Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (36): 7816-7826.doi: 10.12307/2025.534
Previous Articles Next Articles
Effect of a novel cryoprotectant in tissues and cells
Wang Qingfang, Zhang Fen, Chang Guangping, Li Zihan, Xing Lan, Peng Hao, Zeng Xiuping, Zhong Guiqiang, Chen Hui, Liu Bo, Liu Zhenyu, Liang Xiao
- Shenzhen Beike Biotechnology Co., Ltd., Shenzhen 5180601, Guangdong Province, China
-
Received:2024-05-20Accepted:2024-07-15Online:2025-12-28Published:2025-03-13 -
Contact:Liang Xiao, MS, Senior engineer, Shenzhen Beike Biotechnology Co., Ltd., Shenzhen 5180601, Guangdong Province, China -
About author:Wang Qingfang, Shenzhen Beike Biotechnology Co., Ltd., Shenzhen 5180601, Guangdong Province, China. Zhang Fen, Master candidate, Shenzhen Beike Biotechnology Co., Ltd., Shenzhen 5180601, Guangdong Province, China. Wang Qingfang and Zhang Fen contributed equally to this article.
CLC Number:
Cite this article
Wang Qingfang, Zhang Fen, Chang Guangping, Li Zihan, Xing Lan, Peng Hao, Zeng Xiuping, Zhong Guiqiang, Chen Hui, Liu Bo, Liu Zhenyu, Liang Xiao. Effect of a novel cryoprotectant in tissues and cells[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7816-7826.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

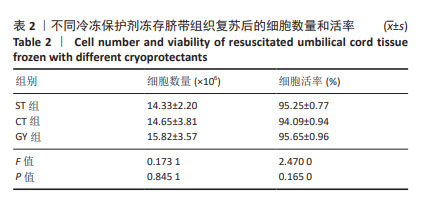
2.1 冷冻前的细胞数量和活率 2.1.1 脐带间充质干细胞冷冻前细胞数量、活率及表面标志物表达 从3个脐带样本中培养扩增脐带间充质干细胞至第2代,细胞浓度分别为4×109 L-1和4×1010 L-1,采用ST、CT、GY 3种冷冻保护剂进行细胞冻存,每支规格为1 mL,细胞活率分别为97.43%,97.41%,95.86%。细胞冻存前进行了细胞表面标志物检测,其中阳性指标CD90、CD73、CD105、CD29均不低于98%,阴性指标CD45、CD34、CD19、CD14、CD79a、HLA-DR均不超过2%。 2.1.2 脐血/外周血来源单个核细胞冷冻前细胞数量和活率 对采集不超过12 h的脐血和外周血进行分离培养后,将脐血单个核细胞按照细胞浓度4×109 L-1采用GY、SB、IM 3种冷冻保护剂进行细胞冻存,每支规格为1 mL,3个样本的细胞活率分别为94.53%,95.45%,93.19%;将外周血单个核细胞按照细胞浓度4×109 L-1采用GY、SB、IM 3种冷冻保护剂进行细胞冻存,每支规格为1 mL,3个样本的细胞活率分别为99.84%,99.62%,99.80%。 2.1.3 NK细胞和CIK细胞冷冻前细胞数量和活率 由脐血/外周血单个核细胞衍生的NK细胞和CIK细胞,按照细胞浓度4×1011 L-1采用GY、SB、IM 3种冷冻保护剂进行细胞冻存,每支规格为1 mL,其中外周血单个核细胞衍生的NK细胞活率分别为94.51%,93.94%,95.50%(平均为94.65%),CIK细胞活率分别为90.03%,89.70%,90.96%(平均为90.23%)。脐血单个核细胞衍生的NK细胞活率分别为94.28%,94.43%,97%.00(平均为94.90%),CIK细胞活率分别为95.8%,94.69%,96.12%(平均为95.54%)。 2.2 GY对脐带华通氏胶组织复苏培养的细胞形态、细胞数量和活率的影响与ST、CT相似 在经过GY、ST和CT冻存2周后,将脐带华通氏胶组织块复苏培养至第11天,通过倒置显微镜(OLYMPUS-IX73,日本)观察3组细胞样本均呈现出长梭形或纺锤形态(图1),且细胞融合度均达到85%。将细胞按照5×106 L-1的细胞浓度培养到第2代进行细胞数量和活率检测(表2),ST组细胞平均收获量为14.33×106,平均活率为95.25%;CT组细胞平均收获量为14.65×106,平均活率为94.09%;GY组细胞平均收获量为15.82×106,平均活率为95.65%。综合细胞数量和活率结果来看,新开发的GY冷冻保护剂略优于其他2种,但3种冷冻保护剂对冻存效果的影响并无显著差异。 "

2.3 GY对细胞数量和活率的影响与其他冷冻保护剂相似 2.3.1 不同冷冻保护剂对脐带间充质干细胞回收率和活率的影响 为了评估新开发的GY冻存保护剂在低冻存浓度(4×109 L-1)和高冻存浓度(4×1010 L-1)下的效果,复苏了分别使用3种冷冻保护剂冻存的第2代脐带间充质干细胞,检测细胞数量和活率,并计算回收率,通过对比回收率来评估冻存效果。低浓度冻存细胞复苏结果显示(图2A),ST组平均获得3.80×106个细胞,平均回收率为95.08%,细胞平均活率为96.82%;CT组平均获得3.68×106个细胞,平均回收率为92.00%,细胞平均活率为95.96%;GY组平均获得3.85×106个细胞,平均回收率为96.25%,细胞平均活率为97.60%。细胞活率与冻存前平均活率96.90%基本一致。3组在细胞回收率和细胞活率上并无显著性差异。高浓度冻存细胞复苏结果显示(图2B),ST组平均获得3.73×107个细胞,回收率为93.25%,细胞平均活率为96.74%;CT组平均获得3.65×107个细胞,平均回收率为91.25%,细胞平均活率为96.54%;GY组平均获得3.80×107个细胞,平均回收率为95%,细胞平均活率为97.47%。细胞活率与冻存前平均活率96.90%基本一致。3组在细胞回收率和细胞活率上也无显著性差异。根据低密度冻存和高密度冻存后的细胞回收率和细胞活率结果,显示新开发的GY冷冻保护剂的冻存效果与其他两组冷冻保护剂无显著性差异。因此,GY冷冻保护剂可应用于脐带华通氏胶组织块和间充质干细胞冻存,为组织和干细胞类细胞冷冻工艺提供了良好的技术参考。 2.3.2 不同冷冻保护剂对脐血/外周血来源单个核细胞回收率和活率的影响 通过检测脐血/外周血来源单个核细胞复苏后的细胞数量和活率,计算出细胞回收率,通过对比细胞回收率评估GY冷冻保护剂的效果。对于脐血单个核细胞,IM组复苏后平均获得3.51×107个细胞,平均活率为93.60%,平均回收率为87.67%;SB组复苏后平均获得3.57×107个细胞,平均活率为94.48%,平均回收率为89.25%;GY组复苏后平均获得3.55×107个细胞,平均活率为94.56%,平均回收率为88.75%。3组之间无论是细胞活率还是细胞回收率均无显著性差异(图3A)。对于外周血单个核细胞,IM组复苏后平均获得3.55×107个细胞,平均活率为93.60%,平均回收率为88.83%;SB组复苏后平均获得3.40×107个细胞,平均活率为94.48%,平均回收率为85.08%;GY组复苏后平均获得3.60×107个细胞,平均活率为94.77%,平均回收率为89.92%。3组之间无论是细胞活率还是细胞回收率均无显著性差异(图3B)。综合细胞数量和活率结果来看,新开发的GY冷冻保护剂对脐血/外周血单个核细胞冻存复苏后的细胞回收率与IM、SB冷冻保护剂无显著性差异,即GY冷冻保护剂适用于脐血和外周血单个核细胞的冻存工艺。 2.3.3 不同冷冻保护剂对NK细胞和CIK细胞回收率和活率的影响 对冻存后的NK和CIK细胞复苏后进行细胞数量和活率检测,对于脐血单个核细胞衍生的NK细胞:IM组复苏后平均获得3.39×108个细胞,平均活率为94.51%,平均回收率为84.70%;SB组复苏后平均获得3.43×108个细胞,平均活率为95.33%,平均回收率为85.68%;GY组复苏后平均获得3.48×108个细胞,平均活率为97.18%,平均回收率为86.88%。使用3种冷冻保护剂冻存脐血单个核细胞衍生NK细胞的细胞回收率无显著性差异(图4A)。对于脐血单个核细胞衍生的CIK细胞:IM组复苏后平均获得3.32×108个细胞,平均活率为92.72%,平均回收率为83.05%;SB组复苏后平均获得3.38×108个细胞,平均活率为92.03%,平均回收率为84.53 %;GY组复苏后平均获得3.39×108个细胞,平均活率为93.04%,平均回收率为84.64%;使用3种冷冻保护剂冻存脐血单个核细胞衍生CIK细胞的细胞回收率无显著性差异(图4B)。对于外周血单个核细胞衍生的NK细胞:IM组复苏后平均获得3.31×108个细胞,平均活率为91.92%,平均回收率为82.87%;SB组复苏后平均获得3.32×108个细胞,平均活率为94.29%,平均回收率为82.89%;GY组复苏后平均获得3.36×108个细胞,平均活率为94.80%,平均回收率为83.99%;使用3种冷冻保护剂冻存外周血单个核细胞衍生NK细胞的细胞回收率无显著性差异(图4C)。对于外周血单个核细胞衍生的CIK细胞:IM组复苏后平均获得3.44×108个细胞,平均活率为95.02%,平均回收率为85.94%;SB组复苏后平均获得3.50×108个细胞,平均活率为95.38%,平均回收率为87.55%;GY组复苏后平均获得3.51×106个细胞,平均活率为96.89%,平均回收率为87.73%。使用3种冷冻保护剂冻存外周血单个核细胞衍生NK细胞的细胞回收率无显著性差异(图4D)。根据细胞数量和活率检测结果显示,无论是脐血单个核细胞还是外周血单个核细胞衍生的CIK细胞和NK细胞,在细胞回收率和活率方面,使用3种冷冻保护剂无显著性差异,这表明新开发的GY冷冻保护剂适用于脐血/外周血来源单个核细胞衍生的NK细胞/CIK细胞的冷冻过程。针对免疫细胞,无论是单个核细胞还是衍生的NK细胞/CIK细胞,均可以采用GY冷冻保护剂,为标准化及规模化推广应用提供了技术基础。 2.4 GY对细胞复苏扩增后表面标志物的影响与其他冷冻保护剂相似 2.4.1 脐带间充质干细胞表面标志物检测 使用3种冷冻保护剂冻存的第2代脐带间充质干细胞复苏后进行表面标志物检测,结果显示,与细胞冷冻前检测结果一致,阳性标志物的平均阳性率达到95%以上,而阴性指标均低于2%,3组细胞表面标志物均无显著性差异(图5)。 "

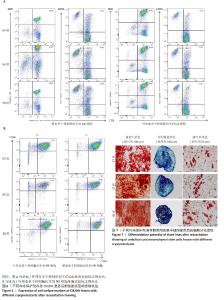
2.4.2 CIK细胞和NK细胞亚群分析 脐血单个核细胞衍生的CIK细胞:GY组平均由57.79%的CD3+CD8+细胞和23.80%的CD3+CD56+细胞组成;SB组平均由53.36%的CD3+CD8+细胞和29.25%的CD3+CD56+细胞组成;IM组平均由51.28%的CD3+CD8+细胞和平均27.09%的CD3+CD56+细胞组成(图6A)。外周血单个核细胞衍生的CIK细胞:GY组平均由58.26%的CD3+CD8+细胞和15.23%的CD3+CD56+细胞组成;SB组平均由54.11%的CD3+CD8+细胞和16.67%的CD3+CD56+细胞组成;IM组平均由53.82%的CD3+CD8+细胞和11.26%的CD3+CD56+细胞组成(图6A)。 脐血单个核细胞衍生的NK细胞:GY组平均由79.05%的CD56+CD16+细胞组成;SB组平均由75.86%的CD56+CD16+细胞组成;IM组平均由71.91%的CD56+CD16+细胞组成(图6B)。外周血单个核细胞衍生的NK细胞:GY组平均由68.40%的CD56+CD16+细胞组成;SB组平均由65.02%的CD56+CD16+细胞组成;IM组平均由61.95%的CD56+CD16+细胞组成(图6B)。 无论是脐血单个核细胞还是外周血单个核细胞衍生的CIK和NK细胞,在同类细胞使用3组冷冻保护剂冷冻复苏后细胞的表面标志物表达无显著性差异,表明所开发的GY冷冻保护剂在冻存效果上与IM、SB一致。 2.5 GY对干细胞冻存后三系分化的影响与ST、CT相似 使用GY、ST、CT冷冻保护剂冻存间充质干细胞复苏后进行三系分化检测,结果显示,3组细胞都具有良好的成骨、成脂和成软骨分化能力(图7),再次验证了新开发的GY冷冻保护剂在冻存效果上与ST和CT一致。 "

2.6 GY对脐血/外周血单个核细胞衍生的NK细胞和CIK细胞杀瘤活性与IM、SB相似 为验证新开发的GY冷冻保护剂对最终细胞制剂有效性方面的影响,复苏了经3种冷冻保护剂保存的脐血/外周血单个核细胞来源的NK细胞和CIK细胞,并进行了体外细胞杀伤毒性检测。在NK细胞/CIK细胞与黑色素瘤细胞系Mel624的效靶比(E∶T)为20∶1共培养后,确认了细胞的杀瘤活性(表3)。结果显示,脐血单个核细胞衍生的NK细胞在杀瘤活性上3组间无显著性差异(P=0.332 4),其中GY组对Mel624的平均致死率为66.48%,略高于SB组的60.91%和IM组的64.86%;外周血单个核细胞衍生的NK细胞在杀瘤活性上3组之间无显著性差异(P=0.118 6),SB组、IM组与GY组对Mel624的致死率分别为59.35%,62.22%和63.63%。SB组、IM组和GY组脐血单个核细胞来源的CIK细胞对Mel624的平均致死率分别为61.94%,64.19%和62.08%(P=0.378 0),SB组、IM组和GY组外周血单个核细胞来源的CIK细胞对Mel624的平均致死率分别为58.62%,61.18%和61.22%(P=0.377 4)。不同冷冻保护剂之间的杀瘤活性基本在50%-70%范围,SB组呈现的结果较GY组略低,可能和细胞冻存的密度相关。SB的细胞浓度范围上限的建议是在1×109 L-1,而实际细胞浓度达到了1×1011 L-1。但是3组的整体结果没有显著性差异,结果说明GY冷冻保护剂与IM、SB在CIK细胞和NK细胞的杀瘤活性方面相似。 "

| [1] MURRAY KA, GIBSON MI. Chemical approaches to cryopreservation. Nat Rev Chem. 2022;6(8):579-593. [2] 胡方方,李延,崔趁趁,等.人卵巢组织玻璃化冷冻及移植的影响因素分析[J].中国计划生育和妇产科,2023,15(9):20-24. [3] SHAHID MA, KIM WH, KWEON OK. Cryopreservation of heat-shocked canine adipose-derived mesenchymal stromal cells with 10% dimethyl sulfoxide and 40% serum results in better viability, proliferation, anti-oxidation, and in-vitro differentiation. Cryobiology. 2020;92:92-102. [4] DING Y, LIU S, LIU J, et al. Cryopreservation with DMSO affects the DNA integrity, apoptosis, cell cycle and function of human bone mesenchymal stem cells. Cryobiology. 2024;114:104847. [5] 赵刚,周学迅,高大勇.细胞低温保存原理与进展[J].中国科学:生命科学,2024,54(6):1109-1128. [6] SUGISHITA Y, MENG L, SUZUKI-TAKAHASHI Y, et al. Quantification of residual cryoprotectants and cytotoxicity in thawed bovine ovarian tissues after slow freezing or vitrification. Hum Reprod. 2022;37(3):522-533. [7] BAHSOUN S, COOPMAN K, AKAM EC. The impact of cryopreservation on bone marrow-derived mesenchymal stem cells: a systematic review. J Transl Med. 2019;17(1):397. [8] 刘代艳,于泊洋,孔群芳,等.海藻酸微囊替代DMSO和FBS的STO细胞冷冻保存研究[J].现代生物医学进展,2012,12(33):6413-6418. [9] MANTRI S, KANUNGO S, MOHAPATRA PC. Cryoprotective Effect of Disaccharides on Cord Blood Stem Cells with Minimal Use of DMSO. Indian J Hematol Blood Transfus. 2015;31(2):206-212. [10] CROWLEY CA, SMITH WPW, SEAH KTM, et al. Cryopreservation of Human Adipose Tissues and Adipose-Derived Stem Cells with DMSO and/or Trehalose: A Systematic Review. Cells. 2021;10(7):1837. [11] FUJITA Y, NISHIMURA M, KOMORI N, et al. Protein-free solution containing trehalose and dextran 40 for cryopreservation of human adipose tissue-derived mesenchymal stromal cells. Cryobiology. 2021;100:46-57. [12] 张鹏,刘宝林.人脐带间充质干细胞新型冻存液实验研究[J/OL].制冷学报,1-10[2024-07-05].http://kns.cnki.net/kcms/detail/11.2182.tb.20240524.1119.002.html. [13] HALME DG, KESSLER DA. FDA regulation of stem-cell-based therapies. N Engl J Med. 2006;355(16):1730-1735. [14] BARRO L, BURNOUF PA, CHOU ML, et al. Human platelet lysates for human cell propagation. Platelets. 2021;32(2):152-162. [15] CHEN MS, WANG TJ, LIN HC, et al. Four types of human platelet lysate, including one virally inactivated by solvent-detergent, can be used to propagate Wharton jelly mesenchymal stromal cells. N Biotechnol. 2019; 49:151-160. [16] LEE S, JOO Y, LEE EJ, et al. Successful expansion and cryopreservation of human natural killer cell line NK-92 for clinical manufacturing. PLoS One. 2024;19(2):e0294857. [17] GAO L, ZHOU Q, ZHANG Y, et al. Dimethyl Sulfoxide-Free Cryopreservation of Human Umbilical Cord Mesenchymal Stem Cells Based on Zwitterionic Betaine and Electroporation. Int J Mol Sci. 2021;22(14):7445. [18] 吕玲,邢义高,张秀涛,等.通用型细胞冻存保护液的探索[J].中国医药生物技术,2022,17(4):347-349. [19] 姬广超,王晓明,王玉琪,等.临床用途细胞冻存保护液探索实验[J].中国医药生物技术,2021,16(2):158-160. [20] MARQUEZ-CURTIS LA, ELLIOTT JAW. Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects: Update from 2015 review. Cryobiology. 2024;115:104856. [21] AL-SAQI SH, SALIEM M, QUEZADA HC, et al. Defined serum- and xeno-free cryopreservation of mesenchymal stem cells. Cell Tissue Bank. 2015; 16(2):181-193. [22] HUANG Z, LIU W, MA T, et al. Slow Cooling and Controlled Ice Nucleation Enabling the Cryopreservation of Human T Lymphocytes with Low-Concentration Extracellular Trehalose. Biopreserv Biobank. 2023;21(4): 417-426. [23] WANG J, SHI X, XIONG M, et al. Trehalose glycopolymers for cryopreservation of tissue-engineered constructs. Cryobiology. 2022;104:47-55. [24] NTAI A, LA SPADA A, DE BLASIO P, et al. Trehalose to cryopreserve human pluripotent stem cells. Stem Cell Res. 2018;31:102-112. [25] AL-SAQI SH, SALIEM M, QUEZADA HC, et al. Correction to: Defined serum- and xeno-free cryopreservation of mesenchymal stem cells. Cell Tissue Bank. 2019;20(2):329-330. [26] 马洁,刘彩霞,谭琴,等.细胞产品质量控制与质量管理[J].药物评价研究,2021,44(2):273-292. [27] 窦蒙家,张明宽,饶伟,等.人体低温保存:通向未来“永生”之路?[J].科学,2017,69(6):1-4+69. [28] 刘威,郭明伟,郭治宇,等.胞内冰形成机理研究进展[J].制冷学报, 2018,39(3):126-134. [29] 邱佳裔,贾晓青,黄岗,等.细胞、组织冻存方法及应用的研究进展[J].中国生物制品学志,2017,30(5):546-550. [30] ARUTYUNYAN I, FATKHUDINOV T, SUKHIKH G. Umbilical cord tissue cryopreservation: a short review. Stem Cell Res Ther. 2018;9(1):236. [31] PAVÓN A, BELOQUI I, SALCEDO JM, et al. Cryobanking Mesenchymal Stem Cells. Methods Mol Biol. 2017;1590:191-196. [32] AWAN M, BURIAK I, FLECK R, et al. Dimethyl sulfoxide: a central player since the dawn of cryobiology, is efficacy balanced by toxicity? Regen Med. 2020;15(3):1463-1491. [33] DAS S, NIEMEYER E, LEUNG ZA, et al. Human Natural Killer Cells Cryopreserved without DMSO Sustain Robust Effector Responses. Mol Pharm. 2024;21(2):651-660. [34] HEISKANEN A, SATOMAA T, TIITINEN S, et al. N-glycolylneuraminic acid xenoantigen contamination of human embryonic and mesenchymal stem cells is substantially reversible. Stem Cells. 2007;25(1):197-202. [35] JURKUNAS UV, YIN J, JOHNS LK, et al. Cultivated autologous limbal epithelial cell (CALEC) transplantation: Development of manufacturing process and clinical evaluation of feasibility and safety. Sci Adv. 2023;9(33):eadg6470. [36] HU Y, LIU X, LIU F, et al. Trehalose in Biomedical Cryopreservation-Properties, Mechanisms, Delivery Methods, Applications, Benefits, and Problems. ACS Biomater Sci Eng. 2023;9(3):1190-1204. [37] 刘宝林,赵子威.红细胞低温保存中海藻糖的加载方法[J].上海理工大学学报,2022,44(1):11-17. [38] WHALEY D, DAMYAR K, WITEK RP, et al. Cryopreservation: An Overview of Principles and Cell-Specific Considerations. Cell Transplant. 2021;30: 963689721999617. [39] WILL RG, IRONSIDE JW, ZEIDLER M, et al. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet. 1996;347(9006):921-925. [40] PEDEN AH, SULEIMAN S, BARRIA MA. Understanding Intra-Species and Inter-Species Prion Conversion and Zoonotic Potential Using Protein Misfolding Cyclic Amplification. Front Aging Neurosci. 2021;13:716452. [41] WENG L. Cell Therapy Drug Product Development: Technical Considerations and Challenges. J Pharm Sci. 2023;112(10):2615-2620. [42] LIANG X, HU X, HU Y, et al. Recovery and functionality of cryopreserved peripheral blood mononuclear cells using five different xeno-free cryoprotective solutions. Cryobiology. 2019;86:25-32. [43] MARESCHI K, ADAMINI A, CASTIGLIA S, et al. Cytokine-Induced Killer (CIK) Cells, In Vitro Expanded under Good Manufacturing Process (GMP) Conditions, Remain Stable over Time after Cryopreservation. Pharmaceuticals (Basel). 2020;13(5):93. [44] XU R, SHI X, HUANG H, et al. Development of a Me2SO-free cryopreservation medium and its long-term cryoprotection on the CAR-NK cells. Cryobiology. 2024;114:104835. [45] YAMATOYA K, NAGAI Y, TERAMOTO N, et al. Dimethyl Sulfoxide-Free Cryopreservation of Differentiated Human Neuronal Cells. Biopreserv Biobank. 2023;21(6):631-634. |
| [1] | Jin Kai, Tang Ting, Li Meile, Xie Yuan. Effects of conditioned medium and exosomes of human umbilical cord mesenchymal stem cells on proliferation, migration, invasion, and apoptosis of hepatocellular carcinoma cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1350-1355. |
| [2] | Li Dijun, Jiu Jingwei, Liu Haifeng, Yan Lei, Li Songyan, Wang Bin. Three-dimensional gelatin microspheres loaded human umbilical cord mesenchymal stem cells for chronic tendinopathy repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1356-1362. |
| [3] | Zheng Yitong, Wang Yongxin, Liu Wen, Amujite, Qin Hu. Action mechanism of intrathecal transplantation of human umbilical cord mesenchymal stem cell-derived exosomes for repair of spinal cord injury under neuroendoscopy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7743-7751. |
| [4] | Ren Shutong, Hao Miao, Liu Yue, Hou Ping, Quan Juanhua. Effect of human umbilical cord mesenchymal stem cells co-culture combined with ginsenoside Rg1 on heart failure cell model [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6625-6633. |
| [5] | Bu Xianmin, Liang Di, Zhang Bin, Xu Yingjie, Ding Hao, Wu Bin, Tian Ronghua. Exosomes derived from human umbilical cord mesenchymal stem cells in treatment of osteoporotic femoral fractures in SD rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6634-6641. |
| [6] | Wei Luxiao, Huang Bingxue, Du Jing, Shi Shuanxia, Wang Jitian, Wang Ling. Therapeutic effects and mechanism of human umbilical cord mesenchymal stem cells combined with melatonin on premature ovarian insufficiency induced by chemotherapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5281-5288. |
| [7] | Tao Chenyue, Chen Shuai, Wang Liping, Meng Defang, Zhou Dongjie, Zhou Luojing. Expression of Rab27A in ovarian tissue of polycystic ovary syndrome model mice treated with human umbilical cord mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5289-5295. |
| [8] | Feng Yirui, Gao Tianyun, Wang Yaping, Huang Yahong, Wang Bin. Interleukin-10 engineered human umbilical cord mesenchymal stem cells for superior treatment of inflammatory bowel disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(23): 4878-4887. |
| [9] | Chen Chunlan, Ye Meiyi, Pan Yuwei, Yuan Jia, Zhou Pengjun. Immunomodulatory effect of umbilical cord mesenchymal stem cells on type 2 diabetes mellitus [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(23): 5031-5040. |
| [10] | Xu Jie, Jiu Jingwei, Liu Haifeng, Zhao Bin. Bio-3D printed bionic scaffold promotes healing after rotator cuff injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(22): 4761-4770. |
| [11] | Ye Li, Tian Chuan, Zhao Xiaojuan, Chen Mengdie, Ye Qianqian, Li Qiang, Liao Zhuyin, Li Ye, Zhu Xiangqing, Ruan Guangping, He Zhixu, Shu Liping, Pan Xinghua. Effect and mechanisms of highly active umbilical cord mesenchymal stem cells on aging spleen in elderly tree shrews [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(19): 4000-4010. |
| [12] | Li Lingyu, Wei Huafeng, Luo Hao, Wang Hao, He Jiahui, Yao Yawei, Lyu Xinghua. Mechanism of human umbilical cord mesenchymal stem cell-derived exosomes against mouse renal ischemia/reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(13): 2706-2712. |
| [13] | Qiu Xiaoyan, Li Bixin, Li Jingdi, Fan Chuiqin, Ma Lian, Wang Hongwu. Differentiation of insulin-producing cells from human umbilical cord mesenchymal stem cells infected by MAFA-PDX1 overexpressed lentivirus [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1000-1006. |
| [14] | Liu Qiwei, Zhang Junhui, Yang Yuan, Wang Jinjuan. Role and mechanism of umbilical cord mesenchymal stem cells on polycystic ovary syndrome [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1015-1020. |
| [15] | Li Ruibo, Kong Ning, Sun Lei, Ma Baodong, Jin Ranran, Zhang Wenjin, Yue Han, Zhang Hui. Erythropoietin-overexpressed umbilical cord mesenchymal stem cells inhibit neuroapoptosis in ischemic-hypoxic SH-SY5Y and its mechanism [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(31): 4937-4944. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||