Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (12): 3156-3170.doi: 10.12307/2026.709
Previous Articles Next Articles
Lactylation-related potential targets and Chinese herbal medicine active ingredients targeting treatment of spinal cord injury: GEO database screening analysis
Liang Liang1, Yan Yulu1, Zheng Yang1, Zhang Xiaoyun2, Wang Lei1, Qi Wen1
- 1Faculty of Chinese Medicine Science, Guangxi University of Chinese Medicine, Nanning 530222, Guangxi Zhuang Autonomous Region, China; 2Ruikang Hospital Affiliated to Guangxi University of Chinese Medicine, Nanning 530011, Guangxi Zhuang Autonomous Region, China
-
Received:2025-05-24Accepted:2025-08-15Online:2026-04-28Published:2025-09-30 -
Contact:Qi Wen, PhD, Professor, Faculty of Chinese Medicine Science, Guangxi University of Chinese Medicine, Nanning 530222, Guangxi Zhuang Autonomous Region, China -
About author:Liang Liang, Faculty of Chinese Medicine Science, Guangxi University of Chinese Medicine, Nanning 530222, Guangxi Zhuang Autonomous Region, China -
Supported by:Research Project of Faculty of Chinese Medicine Science, Guangxi University of Chinese Medicine, No. 2024ZZA001 (to QW); Innovation and Entrepreneurship Training Program for College Students at Sainz New School of Medicine, Guangxi University of Chinese Medicine, No. 202513643001 (to YYL)
CLC Number:
Cite this article
Liang Liang, Yan Yulu, Zheng Yang, Zhang Xiaoyun, Wang Lei, Qi Wen . Lactylation-related potential targets and Chinese herbal medicine active ingredients targeting treatment of spinal cord injury: GEO database screening analysis[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(12): 3156-3170.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
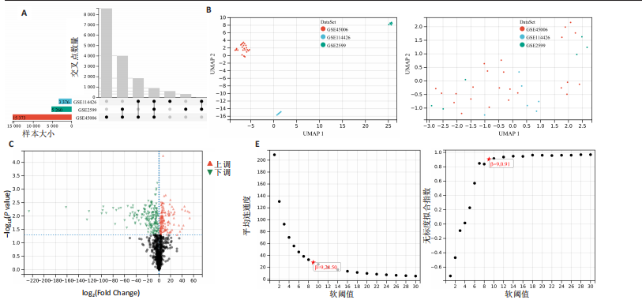
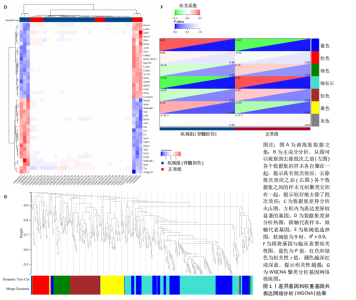
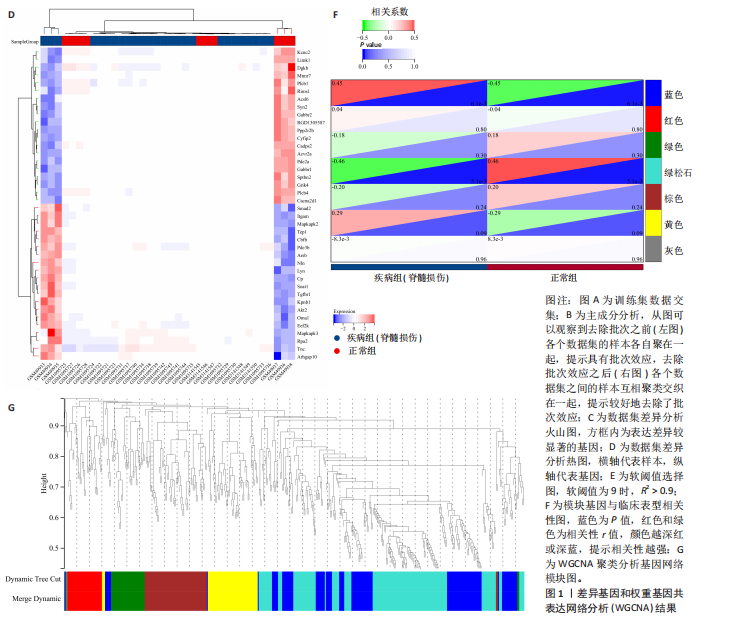
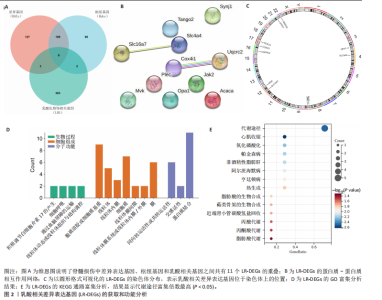
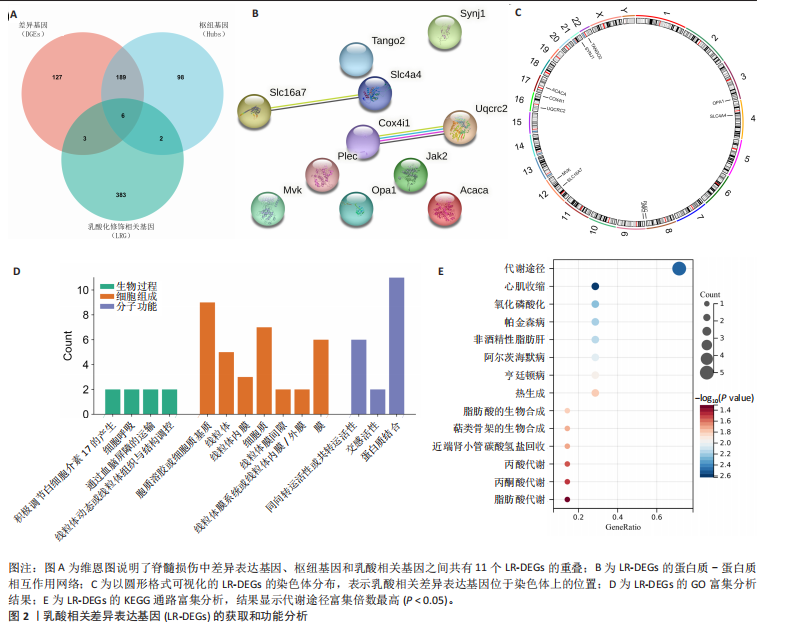
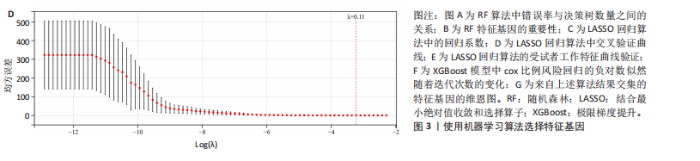
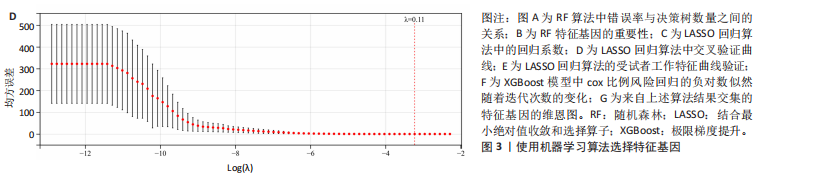
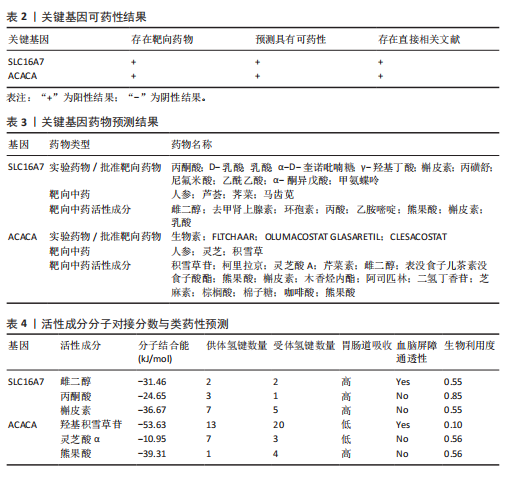
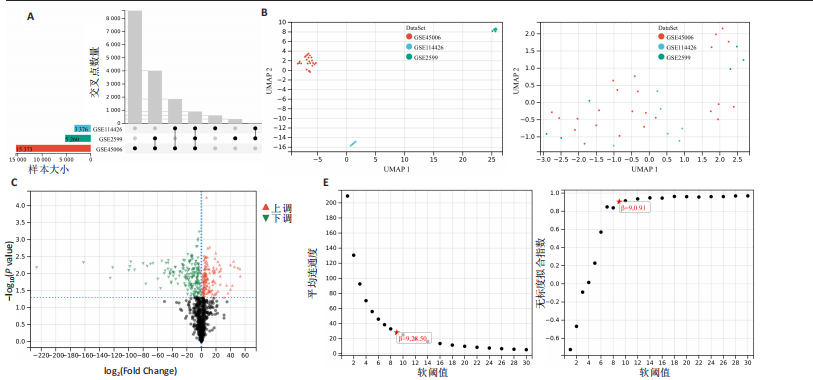
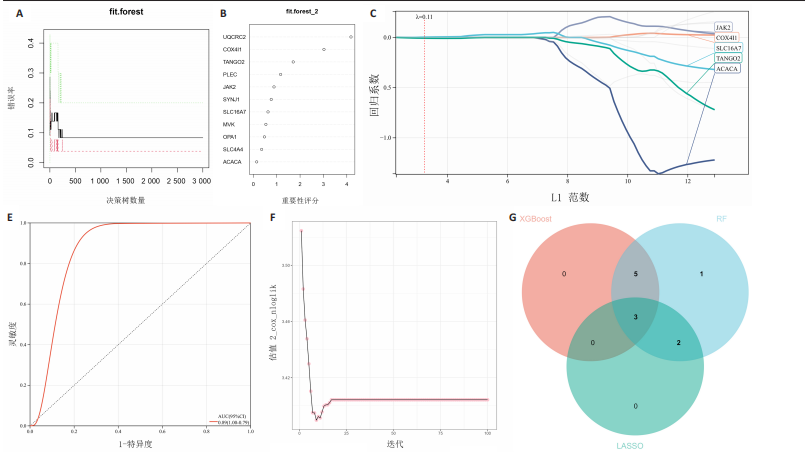
2.1 差异基因和WGCNA分析结果 主成分分析证实合并数据集(训练集)成功去除了批量效应(图1A,B)。在正常组和脊髓损伤组之间共鉴定出325个差异表达基因,其中187个基因下调,138个基因上调。结果以火山图和热图可视化(图1C,D)。通过WGCNA的尺度独立和平均连通比较发现,基因间联系的软阈值为9(图1E)。确定了7个不同的模块(图1F,G)。将模块成员关系阈值设置为0.8,基因显著性阈值设置为0.1,权重阈值设置为0.1,共提取79个与脊髓损伤相关的枢纽基因以供进一步分析。 2.2 乳酸相关差异表达基因的鉴定和功能富集分析 通过交叉差异表达基因、枢纽基因和乳酸相关基因,鉴定了11个LR-DEGs(图2A)。蛋白质-蛋白质相互作用网络显示SLC16A7和SLC4A4,COX4I1和Uqcrc2存在相互作用(图2B)。这些基因分布在3,4,8,9,12,16,17,21,22号染色体上(图2C)。GO分析表明,LR-DEGs主要与线粒体、细胞质及细胞呼吸、蛋白质合成相关(图2D)。KEGG通路分析显示,代谢途径、氧化磷酸化、脂肪酸生物合成、近端微管二碳酸酯代谢、丙烷代谢、丙酮酸代谢、脂肪酸代谢等通路显著富集(图2E)。 2.3 使用机器学习算法筛选特征基因 采用3种不同的机器学习算法来筛选关键的乳酸相关差异表达基因。RF确定了11个相对重要的基因(图3A,"
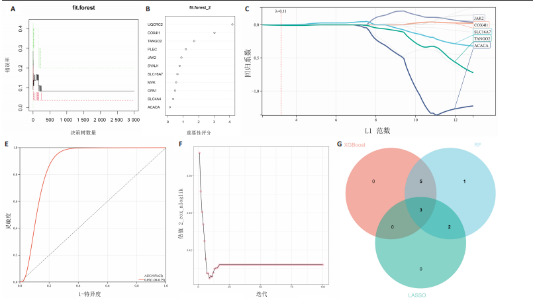
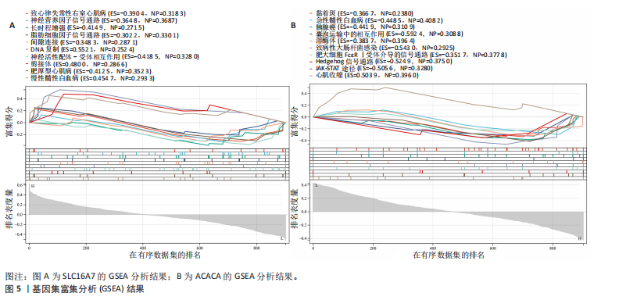
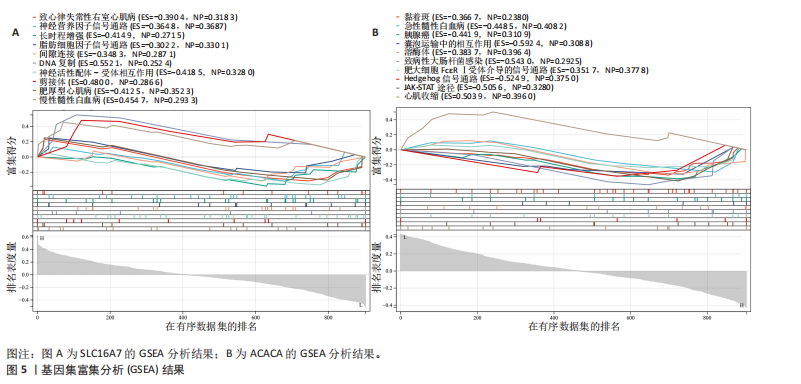
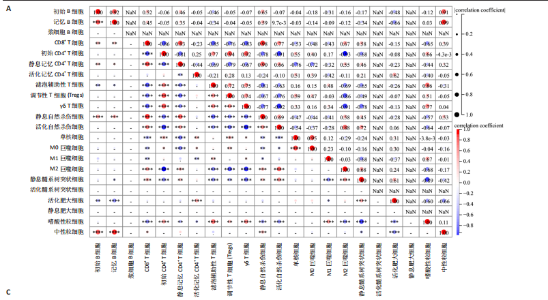
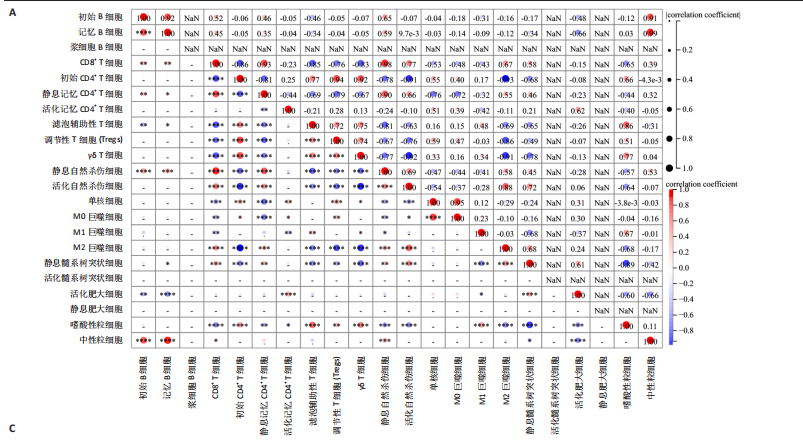
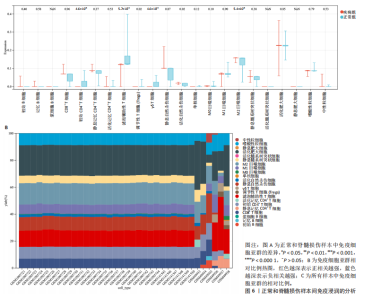
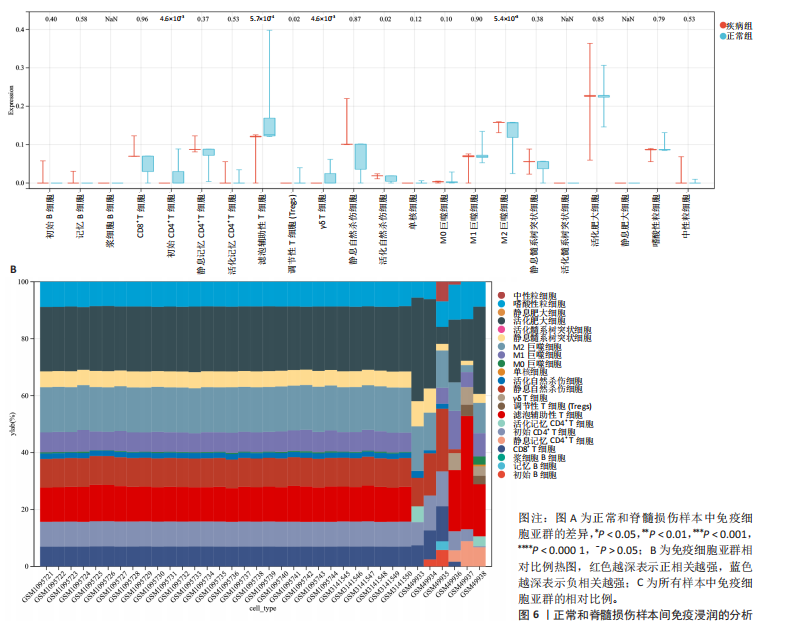
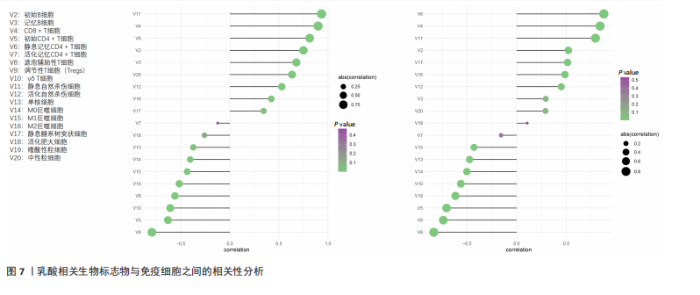
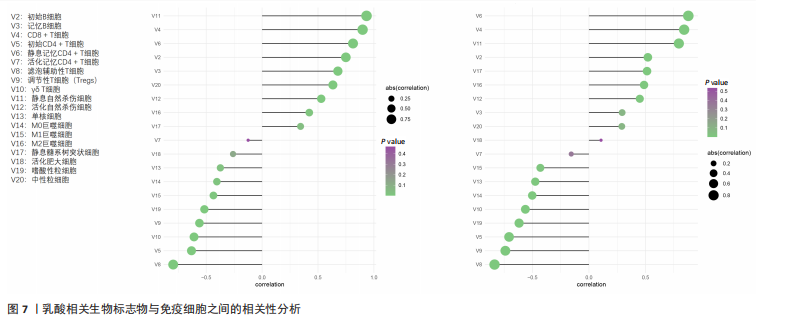
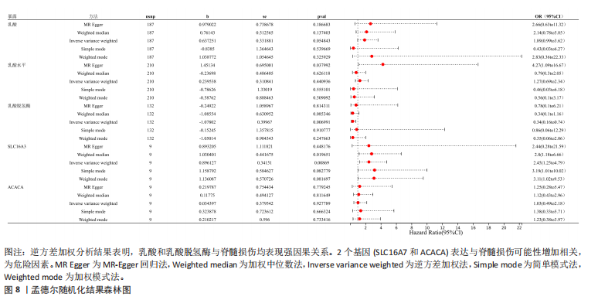
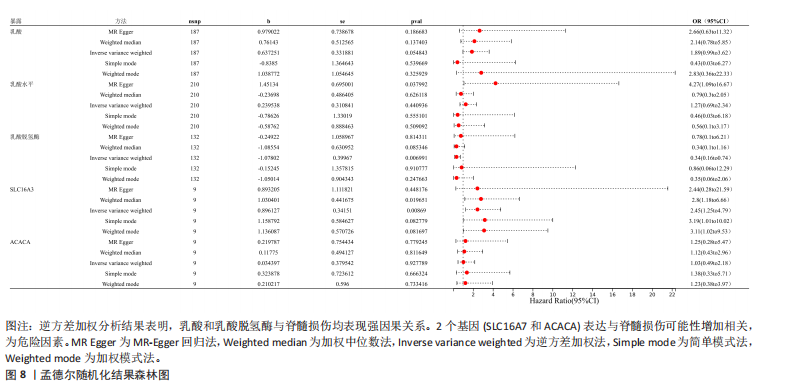
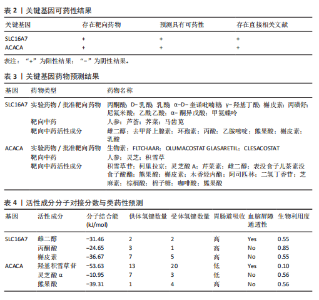
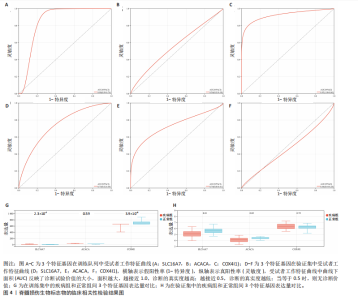
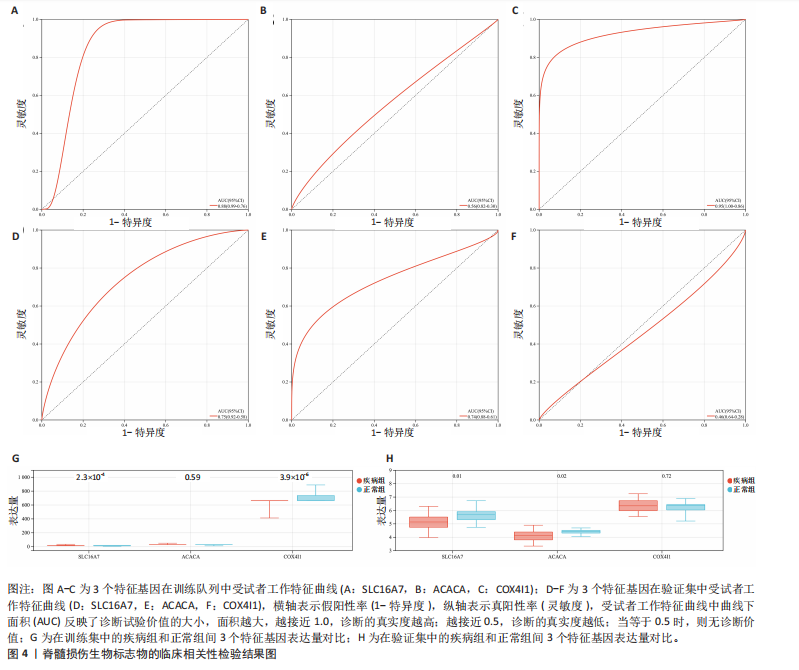
B)。LASSO回归从LR-DEGs中预测了5个基因,受试者工作特征曲线的曲线下面积为0.89(图3C-E)。XGBoost算法鉴定了8个基因(图3F)。通过使用维恩图确定所有3种方法的共同基因,得出3个特征基因SLC16A7、ACACA和COX4I1(图3G)。 2.4 脊髓损伤生物标志物的临床相关性检验 为了进一步研究已鉴定的生物标志物与脊髓损伤的联系。通过受试者工作特征曲线验证了模型的性能和准确性(图4A-F)。其中,SLC16A7和ACACA曲线下面积值均高于0.5,突出了它们对脊髓损伤出色的诊断潜力。COX4I1在验证集中并未表现出具有出色的诊断能力(曲线下面积未高于0.5),这可能与GEO数据集中同一基因的表达组织不同而导致的结果,其诊断能力需要进一步研究验证。同时,评估了此研究使用的数据集中SLC16A7、ACACA和COX4I1的表达,包括训练队列和单独的验证队列。SLC16A7和ACACA表达的趋势在所有数据集中保持一致,但COX4I1表达的趋势未保持一致(图4G,H),故在后续分析中将其排除。只选择SLC16A7和ACACA为关键基因以进行后续分析。 2.5 单基因GSEA分析 为探讨SLC16A7和ACACA在脊髓损伤机制中的作用,进行了单基因GSEA分析。选择KEGG通路富集以揭示了其与关键通路的关联,通路包括“各类信号通路”和“神经活性物质与受体相互作用”等(图5A,B)。 2.6 免疫浸润分析 使用TIMER2.0 (http://timer.cistrome.org/)平台以CIBERSORT算法分析了免疫细胞的组成(图6A,B)。评估训练集中免疫细胞的表达水平(图6C)。值得注意的是,4种免疫细胞类型,包括初始CD4+ T细胞、滤泡辅助性T细胞、γδ T细胞和M2巨噬细胞在脊髓损伤样本之间的丰度显著不同。进一步分析揭示了关键基因(SLC16A7和ACACA)与特异性免疫细胞(尤其是T细胞和M2巨噬细胞)之间的强相关性(图7)。 2.7 双样本孟德尔随机化分析 乳酸[Lactate:OR=1.89;95%CI(0.99-3.62);P=0.054 843]和乳酸脱氢酶[Lactate dehydrogenas:OR=0.34;95%CI (0.16,0.74);P=0.006 991]与脊髓损伤均表现强因果关系。2个基因(SLC16A7和ACACA)的OR值大于1,表明基因表"
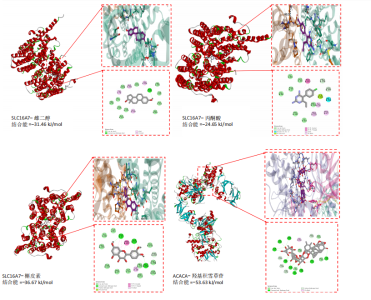
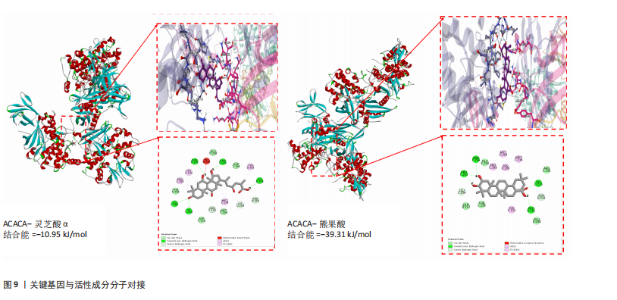
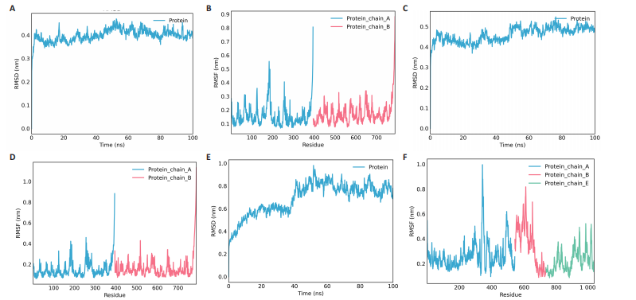
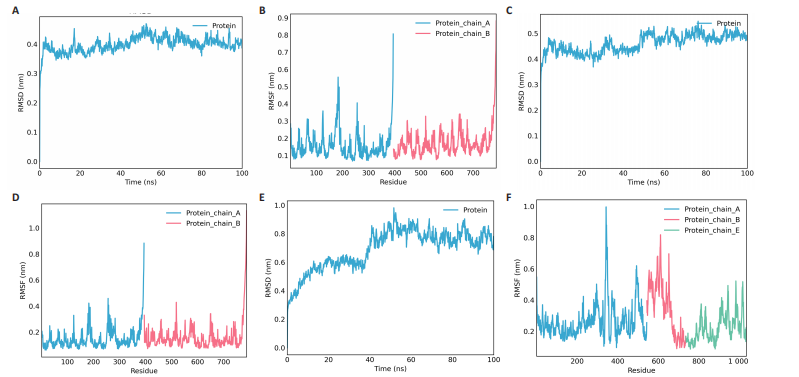
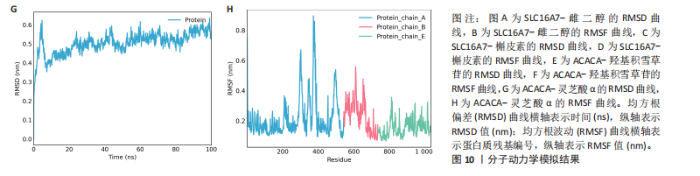
达与脊髓损伤可能性增加相关,为危险因素。敏感性分析显示因果效应的漏斗图近似对称。留一法分析表明,在去除每个单核苷酸多态性后对剩余的单核苷酸多态性进行孟德尔随机化分析会产生一致的结果,表明这一发现的稳健性,见图8。 2.8 关键基因可药性分析 SLC16A7和ACACA均满足得分> 60,具有潜在可药性,见表2。D-乳酸、α-D-喹啉并吡喃糖、尼氟米卡酸、乙酰乙酸和α-酮异戊酸11种实验室药物靶向SLC16A7,靶向ACACA的实验室药物有生物素等,见表3。在相关研究中提到多巴胺也是靶向SLC16A7的潜在药物 [12,19]。 故而通过靶向SLC16A7、ACACA或者其他相关基因的药物帮助脊髓损伤恢复具有一定可行性。同时研究检索到SLC16A7和ACACA与脊髓损伤有显著关系[20]。综上,SLC16A7和ACACA具有成为研究防治脊髓损伤药物的潜在靶点。 2.9 基于脊髓损伤关键基因筛选活性成分及潜在中药 基于ITCM数据库和HERB数据库的检索结果,发现与SLC16A7相关的活性成分有8种,与之相关的中药有4味。与ACACA相关的活性成分有16种,与之相关的中药有3味(表3)。其中,关键基因(SLC16A7和ACACA)与活性成分的分子对接分数前3名化合物组合分别是雌二醇(Estradiol)、丙酮酸(pyruvate,PYR)、槲皮素(quercetin)与SLC16A7;马德卡糖苷(madecassoside)、灵芝酸α(ganodericacidα)、熊果酸(augustic-acid)与ACACA,见图9和表4。 同时对关键基因(SLC16A7和ACACA)与活性成分的分子对接分数前2名化合物组合进行分子动力学模拟,均方根偏差(root mean square deviation,RMSD)曲线表明SLC16A7- Estradiol在5 ns后开始趋于稳定;SLC16A7-quercetin在5 ns后开始趋于稳定;ACACA-madecassoside在50 ns后开始趋于稳定;ACACA-ganodericacidα在30 ns后开始趋于稳定。均方根波动(root mean square fluctuation,RMSF)图表明4对核心成分的峰值范围均在0.01-1.00 nm之间,表明SLC16A7和ACACA在特定压力和温度下十分稳定。总之分子动力学模拟有力地支持了对接结果的有效性,见图10。"
| [1] MCDONALD JW, SADOWSKY C. Spinal-cord injury. Lancet. 2002;359(9304):417-425. [2] ANJUM A, YAZID MD, FAUZI DAUD M, et al. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int J Mol Sci. 2020; 21(20):7533. [3] HU X, XU W, REN Y, et al. Spinal cord injury: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2023;8(1):245. [4] GOLESTANI A, SHOBEIRI P, SADEGHI-NAINI M, et al. Epidemiology of Traumatic Spinal Cord Injury in Developing Countries from 2009 to 2020: A Systematic Review and Meta-Analysis. Neuroepidemiology. 2022;56(4):219-239. [5] SHI Z, YUAN S, SHI L, et al. Programmed cell death in spinal cord injury pathogenesis and therapy. Cell Prolif. 2021;54(3):e12992. [6] ZHENG B, TUSZYNSKI MH. Regulation of axonal regeneration after mammalian spinal cord injury. Nat Rev Mol Cell Biol. 2023;24(6):396-413. [7] NAKAZAKI M, MORITA T, LANKFORD KL, et al. Small extracellular vesicles released by infused mesenchymal stromal cells target M2 macrophages and promote TGF-β upregulation, microvascular stabilization and functional recovery in a rodent model of severe spinal cord injury. J Extracell Vesicles. 2021;10(11):e12137. [8] XIONG W, LI C, KONG G, et al. Treg cell-derived exosomes miR-709 attenuates microglia pyroptosis and promotes motor function recovery after spinal cord injury. J Nanobiotechnology. 2022;20(1):529. [9] WANG Q, YI J, LIU H, et al. Iguratimod promotes functional recovery after SCI by repairing endothelial cell tight junctions. Exp Neurol. 2023;368:114503. [10] WANG J, YANG P, YU T, et al. Lactylation of PKM2 Suppresses Inflammatory Metabolic Adaptation in Pro-inflammatory Macrophages. Int J Biol Sci. 2022;18(16): 6210-6225. [11] ZHANG B, LI F, SHI Y, et al. Single-cell RNA sequencing integrated with bulk RNA sequencing analysis reveals the protective effects of lactate-mediated lactylation of microglia-related proteins on spinal cord injury. CNS Neurosci Ther. 2024;30(9): e70028. [12] HU X, HUANG J, LI Z, et al. Lactate promotes microglial scar formation and facilitates locomotor function recovery by enhancing histone H4 lysine 12 lactylation after spinal cord injury. J Neuroinflammation. 2024;21(1):193. [13] SANDERSON E, GLYMOUR MM, HOLMES MV, et al. Mendelian randomization. Nat Rev Methods Primers. 2022;2:6. [14] CHANG X, ZHENG W, ZHAO Y, et al. Association of Lactate with Risk of Cardiovascular Diseases: A Two-Sample Mendelian Randomization Study. Vasc Health Risk Manag. 2024;20:541-551. [15] RASOOLY D, PELOSO GM, PEREIRA AC, et al. Genome-wide association analysis and Mendelian randomization proteomics identify drug targets for heart failure. Nat Commun. 2023;14(1):3826. [16] RAIES A, TULODZIECKA E, STAINER J, et al. DrugnomeAI is an ensemble machine-learning framework for predicting druggability of candidate drug targets. Commun Biol. 2022;5(1):1291. [17] FANG S, DONG L, LIU L, et al. HERB: a high-throughput experiment- and reference-guided database of traditional Chinese medicine. Nucleic Acids Res. 2021;49(D1): D1197-D1206. [18] TIAN S, ZHANG J, YUAN S, et al. Exploring pharmacological active ingredients of traditional Chinese medicine by pharmacotranscriptomic map in ITCM. Brief Bioinform. 2023;24(2):bbad027. [19] HOEKSTRA SP, KAMIJO YI, MATSUSHITA T, et al. The acute effect of dopamine infusion on lipid and cytokine concentrations in persons with a cervical spinal cord injury-a pilot study. Spinal Cord. 2021;59(3):274-281. [20] GAGLIANI N, AMEZCUA VESELY MC, ISEPPON A, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523(7559): 221-225. [21] ZHANG D, TANG Z, HUANG H, et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019; 574(7779):575-580. [22] WANG X, FAN W, LI N, et al. YY1 lactylation in microglia promotes angiogenesis through transcription activation-mediated upregulation of FGF2. Genome Biol. 2023; 24(1):87. [23] 葛玲玲,黄洪军,罗艳.乳酰化修饰在疾病中的作用及机制研究进展[J].上海交通大学学报(医学版),2023,43(3):374-379. [24] CHEN H, LI Y, LI H, et al. NBS1 lactylation is required for efficient DNA repair and chemotherapy resistance. Nature. 2024;631(8021):663-669. [25] ORIHUELA R, MCPHERSON CA, HARRY GJ. Microglial M1/M2 polarization and metabolic states. Br J Pharmacol. 2016; 173(4):649-665. [26] JOSHI L, PLASTIRA I, BERNHART E, et al. Lysophosphatidic Acid Induces Aerobic Glycolysis, Lipogenesis, and Increased Amino Acid Uptake in BV-2 Microglia. Int J Mol Sci. 2021;22(4):1968. [27] SUHAIL H, NEMATULLAH M, RASHID F, et al. An early glycolysis burst in microglia regulates mitochondrial dysfunction in oligodendrocytes under neuroinflammation. iScience. 2023;26(10):107921. [28] DEVANNEY NA, STEWART AN, GENSEL JC. Microglia and macrophage metabolism in CNS injury and disease: The role of immunometabolism in neurodegeneration and neurotrauma. Exp Neurol. 2020;329: 113310. [29] PROTO JD, DORAN AC, GUSAROVA G, et al. Regulatory T Cells Promote Macrophage Efferocytosis during Inflammation Resolution. Immunity. 2018;49(4):666-677.e6. [30] PINHEIRO C, LONGATTO-FILHO A, SCAPULATEMPO C, et al. Increased expression of monocarboxylate transporters 1, 2, and 4 in colorectal carcinomas. Virchows Arch. 2008;452(2):139-146. [31] PERTEGA-GOMES N, VIZCAINO JR, FELISBINO S, et al. Epigenetic and oncogenic regulation of SLC16A7 (MCT2) results in protein over-expression, impacting on signalling and cellular phenotypes in prostate cancer. Oncotarget. 2015;6(25):21675-21684. [32] HALESTRAP AP. The SLC16 gene family - structure, role and regulation in health and disease. Mol Aspects Med. 2013;34(2-3): 337-349. [33] LEE I, LEE SJ, KANG WK, et al. Inhibition of monocarboxylate transporter 2 induces senescence-associated mitochondrial dysfunction and suppresses progression of colorectal malignancies in vivo. Mol Cancer Ther. 2012;11(11):2342-2351. [34] DONG J, LI M, PENG R, et al. ACACA reduces lipid accumulation through dual regulation of lipid metabolism and mitochondrial function via AMPK- PPARα- CPT1A axis. J Transl Med. 2024;22(1):196. [35] GUO X, CHEN C, LIU B, et al. Genetic variations in monocarboxylate transporter genes as predictors of clinical outcomes in non-small cell lung cancer. Tumour Biol. 2015;36(5):3931-3939. [36] CARMEL JB, GALANTE A, SOTEROPOULOS P, et al. Gene expression profiling of acute spinal cord injury reveals spreading inflammatory signals and neuron loss. Physiol Genomics. 2001;7(2):201-213. [37] LI A, GONG Z, LONG Y, et al. Lactylation of LSD1 is an acquired epigenetic vulnerability of BRAFi/MEKi-resistant melanoma. Dev Cell. 2025. doi: 10.1016/j.devcel.2025.02.016. [38] HU X, HUANG X, YANG Y, et al. Dux activates metabolism-lactylation-MET network during early iPSC reprogramming with Brg1 as the histone lactylation reader. Nucleic Acids Res. 2024;52(10):5529-5548. [39] CHEN X, WANG J, CHAN P, et al. Metabolic Reprogramming in Spinal Cord Injury and Analysis of Potential Therapeutic Targets. J Mol Neurosci. 2025;75(2):50. [40] 周逸敏,李宗洋,许翰勋,等.中药改善脊髓微环境修复血-脊髓屏障的机制研究进展[J].中国中医骨伤科杂志,2023, 31(9):80-83. [41] 郭铁峰,江朔轩,张彦军,等.微血管内皮细胞在脊髓损伤中的作用机制及中药干预脊髓损伤的研究进展[J].中国脊柱脊髓杂志,2024,34(6):647-651. [42] 钟远鸣,叶伟权,邱伟,等.中医药治疗脊髓损伤相关并发症的研究进展[J].海南医学院学报,2023,29(11):866-871. [43] SQUAIR JW, MILANO M, DE COUCY A, et al. Recovery of walking after paralysis by regenerating characterized neurons to their natural target region. Science. 2023;381(6664):1338-1345. [44] SENGELAUB DR, HAN Q, LIU NK, et al. Protective Effects of Estradiol and Dihydrotestosterone following Spinal Cord Injury. J Neurotrauma. 2018;35(6):825-841. [45] MLCEK J, JURIKOVA T, SKROVANKOVA S, et al. Quercetin and Its Anti-Allergic Immune Response. Molecules. 2016;21(5):623. [46] WANG X, FU Y, BOTCHWAY BOA, et al. Quercetin Can Improve Spinal Cord Injury by Regulating the mTOR Signaling Pathway. Front Neurol. 2022;13:905640. [47] LIPINSKI CA, LOMBARDO F, DOMINY BW, et al. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1-3):3-26. [48] 李盛华,柴喜平,王想福,等.中医药治疗脊髓损伤的研究进展[J].中国中医骨伤科杂志,2010,18(11):70-72. [49] SNG KS, LI G, ZHOU LY, et al. Ginseng extract and ginsenosides improve neurological function and promote antioxidant effects in rats with spinal cord injury: A meta-analysis and systematic review. J Ginseng Res. 2022; 46(1):11-22. [50] YUKSEL Y, GUVEN M, KAYMAZ B, et al. Effects of Aloe Vera on Spinal Cord Ischemia-Reperfusion Injury of Rats. J Invest Surg. 2016;29(6):389-398. [51] GOKCE EC, KAHVECI R, ATANUR OM, et al. Neuroprotective effects of Ganoderma lucidum polysaccharides against traumatic spinal cord injury in rats. Injury. 2015; 46(11):2146-2155. |
| [1] | Fu Lyupeng, Yu Peng, Liang Guoyan, Chang Yunbing. Electroactive materials applied in spinal surgery [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2113-2123. |
| [2] | Lai Jiaming, , Song Yuling, Chen Zixi, Wei Jinghuan, Cai Hao, , Li Guoquan, . Screening of diagnostic markers for endothelial cell Senescence in mice with radiation-induced heart disease and analysis of immune infiltration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1450-1463. |
| [3] | Yin Yongcheng, Zhao Xiangrui, Yang Zhijie, Li Zheng, Li Fang, Ning Bin. Effect and mechanism of peroxiredoxin 1 in microglial inflammation after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1106-1113. |
| [4] | Yu Huifen, Mo Licun, Cheng Leping. The position and role of 5-hydroxytryptamine in the repair of tissue injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1196-1206. |
| [5] | Yang Biao, , Wu Zhonghuan, , Jiang Fugui, , He Chenglong, , Li Tingdong, . Conductive hydrogel with cell-free fat extract repairs spinal cord injuries in rats [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(14): 3652-3662. |
| [6] | Tian Minghao, Liao Yehui, Zhou Wenyang, He Baoqiang, Leng Yebo, Xu Shicai, Zhou Jiajun, Li Yang, Tang Chao, Tang Qiang, Zhong Dejun . Neuroprotective regulation of the IRF9 gene after spinal cord injury: bioinformatics analysis combined with experimental validation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(11): 2712-2726. |
| [7] | Tian Meng, Lou Tianwei, Zhang Yongchen, Jia Hongling . Cathepsin F as a potential serum biomarker for stroke risk prediction: GWAS database data analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(10): 2662-2670. |
| [8] | Chi Wenxin, Zhang Cunxin, Gao Kai, Lyu Chaoliang, Zhang Kefeng. Mechanism by which nobiletin inhibits inflammatory response of BV2 microglia [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1321-1327. |
| [9] | Zhao Ruihua, Chen Sixian, Guo Yang, Shi Lei, Wu Chengjie, Wu Mao, Yang Guanglu, Zhang Haoheng, Ma Yong. Wen-Shen-Tong-Du Decoction promoting spinal cord injury repair in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1118-1126. |
| [10] | Wang Rongrong, Huang Yushan, Li Xiangmiao, Bai Jinzhu. Prostaglandin E1 regulates vascular-related factors and protects microcirculatory function during the acute phase of traumatic spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 958-967. |
| [11] | Liu Yani, Yang Jinghuan, Lu Huihui, Yi Yufang, Li Zhixiang, Ou Yangfu, Wu Jingli, Wei Bing . Screening of biomarkers for fibromyalgia syndrome and analysis of immune infiltration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1091-1100. |
| [12] | Yang Bin, Tao Guangyi, Yang Shun, Xu Junjie, Huang Junqing . Visualization analysis of research hotspots of artificial intelligence in field of spinal cord nerve injury and repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 761-770. |
| [13] | Zheng Yitong, Wang Yongxin, Liu Wen, Amujite, Qin Hu. Action mechanism of intrathecal transplantation of human umbilical cord mesenchymal stem cell-derived exosomes for repair of spinal cord injury under neuroendoscopy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7743-7751. |
| [14] | Guo Jia, Ren Yafeng, Li Bing, Huang Jing, Shang Wenya, Yang Yike, Liu Huiyao. Action mechanism of mesenchymal stem cell-derived exosomes carrying miRNAs in improving spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7827-7838. |
| [15] | Yang Dingyan, Yu Zhenqiu, Yang Zhongyu. Machine learning-based analysis of neutrophil-associated potential biomarkers for acute myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7909-7920. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||