Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (13): 3424-3434.doi: 10.12307/2026.325
Previous Articles Next Articles
Gene transfection technology and tissue fibrosis repair
Wu Xianyuan1, Zhang Nini1, Huang Guilin2
- 1Department of Oral and Maxillofacial Surgery, Affiliated Stomatological Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China; 2The Fifth Affiliated Hospital of Zunyi Medical University, Zhuhai 519000, Guangdong Province, China
-
Accepted:2025-07-04Online:2026-05-08Published:2025-12-26 -
Contact:Zhang Nini, MS, Associate professor, Department of Oral and Maxillofacial Surgery, Affiliated Stomatological Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China -
About author:Wu Xianyuan, Master candidate, Department of Oral and Maxillofacial Surgery, Affiliated Stomatological Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China -
Supported by:National Natural Science Foundation of China, No. 81860198 (to ZNN); National Natural Science Foundation of China, No. 81960204 (to HGL)
CLC Number:
Cite this article
Wu Xianyuan, Zhang Nini, Huang Guilin. Gene transfection technology and tissue fibrosis repair[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(13): 3424-3434.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
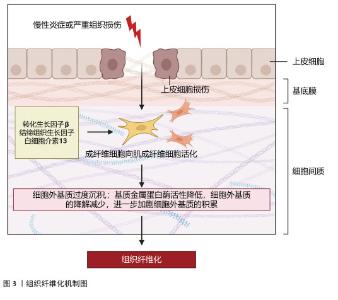
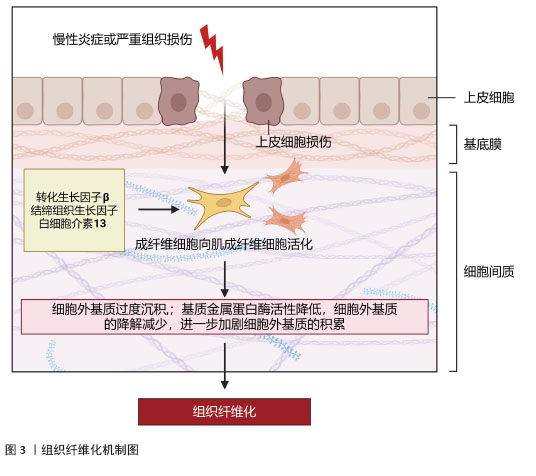
2.1 组织纤维化的机制研究与治疗策略 纤维化是一种以细胞外基质过度沉积和肌成纤维细胞异常活化为核心的病理过程,广泛发生于心脏、肝脏、肾脏等多种器官的慢性疾病终末期,最终导致器官功能衰竭。纤维化的发病机制复杂,涉及多种细胞、分子信号通路及免疫调节的失调。组织纤维化的治疗策略需针对其核心机制(如肌成纤维细胞活化、细胞外基质沉积、炎症反应等)进行多靶点干预。 2.1.1 纤维化的发病机制 (1)核心病理特征:多种内源性和外源性因素可启动机体的纤维化反应,如遗传因素、自身免疫性炎症、慢性感染、反复外源性毒物暴露、肥胖、高血压和糖尿病等。虽然在不同器官和不同的疾病背景下纤维化的致病因素不同,但纤维化过程根据病理特征可分为4个主要阶段:首先,由内源性或外源性因素引起的组织损伤引发炎症反应;其次,来自外周血和外周淋巴组织的免疫细胞被大量募集到受伤部位;第三,激活关键的纤维化效应细胞,即肌成纤维细胞;第四,细胞外基质成分的过度产生和沉积最终会导致组织纤维化及器官衰竭[1],见图3。 "
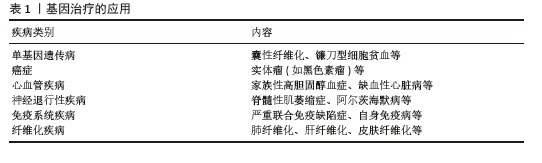
(2)关键信号通路:①转化生长因子β/Smad通路:转化生长因子β1作为主要促纤维化因子,通过激活Smad蛋白家族调控成纤维细胞向肌成纤维细胞转化[2];②非Smad依赖通路:包括磷脂酰肌醇3激酶/蛋白激酶B(phosphoinositide 3 kinase/protein kinase B,PI3K/Akt)、丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)(如p38/MAPK)和腺苷单磷酸活化蛋白激酶(AMP-activated protein kinase,AMPK)通路,参与调控氧化应激和肌成纤维细胞活化[3-5];③Wnt/β-catenin通路:促进巨噬细胞极化并刺激成纤维细胞增殖,在纤维化发病机制的中间过程中发挥重要作用[6-7];④核因子κB(nuclear factor kappa-B,NF-κB)通路:通过调控炎症反应因子,如白细胞介素1β和白细胞介素6和转化生长因子β信号传导影响关键纤维化效应细胞的激活,从而干扰纤维化发病过程[8]。 (3)免疫调节:巨噬细胞可分为两种表型:促炎型M1型和抗炎型 M2 型。M2型巨噬细胞可以分泌转化生长因子β和白细胞介素10以促进成纤维细胞转化为肌成纤维细胞。调节性T细胞是CD4+ T细胞的一个亚群,在放射性纤维化发生中起关键作用。实验发现调节性T细胞通过转化生长因子β和Wnt等细胞外信号分子参与肌成纤维细胞活化,从而促进纤维化的发生[9]。 (4)NLRP3炎性小体:NLRP3炎性小体是先天免疫系统的关键组分,通过介导白细胞介素1β/白细胞介素18分泌促进成纤维细胞向肌成纤维细胞转化,在纤维化发病中起核心作用[10]。 2.1.2 组织纤维化的分子靶向治疗 (1)靶向信号通路:最近的研究表明,miR-29家族(miR-29a,b,c)通过5’非翻译区(UTR)与靶基因mRNA的3’非翻译区配对,使靶基因的mRNA降解,完成抑制靶基因转录和翻译的生理过程。研究发现miR-29与上皮-间充质转化密切相关,miR-29通过靶向转化生长因子β/Smad、PI3K/Akt/哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)等通路抑制上皮-间充质转化和纤维化基因的表达,成为潜在治疗靶点[11]。实验发现转化生长因子β抑制剂如SB431542可阻断转化生长因子β受体,减少细胞外基质沉积[12]。 (2)调节巨噬细胞极化:通过靶向诱导血红素氧合酶1的表达或通过多功能微凝胶递送免疫调节信号诱导巨噬细胞向促再生表型转化[12]。 (3)抗氧化与抗炎通路:血红素氧合酶1作为内源性保护剂,其通过清除活性氧以抑制氧化应激,从而在一定程度上延缓了纤维化进展[13]。 (4)细胞因子作用:白细胞介素10是一种细胞因子,具有抗炎和抗纤维化作用。它可以抑制Ⅰ型和Ⅲ型胶原蛋白的产生,还可以促进基质金属蛋白酶的表达并抑制成纤维细胞向肌成纤维细胞转化。此外,白细胞介素10可促进巨噬细胞分化为再生表型,进一步发挥抗纤维化作用[3]。近年来,研究者在纤维化发生的炎性机制、免疫调控及细胞因子作用方面取得了重要进展,然而如何将这些基础研究成果转化为有效的临床治疗手段仍面临重大挑战[9]。 2.2 基因治疗现状 基因治疗是通过将外源基因引入目标细胞,利用基因本身作为治疗手段,来修复或弥补因缺陷或异常基因引起的疾病,从而实现治疗效果[14]。基因治疗可以用于治疗遗传性疾病、癌症、纤维化类疾病和自身免疫性疾病等多种疾病,见表1。"
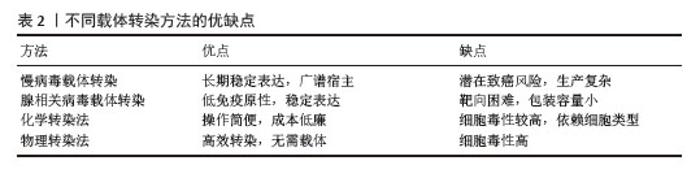
近年来,基因治疗领域发展迅猛,取得了重大突破。来自不同国家的药物监管机构已批准20多种基因疗法用于临床。基因治疗领域包括:离体基因递送与体内基因递送、基因添加与基因组编辑、体细胞基因治疗与种系基因治疗[15]。基因治疗的主要步骤包括:确定治疗靶点基因、设计和构建治疗基因载体、传递治疗基因到患者体内、确保基因的稳定性和安全性、监测治疗效果和不良反应等。常用的基因传递载体主要包括病毒载体和非病毒载体,它们能够将治疗基因有效地传递到细胞内[16]。随着基因工程的不断发展和临床实践的积累,基因治疗有望成为未来治疗许多疾病的重要手段。基因转染技术是基因治疗的关键手段之一,对治疗效果具有重要影响[17]。 2.3 基因转染技术的原理 基因转染技术是通过将外源基因导入目标细胞,使其表达特定蛋白质或RNA,从而实现对细胞功能的调控。常用的转染方法包括病毒载体转染和非病毒载体转染。在病毒载体转染中,常用的载体包括慢病毒、腺相关病毒和反转录病毒等,它们能够高效地将外源基因导入细胞并在细胞内稳定表达。而非病毒载体转染则利用人工制备的载体(如质粒DNA、脂质体或聚合物)向细胞递送外源基因。通过基因转染技术,可以调控细胞的增殖、凋亡、分化等功能,从而影响组织的修复和再生过程。 不同载体转染方法的优缺点见表2。 "


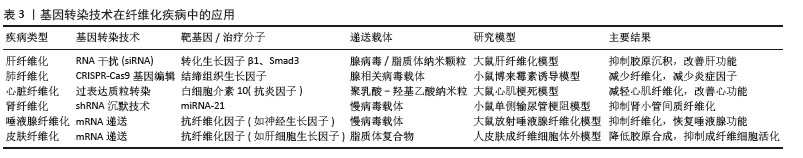
2.3.1 病毒载体转染法 通过将病毒转化为病毒载体,病毒已被重新利用为基因递送工具。最常用的载体是慢病毒载体,其来源于人类免疫缺陷病毒。病毒载体可以有效地将外源基因导入宿主细胞,并利用其复制机制实现基因表达[18]。近年来关于慢病毒的研究受到广泛关注,如何解决转染效率低、基因难以稳定表达、引发宿主细胞免疫反应等问题成为当下研究的主要方向。 (1)慢病毒载体转染:慢病毒载体是一种常用于基因转染的病毒载体,相较于其他病毒载体,慢病毒具有一些独特优点,如能够实现长期稳定的基因表达、适用于广泛的细胞类型和组织、能够转染较大的基因片段等。慢病毒载体介导的基因疗法在肿瘤治疗领域已成为研究热点,但其转导效率不足仍是限制临床转化应用的关键因素[19]。 (2)腺相关病毒载体转染:近年来研究发现重组腺相关病毒可作为有效的基因递送载体,且具有良好的安全性特征[20]。在众多的腺相关病毒载体中,8型腺相关病毒以其高效、稳定的基因转染特性而备受关注。目前,重组8型腺相关病毒已广泛应用于多种疾病的基因治疗研究中,包括遗传性疾病、癌症、自身免疫性疾病和病毒性疾病等[21]。 2.3.2 非病毒载体转染法 非病毒载体法包括化学、物理或生物法。近年来,研究较多的非病毒载体是聚合物、脂质、无机颗粒或不同类型的组合。与病毒载体相比,非病毒载体具有低细胞毒性、低免疫原性和操作简便等优点。然而,研究表明,非病毒载体在基因递送应用中存在明显局限性,主要表现为转染效率低、靶向特异性不足以及外源基因表达持续时间短等问题[22]。 (1)化学转染法:化学转染法主要有阳离子脂质体法、磷酸钙共沉淀法。WANG等[23]用阳离子脂质体进行转染实验时发现人参皂甙作为一种新的稳定剂可以提高阳离子脂质体的转染效率和生物相容性。单独使用化学转染法效果不佳,HADI等[24]研究结果表明,联合应用化学与物理转染方法可显著提高基因转染效率,为癌症治疗提供了一种具有良好应用前景的新型递送策略。 (2)物理转染法:物理转染法作为一种细胞内递送技术,具有操作直接、过程可控、效率高且作用迅速等显著优势。物理转染法包括电穿孔、基因枪、显微注射、激光、高温、超声波和流体动力学基因转移。近年来,基于纳米通道平台进行的物理转染法成为学者们关注的焦点,通过一种非病毒电活性纳米注射平台进行基因转染在一定程度上提高了基因转染效率[25]。 2.4 基因转染技术在纤维化疾病中的应用 基因转染技术为纤维化疾病的干预提供了新思路,该技术通过特异性递送治疗基因或利用基因编辑工具调控关键纤维化信号通路(如转化生长因子β/Smad、Wnt/β-catenin等),从而实现对纤维化进程的靶向调控。基因转染技术在纤维化疾病中的应用详见表3。 "
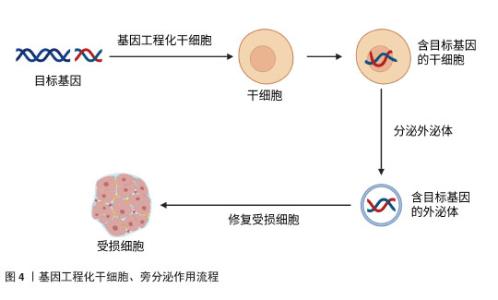
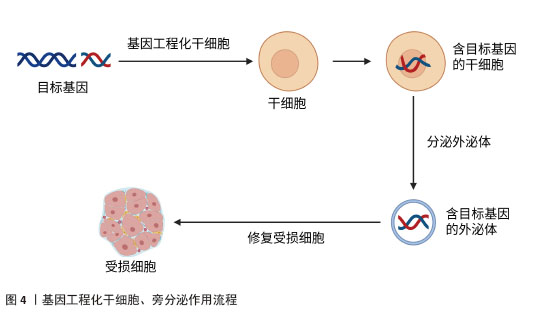
2.4.1 肺纤维化 特发性肺纤维化是一种慢性、进行性且不可逆的间质性肺疾病。虽然肺纤维化的确切病因和发病机制尚未完全阐明,但目前研究普遍认为,肺泡上皮细胞损伤诱发的成纤维细胞向肌成纤维细胞转化,以及细胞外基质成分(如胶原蛋白)的异常沉积,是推动特发性肺纤维化疾病进展的关键病理因素。目前,患者主要依靠口服抗纤维化药物来缓解肺纤维化,如吡非尼酮和尼达尼布等,但口服抗纤维化药物并不能逆转肺纤维化的进展[26]。研究发现,转染CXCR7基因的脐带间充质干细胞通过抑制Wnt/β-catenin-Notch/Jag1信号轴,显著减轻了肺纤维化病理损伤并促进肺泡结构重建[27]。WANG等[28]实验发现,对于博莱霉素诱导的肺纤维化小鼠,通过体内转染上调肺组织中Huntingtin蛋白激酶2的表达,发现肺纤维化减轻;对于转化生长因子β1诱导的小鼠肺成纤维细胞,通过体外转染上调细胞中Huntingtin蛋白激酶2的表达,可抑制小鼠肺成纤维细胞的增殖和迁移,促进小鼠肺成纤维细胞的凋亡,并减少间充质标志物和β-catenin的表达,结果表明体内转染Huntingtin蛋白激酶2通过阻断小鼠肺成纤维细胞中的Wnt/β-catenin通路,抑制成纤维细胞增殖并诱导凋亡,减少胶原沉积,改善纤维化,促进组织修复。有研究表明,在过表达胸腺细胞分化抗原1的肺成纤维细胞中,细胞增殖被抑制且细胞凋亡加速,胸腺细胞分化抗原1通过干扰Wnt信号传导,直接逆转成纤维细胞活化状态[29]。LIU等[30]实验表明,转染miR-27a-3p通过抑制Wnt3a/β-catenin信号通路,下调Ⅰ型胶原和Ⅲ型胶原的表达,从而促进异常细胞外基质的降解。以上实验说明Wnt/β-catenin 信号通路在肺纤维化发展过程中发挥着重要的作用。还有研究显示,转染miR-221通过阻断转化生长因子β1/Smad3/上皮-间充质转化通路,在博莱霉素诱导的肺纤维化模型中展现出显著的组织修复效果[31]。ZHU等[32]通过基因转染实验发现miR-506可能是细胞凋亡和炎症的重要调节因子,可调控细胞凋亡与炎症平衡,创造组织修复微环境。肺纤维化是以肺组织纤维化增生和瘢痕形成为主要病理特征的慢性肺部疾病,其进行性发展可导致不可逆的肺功能损害,目前临床尚缺乏有效的根治性治疗手段。以上研究共同揭示:基因转染技术不仅能阻断纤维化进展,更能通过多靶点干预(抑制异常活化、促进细胞凋亡、重塑细胞外基质平衡)实现功能性组织修复。相较于传统药物的症状控制,基因转染展现出逆转纤维化病理改变的独特优势,为特发性肺纤维化治疗提供了突破性策略。 2.4.2 肝纤维化 肝纤维化是由慢性肝损伤引发的进行性病理过程,可进一步发展为肝硬化和肝细胞癌。近年来的流行病学数据显示,肝纤维化的全球疾病负担持续加重,其发病率与死亡率均呈现显著上升趋势。然而,目前抗肝纤维化的药物存在靶向性差、疗效有限、毒副作用多等问题[33]。近年来越来越多的研究发现miRNA对肝纤维化治疗有一定的作用,然而如何将miRNA递送至受损组织成为治疗的关键。有研究发现,脂肪间充质干细胞分泌的外泌体可将miRNA递送至肝星状细胞,将脂肪间充质干细胞工程化以过表达miR-181-5p,选择性将外泌体归巢至小鼠肝星状细胞或四氯化碳诱导的肝纤维化小鼠模型,使得纤维化基因的表达受到抑制,有效逆转了细胞外基质沉积[34]。PAIK等[35]用硫代乙酰胺处理人肝星状细胞以及向小鼠皮下注射硫代乙酰胺构建肝纤维化的体外和体内模型,然后将转染抗纤维化miR-150的脂肪间充质干细胞来源外泌体作用于肝纤维化模型,发现其通过三重机制(抗纤维化、促增殖、抗氧化)实现肝组织功能重建。还有研究表明,转染miR-10a模拟物也可改善肝纤维化,其作用机制可能是miR-10a通过调节转化生长因子β1/Smads信号转导通路促进肝细胞再生[36]。此外,MOON等[37]研究表明,将转染肝细胞生长因子的人间充质干细胞移植至二甲基亚硝胺诱导的肝纤维化模型大鼠,可显著降低细胞毒性、减少细胞内活性氧水平,同时天冬氨酸氨基转移酶和丙氨酸氨基转移酶水平下降,具有修复肝实质细胞的作用。还有实验发现,转染CXCL9至脐带间充质干细胞可以提高脐带间充质干细胞治疗肝纤维化的效果,主要是脐带间充质干细胞归巢和驻留在肝纤维化部位的能力得到了增强,提高了局部修复效率[38]。XU等[39]实验表明,白细胞介素10基因修饰的树突状细胞通过诱导调节性T细胞和抑制转化生长因子β/Smad信号通路减轻了小鼠肝纤维化,促进肝细胞再生。以上研究表明:基因转染技术不仅能阻断纤维化进展,更通过多维度机制(靶向递送、信号重编程、细胞功能重塑)实现结构性修复。 2.4.3 心脏纤维化 心脏纤维化的特点是细胞外基质过度沉积,导致心脏功能受损并进一步发展成心力衰竭。在病理刺激下,心脏成纤维细胞被激活,然后分化为成肌母细胞,也就是成纤维细胞到肌成纤维细胞的转化,因此心脏成纤维细胞是介导进行性心脏纤维化的主要细胞[40]。传统治疗手段难以逆转已形成的纤维化病变,而基因转染技术通过靶向调控纤维化核心机制,展现出显著的组织修复潜力。LEE等[41]研究显示,达格列净通过调节SGK1信号通路和抑制上皮钠通道蛋白来逆转纤维化,改善心肌僵硬度。用miR-125a-3p模拟物转染糖尿病心肌病小鼠,不仅减轻了心肌纤维化,同时还改善了线粒体功能和抑制了炎症反应,实现心脏结构与功能的协同修复,这些发现为糖尿病心肌病的治疗提供了一个有价值和有前途的治疗靶点[42]。实验研究发现,将miR-548c-3p模拟物通过慢病毒转染大鼠心肌梗死模型,可实现细胞外基质结构重塑,抑制原癌基因c-Myb的蛋白表达,miR-548c-3p通过靶向c-Myb抑制纤维化相关因子(包括α-平滑肌肌动蛋白和胶原蛋白Ⅰ)的表达[43]。还有研究用携带miR-130a的AAV-9转染心肌梗死模型小鼠,发现miR-130a通过直接靶向转化生长因子β受体1调节转化生长因子β/Smad信号传导,抑制缺氧心肌成纤维细胞向肌成纤维细胞转化,使已活化的肌成纤维细胞发生表型逆转,恢复心脏组织弹性[44],因此,对于治疗心脏纤维化,miR-130a是一个很有希望的治疗靶点。此外,ZHANG等[45]实验结果显示,miR-34a能够通过靶向Pin-1信号通路促进心脏成纤维细胞凋亡,同时抑制Ⅰ型胶原蛋白合成、细胞活性和迁移能力,从而改善纤维化。综上,通过基因转染技术转染miRNA等可以通过调节分子通路抑制成纤维细胞向肌成纤维细胞转化,从而逆转心脏纤维化,改善心功能。 2.4.4 肾纤维化 肾纤维化的特征在于肾实质中进行性细胞外基质沉积,最终形成组织瘢痕和不可避免的肾功能恶化。血管稀疏、肾小球硬化和间质性炎症不仅是肾纤维化的典型病理特征,还可能通过多种机制促进其发生发展。基因转染技术通过多靶点干预纤维化进程,实现组织修复。研究表明,胶质细胞源性神经营养因子(glial cell line-derived neurotrophic factor,GDNF)参与肾脏的形态发生并改善肾纤维化,促进组织再生[46]。有研究发现,单侧输尿管梗阻小鼠通过尾静脉注射转染GDNF的人脂肪间充质干细胞,发现其改善了组织缺氧,抑制氧化应激,并通过降低内皮细胞(CD31)和肌成纤维细胞(α-平滑肌肌动蛋白)标志物的共表达来抑制内皮向间充质转化。GDNF基因通过PI3K/Akt/内皮型一氧化氮合酶信号通路增强人脂肪间充质干细胞改善肾脏微循环的能力,进而抑制内皮细胞向间充质细胞转化和肾脏纤维化[47]。CHEN等[48]通过慢病毒转染系统将GDNF转染至人脂肪间充质干细胞,分离出外泌体,发现其可以刺激肾脏纤维化小鼠模型的血管生成以及细胞凋亡从而改善肾纤维化,其机制可能是通过激活SIRT1/内皮型一氧化氮合酶信号通路从而促进肾小管周毛细血管的生成。此外,一些miRNA也被发现作为肾脏纤维化疾病的关键调节因子而发挥重要作用。ZHANG等[49]将含有miR-92d-3p 的慢病毒转染到糖尿病肾病小鼠模型中,发现miR-92d-3p通过抑制C3/HMGB1/转化生长因子β1通路和上皮-间充质转化来减少胶原沉积。有实验发现,在狼疮肾炎小鼠中,转染miR-125a-3p可下调转化生长因子β1,抑制白细胞介素17介导的炎症反应,改善肾小球硬化指数[50]。另一项研究显示,miR-30c-5p通过减少JAK1的表达,抑制高糖诱导的上皮-间质转化和干预糖尿病肾病的纤维化进程以修复组织纤维化[51]。另有实验发现,miR-181可能通过与早期生长反应因子1的结合而成为调控促纤维化标志物的重要递质,有望成为肾纤维化诊断和治疗的新靶点[52]。此外,LEE等[53]实验发现,人促红细胞生成素mRNA转染的间充质干细胞可以抑制上皮-间充质转化,改善肾功能指标。转染肝细胞生长因子的骨髓间充质干细胞也可以有效减轻大鼠肾间质纤维化[54]。以上文献表明,转染GDNF、miRNA以及生长因子等通过调节信号转导通路以实现恢复肾脏微血管架构,改善肾功能。 2.4.5 放射性唾液腺纤维化 放疗通常用于治疗头颈部恶性肿瘤,但放射线也会对周围的正常组织造成损伤,包括唾液腺,引起唾液腺纤维化,从而导致唾液分泌减少、口干、口腔感染等问题,对患者的口腔健康和生活质量造成了严重影响。传统治疗方法为药物治疗,利用药物来改善和刺激唾液的分泌,如氨磷汀、毛果芸香碱、抗氧化剂等,然而,药物治疗缺乏特异性和高效性[55]。许多学者普遍认为间充质干细胞具有多向分化潜能,可以修复放射性损伤的唾液腺,促进组织再生。然而,有研究发现,间充质干细胞移植后1周,仅有<1%的细胞能存活下来[56],因细胞寿命有限、细胞植入效率低以及细胞移植可能带来的免疫排斥反应和肿瘤形成风险,限制了其在临床的广泛应用。近年来,广大学者逐渐把研究的焦点从干细胞治疗过渡到无细胞治疗。基因转移到唾液腺治疗唾液腺功能障碍是一种有前途的方法[57]。LI等[58]通过颌下腺导管将腺相关病毒-神经生长因子逆行灌注到放射性唾液腺损伤模型中,发现腺相关病毒介导的基因治疗可恢复唾液分泌功能,抑制放射诱导的细胞凋亡;在机制上,神经生长因子通过去磷酸化JNK激酶而不是促进AKT磷酸化来抑制放射诱导的细胞凋亡,这为放射性唾液腺纤维化修复的再生医学研究带来了更多的希望。最近,有研究发现脂肪间充质干细胞来源外泌体具有促进放射性唾液腺再生修复的作用,其主要通过外泌体中的miR-199a-3p直接靶向上皮间充质相关转录因子Twist1,从而阻断转化生长因子β1/Smad3通路,减轻上皮-间充质转化,改善唾液腺纤维化,促进组织修复再生[59]。总之,目前放射性唾液腺纤维化治疗的研究方向主要为干细胞治疗和无细胞治疗,越来越多的研究表明通过基因转染可以有效改善放射性唾液腺纤维化,促进组织再生,恢复唾液腺功能。 2.4.6 其他组织纤维化 放射性肠炎、肠纤维化常常于癌症患者放疗后出现。YANG等[60]用慢病毒转染骨髓间充质干细胞以过表达miR-200b,发现miR-200b可以通过微囊泡从转基因骨髓间充质干细胞转移到靶细胞或组织中,从而通过抑制上皮-间充质转化减轻实验性结肠炎相关的肠纤维化。另有研究表明,维生素D受体可以通过Pmaip1介导的途径抑制隐窝干/祖细胞凋亡来改善辐射诱导的肠损伤,维持肠道屏障完整性[61]。此外,大多数接受放射治疗的患者都会出现放射性皮肤损伤,迫切需要有效的治疗,而线粒体超氧化物歧化酶转染实验显示,线粒体超氧化物歧化酶通过抑制铁凋亡可以减轻辐射诱导的皮肤损伤[62]。FUJISAWA等[63]实验发现,转染miR-146b-5p模拟物可减弱皮肤纤维化,而miR-146b-5p抑制剂强烈促进皮肤纤维化,其机制可能是miR-146b-5p抑制脂肪组织细胞中血小板衍生生长因子受体α表达,使促纤维化活性丧失,逆转纤维化。还有研究表明,骨髓间充质干细胞衍生的外泌体通过递送miR-214抑制白细胞介素33/ST2轴减轻皮肤纤维化,改善皮肤弹性[64]。 2.5 干细胞、外泌体作为基因递送载体治疗组织纤维化的潜力及优势 近年来,间充质干细胞和外泌体因具有天然靶向性、低免疫原性和高效递送能力,成为基因治疗的新型载体,在治疗组织纤维化方面展现出巨大潜力和优势,成为广大研究者关注的热点。 2.5.1 干细胞作为基因递送载体治疗组织纤维化的潜力 干细胞,尤其是间充质干细胞,具有自我更新和多向分化能力,越来越多的研究表明,干细胞的治疗效果并不完全依赖于其向受损组织的定向分化能力,而是更多地依赖于其旁分泌作用。旁分泌作用是指干细胞通过分泌多种生物活性分子(如细胞因子、生长因子、miRNA等)来调节周围细胞的行为,从而促进组织修复和再生[65]。基因工程化干细胞通过增强干细胞的抗纤维化能力,为纤维化治疗提供了新的思路。基因工程化干细胞可以通过过表达特定的抗纤维化因子来增强其治疗效果,例如通过基因修饰使间充质干细胞过表达骨形态发生蛋白7,能够显著抑制转化生长因子β1的活性,从而减轻肺纤维化[66]。其次,基因工程化干细胞也可以通过分泌抗炎因子来调节炎症反应,减轻纤维化。在肝纤维化模型中,基因工程化间充质干细胞通过过表达白细胞介素10显著抑制了肝星状细胞的激活,减少纤维化模型中的炎症反应,改善肝纤维化[67]。另外,基因工程化干细胞还可以通过促进组织修复来改善纤维化,例如通过基因修饰使间充质干细胞过表达肝细胞生长因子,促进肺泡上皮细胞修复、减少胶原蛋白沉积,增强其在肺纤维化模型中的治疗效果[68]。总之,基因工程化干细胞通过增强抗纤维化因子的表达、调节炎症反应和促进组织修复等,在纤维化治疗中展现出巨大潜力。 2.5.2 外泌体作为基因递送载体治疗组织纤维化的潜力 近年来的研究发现基于干细胞的疗法在临床应用中仍存在一些局限性,如细胞寿命有限、细胞植入效率低以及细胞移植可能带来的免疫排斥反应和肿瘤形成风险等。基因工程化干细胞通过旁分泌作用从而达到无细胞治疗的方法,成为当下再生医学研究的焦点。外泌体是细胞旁分泌的纳米级膜囊泡,含有蛋白质、脂质、mRNA和miRNA等多种生物分子。外泌体作为基因递送载体的主要作用机制包括:①靶向递送:外泌体表面的特定分子使其能够靶向特定细胞或组织;②细胞摄取:外泌体能够被受体细胞摄取,并在细胞内释放其携带的生物分子;③基因调控:外泌体中的miRNA和mRNA可以直接调节受体细胞的基因表达[69]。为了增强干细胞的旁分泌作用,可采用基因工程化技术,通过基因编辑(如CRISPR/Cas9)或基因转染(如AAV载体),将特定基因导入干细胞中,使其分泌的外泌体中过表达或抑制某些关键分子而发挥治疗作用。基因工程化干细胞产生的外泌体可以通过靶向关键信号通路来抑制细胞外基质的过度沉积,例如在系统性硬化症皮肤纤维化模型中,外泌体通过抑制转化生长因子β1/Smad3信号通路,显著降低纤维化标志物的表达以改善纤维化,修复受损组织[70];其次,通过调节炎症细胞的极化来减轻纤维化,例如间充质干细胞来源外泌体能够促进巨噬细胞从促炎的M1表型向抗炎的M2表型转变,减少炎症因子的分泌,减轻纤维化[71-72];再者,基因工程化干细胞分泌的外泌体还可以通过促进受损细胞的再生来改善纤维化,例如在肺纤维化模型中,工程化外泌体通过激活肺泡上皮细胞的再生,减少胶原蛋白的沉积,促进组织修复。 基因工程化干细胞通过旁分泌作用从而促进组织损伤修复,作用流程见图4。"
| [1] DISTLER JHW, GYÖRFI AH, RAMANUJAM M, et al. Shared and distinct mechanisms of fibrosis. Nat Rev Rheumatol. 2019;15(12): 705-730. [2] ZHAO Y, QU Y, HAO C, et al. PD-1/PD-L1 axis in organ fibrosis. Front Immunol. 2023;14: 1145682. [3] BATLLE E, MASSAGUÉ J. Transforming growth factor-β signaling in immunity andcancer. Immunity. 2019;50(4):924-940. [4] GU X, JIANG YN, WANG WJ, et al. Comprehensive circRNA expression profileand construction of circRNA-related ceRNA network in cardiac fibrosis. Biomed Pharmacother. 2020;125:109944. [5] YANG Q, ZHANG P, LIU T, et al. Magnesium isoglycyrrhizinate ameliorates radiation-induced pulmonary fibrosis by inhibiting fibroblast differentiation via the p38MAPK/Akt/Nox4 pathway. Biomed Pharmacother. 2019;115:108955. [6] FENG Y, REN J, GUI Y, et al. Wnt/β-catenin-promoted macrophage alternative activation contributes to kidney fibrosis. J Am Soc Nephrol. 2018;29(1):182-193. [7] RONG X, LIU J, YAO X, et al. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res Ther. 2019;10(1):98. [8] VERMA S, DUTTA A, DAHIYA A, et al. Quercetin-3-rutinoside alleviates radiation-induced lung inflammation and fibrosisvia regulation of NF-κB/TGF-β1 signaling. Phytomedicine. 2022;99:154004. [9] YU Z, XU C, SONG B, et al. Tissue fibrosis induced by radiotherapy: current understanding of the molecular mechanisms, diagnosis and therapeutic advances. J Transl Med. 2023;21(1):708. [10] LU C, LIU Y, REN F, et al. HO-1: An emerging target in fibrosis. J Cell Physiol. 2025;240(1):e31465. [11] WANG M, HUO Z, HE X, et al. The Role of MiR-29 in the Mechanism of Fibrosis. Mini Rev Med Chem. 2023;23(19):1846-1858. [12] MOHAMMADI S, RAVANBAKHSH H, TAHERI S, et al. Immunomodulatory microgels support proregenerative macrophage activation and attenuate fibroblast collagen synthesis. Adv Healthc Mater. 2022;11(11):e2102366. [13] ARTLETT CM. The Mechanism and Regulation of the NLRP3 Inflammasome during Fibrosis. Biomolecules. 2022;12(5):634. [14] BARHATE A, BAJAJ P, SHIRBHATE U, et al. Implications of Gene Therapy in Dentistry and Periodontics: A Narrative Review. Cureus. 2023;15(11):e49437. [15] TANG R, XU Z. Gene therapy: a double-edged sword with great powers. Mol Cell Biochem. 2020;474(1-2):73-81. [16] CHANCELLOR D, BARRETT D, NGUYEN-JATKOE L, et al. The state of cell and gene therapy in 2023. Mol Ther. 2023;31(12): 3376-3388. [17] SAYED N, ALLAWADHI P, KHURANA A, et al. Gene therapy: Comprehensive overview and therapeutic applications. Life Sci. 2022; 294:120375. [18] GUTIERREZ-GUERRERO A, COSSET FL, VERHOEYEN E. Lentiviral Vector Pseudotypes: PreciousTools to Improve Gene Modification of Hematopoietic Cells for Research and Gene Therapy. Viruses. 2020;12(9):1016. [19] XU F, LIU X, ZHANG D, et al. The Engineered MARCH8-Resistant Vesicular Stomatitis VirusGlycoprotein Enhances Lentiviral Vector Transduction. Hum Gene Ther. 2021; 32(17-18):936-948. [20] SCARROTT JM, JOHARI YB, POHLE TH, et al. Increased recombinant adeno-associated virus production by HEK293 cells using small molecule chemical additives. Biotechnol J. 2023;18(3):e2200450. [21] ZHAO L, YANG Z, ZHENG M, et al. Recombinant adeno-associated virus 8 vectorin genetherapy: Opportunities and challenges. Genes Dis. 2023;11(1):283-293. [22] ZU H, GAO D. Non-viral Vectors in Gene Therapy: Recent Development, Challenges, and Prospects. AAPS J. 2021;23(4):78. [23] WANG MZ, XU Y, XIE JF, et al. Ginsenoside as a new stabilizer enhances the transfection efficiency and biocompatibility of cationic liposome. Biomater Sci. 2021;9(24):8373-8385. [24] HADI A, RASTGOO A, HAGHIGHIPOUR N, et al. Enhanced gene delivery in tumor cells using chemical carriers and mechanical loadings. PLoS One. 2018;13(12):e020919. [25] SHOKOUHI AR, CHEN Y, YOH HZ, et al. Engineering Efficient CAR-T Cells via Electroactive Nanoinjection. Adv Mater. 2023;35(44):e2304122. [26] GUO H, SUN J, ZHANG S, et al. Progress in understanding and treating idiopathic pulmonary fibrosis: recent insights and emerging therapies. Front Pharmacol. 2023;14:1205948. [27] XIAO K, LIU C, WANG H, et al. Umbilical cord mesenchymal stem cells overexpressing CXCR7 facilitate treatment of ARDS-associated pulmonary fibrosis via inhibition of Notch/Jag1 mediated by the Wnt/β-catenin pathway. Biomed Pharmacother. 2023;165:115124. [28] WANG F, ZHANG Y, REN J, et al. HIPK2 attenuates bleomycin-induced pulmonaryfibrosis by suppressing the Wnt/β-catenin signaling pathway. Folia Histochem Cytobiol. 2022;60(3):247-259. [29] CHEN L, TANG RZ, RUAN J, et al. Up-regulation of THY1 attenuates interstitial pulmonary fibrosis and promotes lung fibroblast apoptosis during acute interstitial pneumonia by blockade of the WNT signaling pathway. Cell Cycle. 2019;18(6-7):670-681. [30] LIU L, QIAN H, HU K, et al. miR-27a-3p inhibits pulmonary fibrosis by blocking Wnt3a/β-catenin pathway in rats. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2018;34(11): 1015-1020. [31] WANG YC, LIU JS, TANG HK, et al. miR 221 targets HMGA2 to inhibit bleomycin induced pulmonary fibrosis by regulating TGF β1/Smad3-induced EMT. Int J Mol Med. 2016;38(4):1208-1216. [32] ZHU M, AN Y, ZHANG X, et al. Experimental pulmonary fibrosis was suppressed by microRNA-506 through NF-kappa-mediated apoptosis and inflammation. Cell Tissue Res. 2019;378(2):255-265. [33] CHANG J, HUANG C, LI S, et al. Research Progress Regarding the Effect and Mechanism of Dietary Polyphenols in Liver Fibrosis. Molecules. 2023;29(1):127. [34] QU Y, ZHANG Q, CAI X, et al. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. 2017;21(10):2491-2502. [35] PAIK KY, KIM KH, PARK JH, et al. A novel antifibrotic strategy utilizing conditioned media obtained from miR-150-transfected adipose-derived stem cells: validation of an animal model of liver fibrosis. Exp Mol Med. 2020;52(3):438-449. [36] ZHOU G, LIN W, FANG P, et al. MiR-10a improves hepatic fibrosis by regulating the TGFβl/Smads signal transduction pathway. Exp Ther Med. 2016;12(3):1719-1722. [37] MOON SH, LEE CM, PARK SH, et al. Effects of hepatocyte growth factor gene-transfected mesenchymal stem cells on dimethylnitrosamine-induced liver fibrosis in rats. Growth Factors. 2019;37(3-4):105-119. [38] LI Y, DONG J, ZHOU Y, et al. Therapeutic effects of CXCL9-overexpressing human umbilicalcord mesenchymal stem cells on liver fibrosis in rats. Biochem Biophys Res Commun. 2021;584:87-94. [39] XU Y, TANG X, YANG M, et al. Interleukin 10 Gene-Modified Bone Marrow-Derived Dendritic Cells Attenuate Liver Fibrosis in Mice by Inducing Regulatory T Cells and Inhibiting the TGF-β/Smad Signaling Pathway. Mediators Inflamm. 2019;2019:4652596. [40] XU J, WANG J, LONG F, et al. Inhibition of the cardiac fibroblast-enriched histone methyltransferase Dot1L prevents cardiac fibrosis and cardiac dysfunction. Cell Biosci. 2022;12(1):134. [41] LEE SG, KIM D, LEE JJ, et al. Dapagliflozin attenuates diabetes-induced diastolic dysfunction and cardiac fibrosis by regulating SGK1 signaling. BMC Med. 2022; 20(1):309. [42] LIU Y, ZHU Y, LIU S, et al. NORAD lentivirus shRNA mitigates fibrosis and inflammatory responses in diabetic cardiomyopathy via the ceRNA network of NORAD/miR-125a-3p/Fyn. Inflamm Res. 2021;70(10-12): 1113-1127. [43] ZHANG LX, ZHANG SH, WANG CQ, et al. Role and mechanism of microRNA-548c-3p/c-Myb in myocardial infarction fibrosis in rats. Eur Rev Med Pharmacol Sci. 2019; 23(11):4908-4916. [44] FENG Y, BAO Y, DING J, et al. MicroRNA-130a attenuates cardiac fibrosis after myocardial infarction through TGF-β/Smad signaling by directly targeting TGF-β receptor 1. Bioengineered. 2022;13(3): 5779-5791. [45] ZHANG XL, ZHANG G, BAI ZH. miR-34a attenuates myocardial fibrosis in diabetic cardiomyopathy mice via targeting Pin-1. Cell Biol Int. 2021;45(3):642-653. [46] WAHEED YA, BUBERWA W, SUN D. Glial cell line-derived neurotrophic factor and its role in attenuating renal fibrosis: a review. Korean J Intern Med. 2025;40(2):219-229. [47] LI S, WANG Y, WANG Z, et al. Enhanced renoprotective effect of GDNF-modified adipose-derived mesenchymal stem cells on renal interstitial fibrosis. Stem Cell Res Ther. 2021;12(1):27. [48] CHEN L, WANG Y, LI S, et al. Exosomes derived from GDNF-modified human adipose mesenchymal stem cells ameliorate peritubular capillary loss in tubulointerstitial fibrosis by activating the SIRT1/eNOS signaling pathway. Theranostics. 2020; 10(20):9425-9442. [49] ZHANG Y. MiR-92d-3p suppresses the progression of diabetic nephropathy renal fibrosis by inhibiting the C3/HMGB1/TGF-β1 pathway. Biosci Rep. 2021;41(9): BSR20203131. [50] ZHANG Y, CHEN X, DENG Y. miR-125a-3p decreases levels of interlukin-17 and suppresses renal fibrosis via down-regulating TGF-β1 in systemic lupus erythematosus mediated Lupus nephritic mice. Am J Transl Res. 2019;11(3):1843-1853. [51] GAO BH, WU H, WANG X, et al. MiR-30c-5p inhibits high glucose-induced EMT and renal fibrogenesis by down-regulation of JAK1 in diabetic nephropathy. Eur Rev Med Pharmacol Sci. 2020;24(3):1338-1349. [52] ZHANG X, YANG Z, HENG Y, et al. MicroRNA 181 exerts an inhibitory role during renal fibrosis by targeting early growth response factor 1 and attenuating the expression of profibrotic markers. Mol Med Rep. 2019; 19(4):3305-3313. [53] LEE M, KIM SH, JHEE JH, et al. Microparticles derived from human erythropoietin mRNA-transfected mesenchymal stem cells inhibit epithelial-to-mesenchymal transition and ameliorate renal interstitial fibrosis. Stem Cell Res Ther. 2020;11(1):422. [54] XIE M, WAN J, ZHANG F, et al. Influence of hepatocyte growth factor-transfected bone marrow-derived mesenchymal stem cells towards renal fibrosis in rats. Indian J Med Res. 2019;149(4):508-516. [55] LI SS, WU CZ, QIAO XH, et al. Advances on mechanism and treatment of salivary gland in radiation injury. Hua Xi Kou Qiang Yi Xue Za Zhi. 2021;39(1):99-104. [56] EGGENHOFER E, BENSELER V, KROEMER A, et al. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. 2012;3:297. [57] UPADHYAY A, CAO UMN, HARIHARAN A, et al. Gene Therapeutic Delivery to the Salivary Glands. Adv Exp Med Biol. 2023;1436:55-68. [58] LI SS, WU CZ, ZHANG BW, et al. Nerve growth factor protects salivary glands from irradiation-induced damage. Life Sci. 2021;265:118748. [59] GUO X, HUANG Z, WU F, et al. Exosomes of human adipose stem cells mitigate irradiation injury to salivary glands by inhibiting epithelial-mesenchymal transition through miR-199a-3p targeting Twist1 and regulating TGFβ1/Smad3 pathway. Theranostics. 2025;15(5):1622-1641. [60] YANG J, ZHOU CZ, ZHU R, et al. miR-200b-containing microvesicles attenuate experimental colitis associated intestinal fibrosis by inhibiting epithelial-mesenchymal transition. J Gastroenterol Hepatol. 2017; 32(12):1966-1974. [61] LI W, LIN Y, LUO Y, et al. Vitamin D Receptor Protects against Radiation-Induced Intestinal Injury in Mice via Inhibition of Intestinal Crypt Stem/Progenitor Cell Apoptosis. Nutrients. 2021;13(9):2910. [62] WANG X, LU Y, CHENG X, et al. Local Multiple-site Injections of a Plasmid Encoding Human MnSOD Mitigate Radiation-induced Skin Injury by Inhibiting Ferroptosis. Curr Drug Deliv. 2024;21(5):763-774. [63] FUJISAWA C, HAMANOUE M, KAWANO Y, et al. The Role for miR-146b-5p in the Attenuation of Dermal Fibrosis and Angiogenesis by Targeting PDGFRα in Skin Wounds. J Invest Dermatol. 2022;142(7): 1990-2002.e4. [64] XIE L, LONG X, MO M, et al. Bone marrow mesenchymal stem cell-derived exosomes alleviate skin fibrosis in systemic sclerosis by inhibiting the IL-33/ST2 axis via the delivery of microRNA-214. Mol Immunol. 2023;157:146-157. [65] YADAV S, MAITY P, KAPAT K. The Opportunities and Challenges of Mesenchymal Stem Cells-Derived Exosomes in Theranostics and Regenerative Medicine. Cells. 2024;13(23):1956. [66] MEHRABANI M, MOHAMMADYAR S, RAJIZADEH MA, et al. Boosting therapeutic efficacy of mesenchymal stem cells in pulmonary fibrosis: The role of genetic modification and preconditioning strategies. Iran J Basic Med Sci. 2023;26(9):1001-1015. [67] LI C, WANG B. Mesenchymal Stem/Stromal Cells in Progressive Fibrogenic Involvement and Anti-Fibrosis Therapeutic Properties. Front Cell Dev Biol. 2022;10:902677. [68] YANG S, LIU P, JIANG Y, et al. Therapeutic Applications of Mesenchymal Stem Cells in Idiopathic Pulmonary Fibrosis. Front Cell Dev Biol. 2021;9:639657. [69] LI T, LI X, HAN G, et al. The Therapeutic Potential and Clinical Significance of Exosomes as Carriers of Drug Delivery System. Pharmaceutics. 2022;15(1):21. [70] XIAO Y, XIANG Q, WANG Y, et al. Exosomes carrying adipose mesenchymal stem cells function alleviate scleroderma skin fibrosis by inhibiting the TGF-β1/Smad3 axis. Sci Rep. 2025;15(1):7162. [71] LIU Y, ZHENG Y, YANG Y, et al. Exosomes in liver fibrosis: The role of modulating hepatic stellate cells and immune cells, and prospects for clinical applications. Front Immunol. 2023;14:1133297. [72] TAN F, LI X, WANG Z, et al. Clinical applications of stem cell-derived exosomes. Signal Transduct Target Ther. 2024;9(1):17. [73] ZHANG M, HU S, LIU L, et al. Engineered exosomes from different sources for cancer-targeted therapy. Signal Transduct Target Ther. 2023;8(1):124. [74] FAN M, LIU H, YAN H, et al. A CAR T-inspiring platform based on antibody-engineered exosomes from antigen-feeding dendritic cells for precise solid tumor therapy. Biomaterials. 2022;282:121424. [75] ZHANG J, JI C, ZHANG H, et al. Engineered neutrophil-derived exosome-like vesicles for targeted cancer therapy. Sci Adv. 2022; 8(2):eabj8207. [76] BALARAMAN AK, BABU MA, MOGLAD E, et al. Exosome-mediated delivery of CRISPR-Cas9: A revolutionary approach to cancer gene editing. Pathol Res Pract. 2025;266:155785. [77] BAHADORANI M, NASIRI M, DELLINGER K, et al. Engineering Exosomes for Therapeutic Applications: Decoding Biogenesis, Content Modification, and Cargo Loading Strategies. Int J Nanomedicine. 2024;19:7137-7164. [78] LIU W, LIU Q, LI Z, et al. Multifunctional magneto-electric and exosome-loaded hydrogel enhances neuronal differentiation and immunoregulation through remote non-invasive electrical stimulation for neurological recovery after spinal cord injury. Bioact Mater. 2025;48:510-528. [79] JUNG I, SHIN S, BAEK MC, et al. Modification of immune cell-derived exosomes for enhanced cancer immunotherapy: current advances and therapeutic applications. Exp Mol Med. 2024;56(1):19-31. [80] HUANG J, CHEN H, LUO Z, et al. Genetically Engineered Stromal Cell Exosomes from High-Throughput Herringbone Microfluidics. ACS Nano. 2025;19(10):10568-10577. |
| [1] | Tan Jing, Li Li, Wang Liangliang, Qin Xiangyu. Bionic functional coating improves the integration of titanium implants and skin tissue interface [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2014-2022. |
| [2] | Fu Lyupeng, Yu Peng, Liang Guoyan, Chang Yunbing. Electroactive materials applied in spinal surgery [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2113-2123. |
| [3] | Jiang Xinghai, Song Yulin, Li Dejin, Shao Jianmin, Xu Junzhi, Liu Huakai, Wu Yingguo, Shen Yuehui, Feng Sicheng. Vascular endothelial growth factor 165 genes transfected into bone marrow mesenchymal stem cells to construct a vascularized amphiphilic peptide gel module [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1903-1911. |
| [4] | Wang Baiyan, Yang Shu, Wang Yiming, Wu Mengqing, Xiao Yu, Guo Zixuan, Zhang Boyi, Feng Shuying. Exosome-delivered CRISPR/Cas system enables gene editing in target cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1839-1849. |
| [5] | Peng Zhiwei, Chen Lei, Tong Lei. Luteolin promotes wound healing in diabetic mice: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1398-1406. |
| [6] | Hou Chaowen, Li Zhaojin, Kong Jianda, Zhang Shuli. Main physiological changes in skeletal muscle aging and the multimechanism regulatory role of exercise [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1464-1475. |
| [7] | Liu Kexin, , Hao Kaimin, Zhuang Wenyue, , Li Zhengyi. Autophagy-related gene expression in pulmonary fibrosis models: bioinformatic analysis and experimental validation [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1129-1138. |
| [8] | Yu Huifen, Mo Licun, Cheng Leping. The position and role of 5-hydroxytryptamine in the repair of tissue injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1196-1206. |
| [9] | Yu Shiyu, Yu Sutong, Xu Yang, Zhen Xiangyan, Han Fengxuan. Advances in research and application of tissue engineering therapeutic strategies in oral submucous fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 936-948. |
| [10] | Cao Yuqing, Guo Meiling, Liu Feng, Wei Junchao. Preparation, classification and application of polysaccharide-based hydrogels in skin damage repair [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5257-5269. |
| [11] | Diao Youlu, Gao Jia, Pan Guoqing. Recruitable tissue repair biomaterials: advantages of regulating cell and factor migration and improving tissue integration [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5270-5281. |
| [12] | Wang Jieyan, Yao Jiayi, Xin Yingtong, Zhang Xinwen, Li Riwang, Liu Dahai. Chitosan hydrogel drug delivery system is a safer and more effective solution for treating oral ulcers [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5331-5340. |
| [13] | Xu Yixuan, Yao Jun, Liu Xulu, Li Xinlian, Liu Zhixiong, Zhang Zhihong. Vancomycin-containing porcine skin acellular extracellular matrix hydrogel promotes wound healing in skin infections [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(20): 5214-5228. |
| [14] | Wang Xinyue, Li Hongli, Guo Chunhui, Chen Jibing, Yu Hua. Changes in the expression of six microRNAs in ovarian tissue from animal models of premature ovarian failure and in peripheral blood of patients with premature ovarian failure [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(18): 4675-4684. |
| [15] | Huang Sijing, Cui Rui, Geng Longyu, Gao Beiyao, Ge Ruidong, Jiang Shan. Application and molecular mechanism of extracorporeal shock wave for anti-fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(17): 4417-4429. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||