out分析显示结果稳健。"

| [1] BURISCH J, ZHAO M, ODES S, et al. The cost of inflammatory bowel disease in high-income settings: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol. 2023;8(5):458-492. [2] BUIE MJ, QUAN J, WINDSOR JW, et al. Global Hospitalization Trends for Crohn’s Disease and Ulcerative Colitis in the 21st Century: A Systematic Review With Temporal Analyses. Clin Gastroenterol Hepatol. 2023;21(9):2211-2221. [3] MASSIRONI S, VIGANÒ C, PALERMO A, et al. Inflammation and malnutrition in inflammatory bowel disease. Lancet Gastroenterol Hepatol. 2023;8(6):579-590. [4] CRUZ-JENTOFT AJ, SAYER AA. Sarcopenia. Lancet. 2019;393(10191):2636-2646. [5] ZHANG Y, ZHANG L, GAO X, et al. Impact of malnutrition and sarcopenia on quality of life in patients with inflammatory bowel disease: A multicentre study. J Cachexia Sarcopenia Muscle. 2023;14(6):2663-2675. [6] VALVANO M, CAPANNOLO A, CESARO N, et al. Nutrition, Nutritional Status, Micronutrients Deficiency, and Disease Course of Inflammatory Bowel Disease. Nutrients. 2023;15(17):3824. [7] VOEGTLIN M, VAVRICKA SR, SCHOEPFER AM, et al. Prevalence of anaemia in inflammatory bowel disease in Switzerland: a cross-sectional study in patients from private practices and university hospitals. J Crohns Colitis. 2010;4(6):642-648. [8] GODALA M, GASZYŃSKA E, WALCZAK K, et al. Evaluation of Albumin, Transferrin and Transthyretin in Inflammatory Bowel Disease Patients as Disease Activity and Nutritional Status Biomarkers. Nutrients. 2023;15(15):3479. [9] ZHENG Y, LIAO Y, OUYANG Y, et al. The effects and predictive value of calcium and magnesium concentrations on nutritional improvement, inflammatory response and diagnosis in patients with Crohn’s disease. J Hum Nutr Diet. 2023;36(5):1649-1660. [10] ALGIERI F, RODRIGUEZ-NOGALES A, GARRIDO-MESA J, et al. Intestinal anti-inflammatory activity of calcium pyruvate in the TNBS model of rat colitis: Comparison with ethyl pyruvate. Biochem Pharmacol. 2016;103:53-63. [11] CHA KH, YANG JS, KIM KA, et al. Improvement in host metabolic homeostasis and alteration in gut microbiota in mice on the high-fat diet: A comparison of calcium supplements. Food Res Int. 2020; 136:109495. [12] OPSTELTEN JL, LEENDERS M, DIK VK, et al. Dairy Products, Dietary Calcium, and Risk of Inflammatory Bowel Disease: Results From a European Prospective Cohort Investigation. Inflamm Bowel Dis. 2016;22(6):1403-1411. [13] CHEN YH, WANG L, FENG SY, et al. The Relationship between C-Reactive Protein/Albumin Ratio and Disease Activity in Patients with Inflammatory Bowel Disease. Gastroenterol Res Pract. 2020;2020:3467419. [14] PEDERSEN M, CROMWELL J, NAU P. Sarcopenia is a Predictor of Surgical Morbidity in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017;23(10):1867-1872. [15] ZHU Z, HASEGAWA K, CAMARGO CA JR, et al. Investigating asthma heterogeneity through shared and distinct genetics: Insights from genome-wide cross-trait analysis. J Allergy Clin Immunol. 2021;147(3):796-807. [16] CRUZ-JENTOFT AJ, BAHAT G, BAUER J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16-31. [17] DENNIS JK, SEALOCK JM, STRAUB P, et al. Clinical laboratory test-wide association scan of polygenic scores identifies biomarkers of complex disease. Genome Med. 2021;13(1):6. [18] MANOUSAKI D, MITCHELL R, DUDDING T, et al. Genome-wide Association Study for Vitamin D Levels Reveals 69 Independent Loci. Am J Hum Genet. 2020;106(3):327-337. [19] MBATCHOU J, BARNARD L, BACKMAN J, et al. Computationally efficient whole-genome regression for quantitative and binary traits. Nat Genet. 2021;53(7):1097-1103. [20] DAVYSON E, SHEN X, GADD DA, et al. Metabolomic Investigation of Major Depressive Disorder Identifies a Potentially Causal Association With Polyunsaturated Fatty Acids. Biol Psychiatry. 2023;94(8):630-639. [21] PEI YF, LIU YZ, YANG XL, et al. The genetic architecture of appendicular lean mass characterized by association analysis in the UK Biobank study. Commun Biol. 2020; 3(1):608. [22] JUNG H, JUNG HU, BAEk EJ, et al. Integration of risk factor polygenic risk score with disease polygenic risk score for disease prediction. Commun Biol. 2024;7(1):180. [23] BULIK-SULLIVAN BK, LOH PR, FINUCANE HK, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015; 47(3):291-295. [24] FINUCANE HK, BULIK-SULLIVAN B, GUSEV A, et al. Partitioning heritability by functional annotation using genome-wide association summary statistics. Nat Genet. 2015;47(11):1228-1235. [25] AUTON A, BROOKS LD, DURBIN RM, et al. A global reference for human genetic variation. Nature. 2015;526(7571):68-74. [26] WIELSCHER M, AMARAL AFS, VAN DER PLAAT D, et al. Genetic correlation and causal relationships between cardio-metabolic traits and lung function impairment. Genome Med. 2021; 13(1):104. [27] ZHU X, FENG T, TAYO BO, et al. Meta-analysis of correlated traits via summary statistics from GWASs with an application in hypertension. Am J Hum Genet. 2015; 96(1):21-36. [28] PURCELL S, NEALE B, TODD-BROWN K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559-575. [29] CHOU MH, YANG YK, WANG JD, et al. The Association of Serum and Dietary Magnesium with Depressive Symptoms. Nutrients. 2023;15(3):774. [30] PIERCE BL, AHSAN H, VANDERWEELE TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. 2011;40(3):740-752. [31] LAWLOR DA, HARBORD RM, STERNE JA, et al. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133-1163. [32] BOWDEN J, DAVEY SMITH G, BURGESS S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512-525. [33] BOWDEN J, DAVEY SMITH G, HAYCOCK PC, et al. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016; 40(4):304-314. [34] WEERSMA RK, CRUSIUS JB, ROBERTS RL, et al. Association of FcgR2a, but not FcgR3a, with inflammatory bowel diseases across three Caucasian populations. Inflamm Bowel Dis. 2010;16(12):2080-2089. [35] RAN Y, WU K, HU C, et al. Downregulated APOD and FCGR2A correlates with immune infiltration and lipid-induced symptoms of irritable bowel syndrome. Sci Rep. 2023; 13(1):14211. [36] EL YOUSFI M, BREUILLÉ D, PAPET I, et al. Increased tissue protein synthesis during spontaneous inflammatory bowel disease in HLA-B27 rats. Clin Sci (Lond). 2003;105(4):437-446. [37] KARINEN H, KÄRKKÄINEN P, PIHLAJAMÄKI J, et al. Gene dose effect of the DQB1*0201 allele contributes to severity of coeliac disease. Scand J Gastroenterol. 2006;41(2):191-199. [38] ROJAS-CRUZ AF, MARTÍN-JIMÉNEZ CA, GONZÁLEZ J, et al. Palmitic Acid Upregulates Type I Interferon-Mediated Antiviral Response and Cholesterol Biosynthesis in Human Astrocytes. Mol Neurobiol. 2023; 60(8):4842-4854. [39] FAN X, LI Q, WANG Y, et al. Non-canonical NF-κB contributes to endothelial pyroptosis and atherogenesis dependent on IRF-1. Transl Res. 2023;255:1-13. [40] MA H, HU T, TAO W, et al. A lncRNA from an inflammatory bowel disease risk locus maintains intestinal host-commensal homeostasis. Cell Res. 2023;33(5):372-388. [41] NOVAKOVA K, TÖRÖK M, PANAJATOVIC M, et al. PGC-1α and MEF2 Regulate the Transcription of the Carnitine Transporter OCTN2 Gene in C2C12 Cells and in Mouse Skeletal Muscle. Int J Mol Sci. 2022;23(20): 12304. [42] DUTEIL D, CHAMBON C, ALI F, et al. The transcriptional coregulators TIF2 and SRC-1 regulate energy homeostasis by modulating mitochondrial respiration in skeletal muscles. Cell Metab. 2010;12(5):496-508. [43] PÉREZ-JELDRES T, BUSTAMANTE ML, SEGOVIA-MELERO R, et al. Genotype Prevalence of Lactose Deficiency, Vitamin D Deficiency, and the Vitamin D Receptor in a Chilean Inflammatory Bowel Disease Cohort: Insights from an Observational Study. Int J Mol Sci. 2023;24(19):14866. [44] TOPALOVA-DIMITROVA A, DIMITROV IV, NIKOLOV R. Lower vitamin D levels are associated with the pathogenesis of inflammatory bowel diseases. Medicine (Baltimore). 2023;102(41):e35505. [45] SONG F, LU J, CHEN Z, et al. Vitamin D and CRP are associated in hospitalized inflammatory bowel disease (IBD) patients in Shanghai. Asia Pac J Clin Nutr. 2024;33(3):370-380. [46] LI Y, GUO Y, GENG C, et al. Vitamin D/vitamin D receptor protects intestinal barrier against colitis by positively regulating Notch pathway. Front Pharmacol. 2024;15:1421577. [47] GAO H, ZHOU H, ZHANG Z, et al. Vitamin D3 alleviates inflammation in ulcerative colitis by activating the VDR-NLRP6 signaling pathway. Front Immunol. 2023; 14:1135930. [48] WU Z, MA B, XIAO M, et al. Vitamin D Modified DSS-Induced Colitis in Mice via STING Signaling Pathway. Biology (Basel). 2025;14(6):715. [49] KARIMI S, TABATABA-VAKILI S, YARI Z, et al. The effects of two vitamin D regimens on ulcerative colitis activity index, quality of life and oxidant/anti-oxidant status. Nutr J. 2019;18(1):16. [50] AHAMED Z R, DUTTA U, SHARMA V, et al. Oral Nano Vitamin D Supplementation Reduces Disease Activity in Ulcerative Colitis: A Double-Blind Randomized Parallel Group Placebo-controlled Trial. J Clin Gastroenterol. 2019;53(10):e409-e415. [51] VALVANO M, MAGISTRONI M, CESARO N, et al. Effectiveness of Vitamin D Supplementation on Disease Course in Inflammatory Bowel Disease Patients: Systematic Review With Meta-Analysis. Inflamm Bowel Dis. 2024;30(2):281-291. [52] WALLACE C, GORDON M, SINOPOULOU V, et al. Vitamin D for the treatment of inflammatory bowel disease. Cochrane Database Syst Rev. 2023;10(10):CD011806. [53] GIUSTINA A, BILEZIKIAN JP, ADLER RA, et al. Consensus Statement on Vitamin D Status Assessment and Supplementation: Whys, Whens, and Hows. Endocr Rev. 2024;45(5):625-654. [54] HAMILTON FW, HUGHES DA, SPILLER W, et al. Non-linear Mendelian randomization: detection of biases using negative controls with a focus on BMI, Vitamin D and LDL cholesterol. Eur J Epidemiol. 2024;39(5):451-465. [55] GRANT RK, JONES GR, PLEVRIS N, et al. Validation of the ACE [Albumin, CRP, and Endoscopy] Index in Acute Colitis: Analysis of the CONSTRUCT dataset. J Crohns Colitis. 2024;18(2):286-290. [56] ZHENG J, FAN Z, LI C, et al. Predictors for colectomy in patients with acute severe ulcerative colitis: a systematic review and meta-analysis. BMJ Open Gastroenterol. 2024;11(1):e001587. [57] MUNDHRA SK, MADAN D, GOLLA R, et al. Intravenous Albumin Infusion Does not Augment the Response Rate to a Combination of Exclusive Enteral Nutrition and Intravenous Steroids in Acute Severe Ulcerative Colitis: A Randomised Controlled Trial. J Crohns Colitis. 2024;18(11):1870-1878. [58] SUN Q, YU Z, LUO L, et al. Modulation of Inflammation Levels and the Gut Microbiota in Mice with DSS-Induced Colitis by a Balanced Vegetable Protein Diet. Plant Foods Hum Nutr. 2024;80(1):19. [59] CHEN J, RUAN X, YUAN S, et al. Antioxidants, minerals and vitamins in relation to Crohn’s disease and ulcerative colitis: A Mendelian randomization study. Aliment Pharmacol Ther. 2023;57(4):399-408. [60] CUI J, LI Y, JIAO C, et al. Improvement of magnesium isoglycyrrhizinate on DSS-induced acute and chronic colitis. Int Immunopharmacol. 2021;90:107194. [61] DEL CHIERICO F, TRAPANI V, PETITO V, et al. Dietary Magnesium Alleviates Experimental Murine Colitis through Modulation of Gut Microbiota. Nutrients. 2021;13(12):4188. [62] JAHNEN-DECHENT W, KETTELER M. Magnesium basics. Clin Kidney J. 2012; 5(Suppl 1):i3-i14. [63] DUNCAN A, TALWAR D, MCMILLAN DC, et al. Quantitative data on the magnitude of the systemic inflammatory response and its effect on micronutrient status based on plasma measurements. Am J Clin Nutr. 2012;95(1):64-71. [64] CHO JM, YANG HR. Hair Mineral and Trace Element Contents as Reliable Markers of Nutritional Status Compared to Serum Levels of These Elements in Children Newly Diagnosed with Inflammatory Bowel Disease. Biol Trace Elem Res. 2018; 185(1):20-29. [65] JIN L, CHEN C, LI Y, et al. A Biodegradable Mg-Based Alloy Inhibited the Inflammatory Response of THP-1 Cell-Derived Macrophages Through the TRPM7-PI3K-AKT1 Signaling Axis. Front Immunol. 2019; 10:2798. [66] FENG Y, FENG W, XU M, et al. Sarcopenia and treatment failure in inflammatory bowel disease: a systematic review and meta-analysis. Rev Esp Enferm Dig. 2024;116(2):68-76. [67] KOHLI I, THIND N, BHALLA A, et al. Sarcopenia is associated with worse outcomes in patients with inflammatory bowel disease: insights from US national hospitalization data. Eur J Gastroenterol Hepatol. 2025;37(1):55-61. [68] CHAVEZ C, LIN K, MALVEAUX A, et al. IRF1 cooperates with ISGF3 or GAF to form innate immune de novo enhancers in macrophages. Sci Signal. 2025;18(868): eado8860. [69] RAVI SUNDAR JOSE GEETHA A, FISCHER K, BABADEI O, et al. Dynamic control of gene expression by ISGF3 and IRF1 during IFNβ and IFNγ signaling. EMBO J. 2024;43(11):2233-2263. [70] TAN G, HUANG C, CHEN J, et al. An IRF1-dependent Pathway of TNFα-induced Shedding in Intestinal Epithelial Cells. J Crohns Colitis. 2022;16(1):133-142. [71] XU X, LV X, ZENG R, et al. Elevated levels of IRF1 and CASP1 as pyroptosis-related biomarkers for intestinal epithelial cells in Crohn’s disease. Front Immunol. 2025; 16:1551547. [72] PINTO-SANCHEZ MI, BLOM JJ, GIBSON PR, et al. Nutrition Assessment and Management in Celiac Disease. Gastroenterology. 2024; 167(1):116-131.e1. [73] DE LA CONCHA EG, FERNANDEZ-ARQUERO M, SANTA-CRUZ S, et al. Positive and negative associations of distinct HLA-DR2 subtypes with ulcerative colitis (UC). Clin Exp Immunol. 1997;108(3):392-395. [74] WANG Y, ZHANG F, YAO B,et al. Notch4 participates in mesenchymal stem cell-induced differentiation in 3D-printed matrix and is implicated in eccrine sweat gland morphogenesis. Burns Trauma. 2023;11:tkad032. [75] SHEKHAWAT PS, SRINIVAS SR, MATERN D, et al. Spontaneous development of intestinal and colonic atrophy and inflammation in the carnitine-deficient jvs (OCTN2(-/-)) mice. Mol Genet Metab. 2007;92(4):315-324. [76] MULLANY LK, LONARD DM, O’MALLEY BW. Wound Healing-related Functions of the p160 Steroid Receptor Coactivator Family. Endocrinology. 2021;162(3):bqaa232. |
| [1] | Liu Hongtao, Wu Xin, Jiang Xinyu, Sha Fei, An Qi, Li Gaobiao. Causal relationship between age-related macular degeneration and deep vein thrombosis: analysis based on genome-wide association study data [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1602-1608. |
| [2] | Zhou Jian, Zhang Tao, Zhou Weili, Zhao Xingcheng, Wang Jun, Shen Jie, Qian Li, Lu Ming. Effects of resistance training on quadriceps mass and knee joint function in patients with osteoporosis and sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1081-1088. |
| [3] | Sun Jiahe, Shi Jipeng, Zhu Tianrui, Quan Helong, Xu Hongqi. Effect of exercise intervention in elderly individuals with sarcopenia and its comorbidities: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 997-1007. |
| [4] | He Qiwang, , , Chen Bo, Liang Fuchao, Kang Zewei, Zhou Yuan, Ji Anxu, Tang Xialin, . Relationship between Alzheimer’s disease and sarcopenia and body mass index: analysis of GWAS datasets for European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1036-1046. |
| [5] | Ding Yu, Chen Jingwen, Chen Xiuyan, Shi Huimin, Yang Yudie, Zhou Meiqi, Cui Shuai, . Circulating inflammatory proteins and myocardial hypertrophy: large sample analysis of European populations from GWAS Catalog and FinnGen databases [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1047-1057. |
| [6] | Li Guangzheng, Li Wei, Zhang Bochun, Ding Haoqin, Zhou Zhongqi, Li Gang, Liang Xuezhen. A prediction model for sarcopenia in postmenopausal women: information analysis based on the China Health and Retirement Longitudinal Study database [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 849-857. |
| [7] | Liu Chu, Qiu Boyuan, Tong Siwen, He Linyuwei, Chen Haobo, Ou Zhixue. A genetic perspective reveals the relationship between blood metabolites and osteonecrosis: an analysis of information from the FinnGen database in Finland [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 785-794. |
| [8] | Huang Zhe, Shang Baoling, Yao Gengzhen, Pan Guangming. Association between immune cells and cardiovascular disease risk: a genome-wide association study in European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(23): 5992-5999. |
| [9] | Zhang Zheng, Zhang Yibo, Xu Bin, Yan Shichao, Guo Hui. Sarcopenia and non-alcoholic fatty liver disease: analysis of the gut microbiota [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(23): 6000-6009. |
| [10] | Yin Xingxiao, Jiang Yang, Song Yanping, Yao Na, Shen Zhen, Li Yanqi, Song Yueyu, Peng Hao, Chen Qigang. Association between sarcopenia and osteoporosis: a genome-wide data analysis in European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(23): 6030-6039. |
| [11] | Wei Wei, Liu Hongfei, Qi Xiaonan, Liu Yantong, Wang Deyu, Yu Zhitong, Qiao Chunlin, Wang Shixuan, Teng Hai. Gushukang Granule-containing drug serum improves dexamethasone-induced atrophy of C2C12 myotubes via regulating mitochondrial homeostasis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(22): 5682-5693. |
| [12] | Wang Siwei, Yao Xiaosheng, Qi Xiaonan, Wang Yu, Cui Haijian, Zhao Jiaxuan. Matrix metalloproteinase 9 mediates mitophagy to regulate osteogenesis and myogenesis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(18): 4557-4567. |
| [13] | Han Jie, Yao Guojun, Huang Yebao, Xu Zhiwei, Shao Weigang, Shang Kebin, Wu Yachao, Liao Zhen. Genetic structure of co-morbidity between frailty and rheumatoid arthritis: a genome-wide association analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(17): 4548-4556. |
| [14] | Liang Liang, Yan Yulu, Zheng Yang, Zhang Xiaoyun, Wang Lei, Qi Wen . Lactylation-related potential targets and Chinese herbal medicine active ingredients targeting treatment of spinal cord injury: GEO database screening analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(12): 3156-3170. |
| [15] | Wang Tao, Min Youjiang, Wang Min, Wang Shunpu, Li Le, Zhang Chen, Xiao Weiping, Yu Yiping. Causal relationship between gut microbiota and amyotrophic lateral sclerosis: sample analysis from the IEU Open GWAS Database [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(12): 3182-3189. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||