Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (32): 6947-6954.doi: 10.12307/2025.989
Previous Articles Next Articles
Integration of pathways for interaction mechanism between exercise and proteins
Wei Huqiang, Wu Hebin, Hou Yali, Zhang Xiangyong, Wang Zixuan, Wang Wenxuan, Bai Caiqin
- School of Physical Education, Shanxi Normal University, Taiyuan 030031, Shanxi Province, China
-
Received:2024-11-21Accepted:2024-12-24Online:2025-11-18Published:2025-04-28 -
Contact:Bai Caiqin, Master’s supervisor, Professor, School of Physical Education, Shanxi Normal University, Taiyuan 030031, Shanxi Province, China -
About author:Wei Huqiang, Master candidate, School of Physical Education, Shanxi Normal University, Taiyuan 030031, Shanxi Province, China -
Supported by:National Social Science “Thirteenth Five-Year Plan” 2020 Education Project (General), No. BLA200219 (to BCQ)
CLC Number:
Cite this article
Wei Huqiang, Wu Hebin, Hou Yali, Zhang Xiangyong, Wang Zixuan, Wang Wenxuan, Bai Caiqin. Integration of pathways for interaction mechanism between exercise and proteins[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6947-6954.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
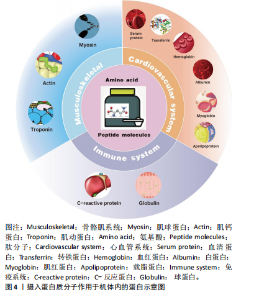
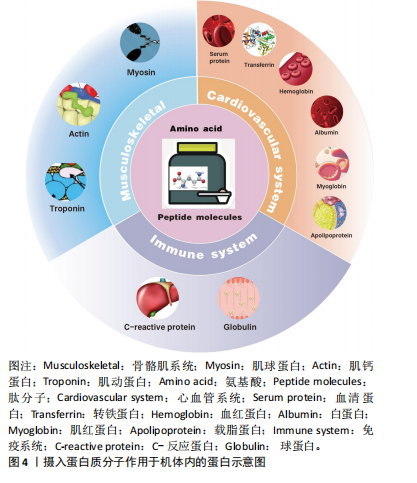
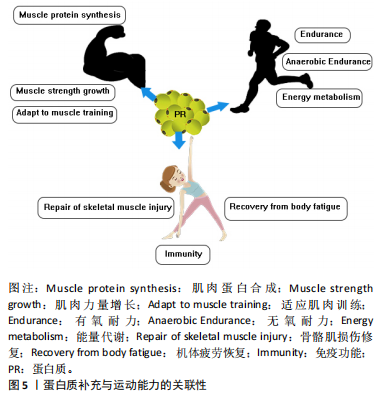
2.2 蛋白质补充在促进运动表现中的重要性 田麦久等[16]认为运动表现是人体在训练或比赛中所展现出的竞技能力,包括体能、技战术能力和心智能力等多个方面,也称其为竞技表现。在进行身体运动或日常锻炼中,人体依靠肌肉收缩来产生运动行为[17]。不同的项目对肌肉发力模式的要求不同,肌肉力量储备越充分,参与有效运动的时间就越长。蛋白质摄入能够对骨骼肌系统内相关蛋白产生积极的促进作用。在高强度运动中,肌肉蛋白消耗量增加,为了获得良好的身体状态和运动能力,运动人群应进行蛋白质补充以改善由大强度运动而出现的肌肉酸痛和身体疲劳[18]。 补充蛋白质能够促进心血管系统中相关蛋白的生成速率,提高机体的整体功能。在不同练习强度下,蛋白质补充干预需求也是不同的,进行蛋白质补充干预并不能用量过大,需要根据科学的方案来进行补充[19]。运动前或运动后进行蛋白质补充干预对运动能力的作用是肯定的,补充蛋白质能够促进机体免疫蛋白的产生,提高机体免疫功能。图4显示摄入蛋白质分子作用于机体内的蛋白。 2.3 蛋白质补充与运动能力的关联性 蛋白质补充与运动能力之间存在密切的关联性。运动能力是指人体在特定"
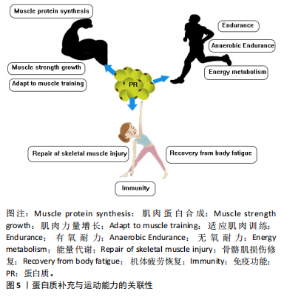
运动项目中所表现出来的综合能力,涵盖了力量、速度和耐力等多个方面,这些能力共同构成了人体提高运动表现的基础。运动能力的提升需要长期的训练、科学的营养补充策略。蛋白质是肌肉的重要组成部分,对于维持肌肉结构和功能至关重要。通过补充蛋白质,参与高强度练习的运动人群可以为肌肉提供充足的支持,促进肌肉生长和恢复,有关蛋白质补充营养品的主要成分为蛋白质及其水解物,能够满足自身机体的生长修复需要[20]。然而,如何科学精准地摄入蛋白质、避免潜在的健康风险等问题仍然需要深入研究和探讨。图5展示蛋白质补充与运动能力的关联性。 2.4 运动与蛋白质交互作用机制的关联性 2.4.1 运动与蛋白质交互作用对肌肉力量的影响 肌肉蛋白质是构成肌肉组织的基本单元,而肌肉蛋白质的合成是肌肉生长和恢复的关键过程[21]。在完成训练后,与补充单一蛋白质或碳水化合物相比,补充具有碳水化合物的蛋白质食物或者补充剂在促进肌肉蛋白质合成方面效果更显著[22]。优质蛋白含量按照20-40 g或0.25-0.40 g/kg的剂量摄入对机体肌肉蛋白合成的促进效果最佳[23]。使用碱性糖和蛋白质能够防止肌肉损失和力量下降[24]。 单靠蛋白补充来提高效益显然是不够的,需要配合适当锻炼才能够获得最佳效果。LOCKWOOD等[25]认为简单地补充蛋白质而不搭配力量训练或者其他种类运动,可能会产生一定的增肌效果,但效果并不显著,无法提高身体的脂肪含量。力量训练是提升肌肉力量的重要手段,而蛋白质补充则有助于增强运动员对力量训练的适应性。在力量训练过程中肌肉会经历微损伤,但可以通过修复"
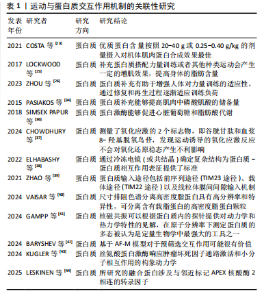
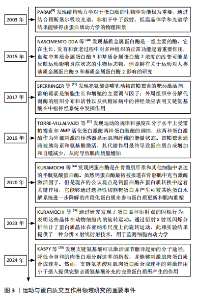
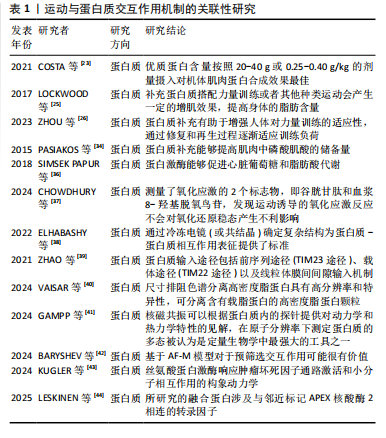
和再生过程逐渐适应训练负荷,从而实现肌肉力量的增长[26]。MACKAY-PHILLIPS等[27]通过对16名健康参与者进行α-乳清蛋白补充干预,得出α-乳清蛋白的使用能够刺激运动神经元的输入输出增益、增加疲劳肌肉收缩时的握力变异性、降低肌肉收缩的表现,但对健康个体的短暂最大收缩和心理参数没有影响。运动过程中肌肉纤维可能会遭受微损伤,而及时修复是保持肌肉功能和防止过度劳损的关键。对于蛋白摄入时机,有研究认为运动后补充蛋白质是缓解疲劳和修复损伤的一种合理选择[28]。KRITIKOS等[29]在试验中将补充大豆蛋白的实验组与补充乳清蛋白的对照组进行耐力训练恢复比较,发现在为期1周的训练中连续补充乳清蛋白或大豆蛋白并不能减少运动引起的肌肉酸痛、肌肉损伤和氧化还原状态标志物指数,不排除是由于蛋白摄入量不足所致。 2.4.2 运动与蛋白质交互作用对能量代谢的影响 有氧运动会使机体产生糖原消耗,造成乳酸和血尿素氮堆积,而补充单蛋白质能够增加葡萄糖和肝糖原含量,在提升氧化应激和抑制炎症通路方面能够发挥关键作用[30]。CAMERA等[31]发现耐力运动结束后补充蛋白质能够促进肌肉蛋白质的合成反应。TAN等[32]发现在碳水化合物饮料中添加蛋白质对耐力表现和抗氧化能力产生的影响不明显,但可以改善运动后产生的疲劳恢复。LI等[33]发现每天摄入40 g大豆分离蛋白补充剂还不足以提高人体的耐力运动表现,需要增加摄入量。蛋白质补充还可能通过提高肌肉中磷酸肌酸的储备量进一步提高人体无氧运动的能量供应和耐力表现[34]。能量代谢在细胞的生长和维持中起着关键作用,而合理的蛋白质分配能够提升能量代谢的效率[35];蛋白激酶能够促进心脏葡萄糖和脂肪酸代谢[36]。蛋白质补充可以通过影响人体的肌肉代谢和能量生成途径优化能量利用效率,还可能通过影响人体的胰岛素敏感性和脂肪氧化等过程进一步调节能量代谢。 2.4.3 运动与蛋白质交互作用在动力学方面的研究 由于运动通常能够与自由基的产生和以强度依赖性方式诱导氧化应激产生效应,有研究在试验过程中测量了氧化应激的2个标志物,即谷胱甘肽和血浆8-羟基脱氧鸟苷,发现运动诱导的氧化应激反应不会对氧化还原稳态产生不利影响[37]。现有的高精度蛋白质结构预测是将实验图与原子模型数据库对比而不是将序列进行比较,从而有助于以较低分辨率开展蛋白质鉴定。虽然通过基于模板的建模和对接获得的界面可能会存在较大的不确定性,但通过冷冻电镜(或共结晶)确定复杂结构为蛋白质-蛋白质相互作用表征提供了标准。共结晶和冷冻电镜可以提供通过相互作用形成复合物的最高分辨率,但它们也是最耗费人力的,并且成功率不确定。交联质谱法和序列协同进化等方法的劳动强度较低,并且具有更高的可计划成功率,但它们提供了与实验相关的中等分辨率数据,并且必须要与单个蛋白质结构相结合才能组成复合物[38]。 ZHAO等[39]发现线粒体蛋白质输入系统不仅能够作为蛋白质易位的独立单位发挥作用,而且还深度集成到线粒体生物能量学、蛋白质质量控制、线粒体动力学和形态以及与其他细胞器相互作用的功能网络中,得出关键的蛋白质输入途径包括前序列途径(TIM23途径)、载体途径(TIM22途径)以及线粒体膜间间隙输入和组装机制、相关的转位酶和蛋白酶。线粒体对于真核细胞的能量产生至关重要,通过氧化磷酸化能够产生细胞ATP。VAISAR等[40]在研究中通过尺寸排阻色谱分离高密度脂蛋白,之后将其与硅酸钙水合物结合,这种方法可能错过了捕获非常小的贫脂前β1颗粒,以及通过载脂蛋白通路获取胆固醇外排的主要介质。该研究的优势是一种新颖的3D分离方法,与其他分离技术相比具有高分辨率和特异性,可分离含有载脂蛋白的高密度脂蛋白颗粒。GAMPP等[41]提出由于溶液态核磁共振是在水溶液生理条件下测量的,因此核磁共振波谱的发展在人们对蛋白质变构的理解中发挥了关键作用。除了蛋白质结构的原子分辨率信息外,核磁共振波谱还可以根据蛋白质内的探针提供对动力学和热力学特性的见解,在原子分辨率下通过实验测定蛋白质的多态被认为是定量生物学中最强大的工具之一。 BARYSHEV等[42]发现了脱氧核糖核酸条形码与特定的蛋白质相关联,并且可以通过测序读取条形码富集,从而直接测量相互作用强度,实验发现MP3-seq是高度定量的且可以扩展到超过100 000 次交互。KUGLER等[43]应用MP3-seq来表明合理设计的异二聚体家族之间的相互作用,并且研究了赋予卷曲螺旋相互作用特异性的元件;使用人工智能预测卷曲异二聚体结构,并根据基于物理的线性模型来预测MP3-seq值,研究得出基于人工智能的模型对于预筛选交互作用可能很有价值,探讨了丝氨酸蛋白激酶响应肿瘤坏死因子通路激活和促进小分子相互作用的构象动力学。LESKINEN等[44]发现转录因子之间动态相互作用控制基因表达的变化,这些基因表达介导伴随损伤反应和再生细胞状态的变化,并且所研究的融合蛋白涉及与邻近标记APEX核酸酶2相连的转录因子。表1为运动与蛋白质交互作用机制的关联性研究的重要"
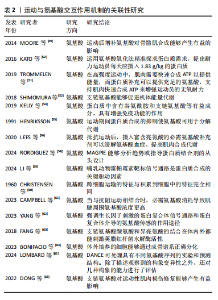
结论。 2.5 运动与氨基酸交互作用机制的关联性 2.5.1 运动与氨基酸交互作用对机体恢复的影响 氨基酸是构成蛋白质的基本单位,也是生命体系中非常重要的有机分子,由一个氨基(NH2)、一个羧基(COOH)和一个R基团组成。氨基酸是通过肽键互相连接而形成的肽链构架,在肽链中,一个氨基酸的羧基通过其碳氧键与另一个氨基酸的氨基连接。植物和动物体内的氨基酸基本上是18种,这是构成动植物蛋白的种类,但并不是说氨基酸的种类只有18种,目前世界上发现的氨基酸有300余种。 乳清蛋白中含有丰富的亮氨酸,能产生提高力量素质的效益。大豆蛋白肽中也含有亮氨酸,也能产生促进肌肉力量增长的效益[45]。小麦蛋白中含有赖氨酸、异亮氨酸和苯丙氨酸,能产生增加肌肉合成速率的效益[46]。上述蛋白质在长时间、高强度的运动中能够作为能源物质为机体提供能量,通过其分解为肌肉收缩和其他活动提供必要的动力。大豆蛋白的氨基酸成分与牛奶蛋白的氨基酸成分相近似,能产生提升耐力素质的效益[47]。HOLWERDA等[48]研究发现,进行抗阻运动后,在睡前补充蛋白质能提高老年男性的夜间肌肉结缔组织蛋白质合成率,而运动促进了膳食蛋白质转化为氨基酸。MOORE等[49]发现骨骼肌蛋白质的周转会根据单次的耐力运动而上升,运动后增补氨基酸对骨骼肌合成有益,摄入膳食蛋白质会对蛋白质周转产生有利影响。KATO等[50]运用氨基酸氧化法精准探求试验群体的蛋白质需求,提出耐力运动员每天应摄入1.83 g/kg的蛋白质。在高强度运动中肌肉需要快速合成ATP以提供能量,而蛋白质补充可以提供充足的氨基酸,支持肌肉快速合成ATP来增强运动员的无氧耐力[51]。支链氨基酸能够促进机体能量代谢[52];SAUNDERS等[53]发现β-丙氨酸具有明显的总体影响,而亚组分析结果能够促使人们根据自身选择的运动方式,有针对性地补充β-丙氨酸[53]。 运动人群在训练和比赛中经常面临免疫系统的挑战,免疫功能下降可能导致身体出现健康问题。蛋白质中含有谷氨酰胺和支链氨基酸等有益成分,具有增强免疫功能的作用[54]。运动期间氨基酸分解代谢率仍显著增加,这是运动引起多种代谢过程增加的继发因素,例如利用氨基酸碳的肝糖异生和柠檬酸循环来产生效益。运动期间蛋白质合成的抑制使氨基酸能够在分解代谢中发挥作用[55]。抵抗运动后,补充富含亮氨酸的必需氨基酸补充剂能够在缓解氨基酸血症和促进肌肉合成代谢方面发挥作用[56]。摄入游离形式的必需氨基酸比摄入等量的完整蛋白质更能刺激肌肉蛋白质合成。J?GER等[57]发现在不运动的情况下,补充必需氨基酸可以增强人体的肌肉合成代谢;如果热量不足,必需氨基酸需求量会增加,在热量不足期间必须满足全身必需氨基酸要求,从而保持骨骼肌的合成代谢敏感性。 2.5.2 运动与氨基酸交互作用的信号通路研究 RORDIGUEZ等[58]研究指出MAGPIE(是一个公开可用的软件包)可用于可视化和分析单个蛋白质,其将最常见的靶点-结合剂相互作用整理为“热点”列表,能够用于分析趋势或指导蛋白质结合剂的从头设计。 LI等[59]提出了支链氨基酸能够通过复杂的细胞内信号通路网络,是骨骼肌蛋白代谢调节不可或缺的一部分。雷帕霉素靶标信号通路是哺乳动物蛋白质合成的关键驱动因素,真核翻译起始因子4E结合蛋白1和S6激酶1作为雷帕霉素靶标1的磷酸化底物能够起到关键作用。亮氨酸通过多种机制激活雷帕霉素靶标1信号通路,包括蛋白复合物的破坏、亮氨酸tRNA合成酶的激活以及蛋白激酶的去磷酸化,这些都是由其代谢物β-羟基-β-甲基丁酸酯促进的。 CHRISTENSEN等[60]采用简单的形式研究跨肠黏膜或类似屏障的运输问题,发现细胞可以排列成一个膜,能够将氨基酸从一个区域浓缩到另一个由膜隔开的2个区域中,这是通过在第一区域中添加吡哆醛或磷酸吡哆醛,或通过在第二区域添加过量的钾离子来实现的。 食用富含氨基酸的化合物可以提高肌腱胶原蛋白含量和生物力学特性。当不与运动相结合时,口服必需氨基酸仅导致跟腱周围氨基酸浓度适度增加;当与抗阻运动相结合时,必需氨基酸消耗导致肌腱周围氨基酸浓度更高[61]。YANG等[62]通过研究发现,中枢神经系统中髓鞘形成的少突胶质细胞和周围神经系统中的施万细胞对于维护神经组织的结构和功能稳态至关重要。 FANG等[63]的实验表明,存在于荚膜红杆菌酶中的ACT结构域结合支链氨基酸缬氨酸和异亮氨酸,这些氨基酸的结合在体内外都刺激荚膜红杆菌水解酶活性;此外,研究发现许多不同物种的荚膜红杆菌蛋白中存在的ACT结构域也结合支链氨基酸,细胞中氨基酸的浓度可以直接影响荚膜红杆菌合成酶的活性,更好地控制细胞模型的严格反应。 BONIFACIO等[64]发现在透明质酸和氨基酸存在下,成骨细胞的代谢活性增强和细胞周期进程加快,并且透明质酸和氨基酸的组合也增强了碱性磷酸酶活性,证实体外培养的细胞能够通过成骨谱系正确分化。 2.5.3 运动与氨基酸交互作用的动力学研究 LOMBARD等[65]描述了不同序列同源水平蛋白质构象变异性的蛋白质数据库范围分析,DANCE可处理具有不同氨基酸序列的实验和预测结构,除了描述观察到的构象变异性之外还评估了几种构象的能力,为此,研究确定了一组10个构象集合,这些构象集合的运动复杂程度不同,它们包含20-3 300多个构象,其参考链包含80-1 200个残基。DONG等[66]的研究结果表明,支链氨基酸对运动性肌肉损伤修复能够产生有益影响,可以刺激肌肉细胞的增殖和分化,认为支链氨基酸在改善肌肉损伤方面具有潜在营养价值。表2为运动与氨基酸交互作用机制关联性研究的重要结论。"
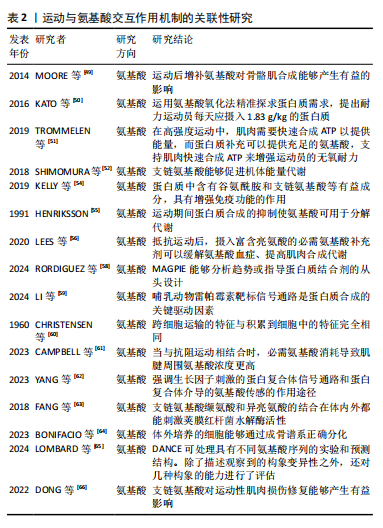

2.6 运动与多肽交互作用机制的关联性 2.6.1 运动与多肽交互作用对能量代谢的影响 多肽是由氨基酸通过肽键连接而成的长链分子。肽键是氨基酸之间形成的共价键,连接一个氨基酸的氨基和另一个氨基酸的羧基。多肽链可以进一步折叠和卷曲,形成具有特定功能的蛋白质。蛋白质是生命体中最重要的生物大分子之一,参与了几乎所有的生物过程,如催化化学反应、传递信号和提供结构支持。与氨基酸和蛋白质相比,小分子肽更容易被人体分解和吸收,可以加快人体在大量运动后的恢复速度,调节骨骼肌蛋白质的负平衡[67]。 运动会改变人体血浆氨基酸谱,从而导致中枢性疲劳;支链氨基酸可以改善运动疲劳,那么在运动后依靠植物蛋白肽向人体内补充支链氨基酸能提高肌肉组织中氨基酸的利用效率,减少肌肉糖原的消耗,降低肌肉分解率。在特殊状态下活性肽能为人体供能[68]。ZHANG等[69]研究发现豌豆肽属于小分子蛋白质,在人体内吸收速度快,吸收速度是正常氨基酸的3倍以上,约为正常蛋白质的20倍,能给人体补充能量,从而恢复体力,进一步证实了补充蛋白质能够改善疲劳恢复的观点。HA等[70]检测到血清中2个含d-天门冬氨酸的肽,全部来自于纤维蛋白原β链,这两种肽中的一种是纤维蛋白肽B,可防止纤维蛋白原形成纤维蛋白聚合物。口服补充特定胶原蛋白肽可以缓解跟腱病患者的临床症状,帮助患者恢复跑步计划[71]。 2.6.2 运动与多肽交互作用的序列研究 肽“药物”最初仅仅依靠发挥激素类似物的作用来平衡疾病,如今它们完成了许多生物医学任务,可以穿过膜或到达细胞内靶标。肽在生物过程中的作用几乎无法被其他化学物质所模仿[72]。YOO等[73]研究肽的形态特征、分子堆积结构和物理性质,通过粉末X射线衍射发现分子间氢键以从头到尾的方式连接折叠体,折叠体构建块中的每个堆积结构都具有不同的对称元素,这些元素直接以3D形态表示。"

| [1] DI MEO A, SOHAEI D, BATRUCH I, et al. Proteomic Profiling of the Human Tissue and Biological Fluid Proteome. J Proteome Res. 2021; 20(1):444-452. [2] CAMERA DM. Evaluating the Effects of Increased Protein Intake on Muscle Strength, Hypertrophy and Power Adaptations with Concurrent Training: A Narrative Review. Sports Med. 2022;52(3):441-461. [3] ZELENKOVA IE, ZOTKIN SV, KORNEEV PV, et al. Relationship between total hemoglobin mass and competitive performance in endurance athletes. J Sports Med Phys Fitness. 2019;59(3):352-356. [4] MAUNDER E, ROTHSCHILD JA, FRITZEN AM, et al. Skeletal muscle proteins involved in fatty acid transport influence fatty acid oxidation rates observed during exercise. Pflugers Arch. 2023;475(9):1061-1072. [5] LI C, LU Y, WANG J, et al. Immunoregulation of bovine lactoferrin together with osteopontin promotes immune system development and maturation. Food Funct. 2024;15(2):866-880. [6] 李世成,杨则宜.活性肽及其在运动中的应用[J].中国运动医学杂志,2003,22(2):174-176,149. [7] MASTER PBZ, MACEDO RCO. Effects of dietary supplementation in sport and exercise: a review of evidence on milk proteins and amino acids. Crit Rev Food Sci Nutr. 2021;61(7):1225-1239. [8] MANORE MM, PATTON-LOPEZ MM, MENG Y, et al. Sport Nutrition Knowledge, Behaviors and Beliefs of High School Soccer Players. Nutrients. 2017;9(4):350. [9] PARAK FG. Proteins in action: the physics of structural fluctuations and conformational changes. Curr Opin Struct Biol. 2003;13(5):552-557. [10] NASCIMENTO DDA C, DURIGAN RDE C, TIBANA RA, et al. The response of matrix metalloproteinase-9 and -2 to exercise. Sports Med. 2015; 45(2):269-278. [11] SPERRINGER JE, ADDINGTON A, HUTSON SM. Branched-Chain Amino Acids and Brain Metabolism. Neurochem Res. 2017;42(6):1697-1709. [12] TORRE-VILLALVAZO I, ALEMAN-ESCONDRILLAS G, VALLE-RIOS R, et al. Protein intake and amino acid supplementation regulate exercise recovery and performance through the modulation of mTOR,AMPK,FGF21,and immunity. Nutr Res. 2019;72:1-17. [13] KURAMOCHI M, HYATT HW, POWERS SK. The Role of Calpains in Skeletal Muscle Remodeling with Exercise and Inactivity-induced Atrophy. Int J Sports Med. 2020;41(14):994-1008. [14] KURAMOCHI M, SUGAWARA I, SHINKAI Y, et al. Time-Resolved X-ray Observation of Intracellular Crystallized Protein in Living Animal. Int J Mol Sci. 2023;24(23):16914. [15] KASPY MS, HANNAIAN SJ, BELL ZW, et al. The effects of branched-chain amino acids on muscle protein synthesis,muscle protein breakdown and associated molecular signalling responses in humans: an update. Nutr Res Rev. 2024;37(2):273-286. [16] 田麦久,田烈,高玉花.运动训练理论核心概念的界定及认知的深化[J].天津体育学院学报,2020,35(5):497-505,512 [17] HERZOG W, SCHAPPACHER-TILP G. Molecular mechanisms of muscle contraction: A historical perspective. J Biomech. 2023;155:111659. [18] 冯兰兰,窦燕,陈嘉威,等.高蛋白运动补剂对空手道运动员骨密度的影响[J].食品研究与开发,2023,44(23):234-236. [19] 李良,苏浩.运动后联合补充糖与蛋白质对肌糖原合成效果的研究进展[J].体育科学,2015,35(9):84-89. [20] PEARSON AG, HIND K, MACNAUGHTON LS. The impact of dietary protein supplementation on recovery from resistance exercise-induced muscle damage: A systematic review with meta-analysis. Eur J Clin Nutr. 2023;77(8):767-783. [21] DIRKS ML, GROEN BB, FRANSSEN R, et al. Neuromuscular electrical stimulation prior to presleep protein feeding stimulates the use of protein-derived amino acids for overnight muscle protein synthesis. J Appl Physiol (1985). 2017;122(1):20-27. [22] KOVACS MS, BAKER LB. Recovery interventions and strategies for improved tennis performance. Br J Sports Med. 2014;48 Suppl 1 (Suppl 1):i18-21. [23] COSTA JV, MICHEL JM, MADZIMA TA. The Acute Effects of a Relative Dose of Pre-Sleep Protein on Recovery Following Evening Resistance Exercise in Active Young Men. Sports (Basel). 2021;9(4):44. [24] UR REHMAN H, AZAM N, YAO J, et al. A three-way approach for protein function classification. PLoS One. 2017;12(2):e0171702. [25] LOCKWOOD CM, ROBERTS MD, DALBO VJ, et al. Effects of Hydrolyzed Whey versus Other Whey Protein Supplements on the Physiological Response to 8 Weeks of Resistance Exercise in College-Aged Males. J Am Coll Nutr. 2017;36(1):16-27. [26] ZHOU HH, LIAO Y, ZHOU X, et al. Effects of Timing and Types of Protein Supplementation on Improving Muscle Mass,Strength,and Physical Performance in Adults Undergoing Resistance Training: A Network Meta-Analysis. Int J Sport Nutr Exerc Metab. 2023;34(1):54-64. [27] MACKAY-PHILLIPS K, ORSSATTO LBR, POLMAN R, et al. Effects of α-lactalbumin on strength,fatigue and psychological parameters: a randomised double-blind cross-over study. Eur J Appl Physiol. 2023; 123(2):381-393. [28] ARENT SM, CINTINEO HP, MCFADDEN BA, et al. Nutrient Timing: A Garage Door of Opportunity? Nutrients. 2020;12(7):1948. [29] KRITIKOS S, PAPANIKOLAOU K, DRAGANIDIS D, et al. Effect of whey vs. soy protein supplementation on recovery kinetics following speed endurance training in competitive male soccer players: a randomized controlled trial. J Int Soc Sports Nutr. 2021;18(1):23. [30] ZHAO C, GONG Y, ZHENG L, et al. Whey protein hydrolysate enhances exercise endurance, regulates energy metabolism, and attenuates muscle damage in exercise mice. Food Bioscience. 2023;52:102453. [31] CAMERA DM, ONG JN, COFFEY VG, et al. Selective Modulation of MicroRNA Expression with Protein Ingestion Following Concurrent Resistance and Endurance Exercise in Human Skeletal Muscle. Front Physiol. 2016;7:87. [32] TAN AY, HAMZAH SH, HUANG CY, et al. Pre-exercise Carbohydrate Drink Adding Protein Improves Post-exercise Fatigue Recovery. Front Physiol. 2021;12:765473. [33] LI F, HSUEH YT, HSU YJ, et al. Effects of Isolated Soy Protein Supplementation Combined with Aerobic Exercise Training on Improving Body Composition,Anthropometric Characteristics and Cardiopulmonary Endurance in Women: A Pilot Study. Int J Environ Res Public Health. 2021;18(22):11798. [34] PASIAKOS SM, MCLELLAN TM, LIEBERMAN HR. The effects of protein supplements on muscle mass,strength,and aerobic and anaerobic power in healthy adults: a systematic review. Sports Med. 2015;45(1): 111-131. [35] CHEN Y, NIELSEN J. Energy metabolism controls phenotypes by protein efficiency and allocation. Proc Natl Acad Sci U S A. 2019;116(35): 17592-17597. [36] SIMSEK PAPUR O, SUN A, GLATZ JFC, et al. Acute and Chronic Effects of Protein Kinase-D Signaling on Cardiac Energy Metabolism. Front Cardiovasc Med. 2018;5:65.
[37] CHOWDHURY MMH, FONTAINE MN, LORD SE, et al. Impact of a tailored exercise regimen on physical capacity and plasma proteome profile in post-COVID-19 condition. Front Physiol. 2024;15:1416639. [38] ELHABASHY H, MERINO F, ALVA V, et al. Exploring protein-protein interactions at the proteome level. Structure. 2022;30(4):462-475. [39] ZHAO F, ZOU MH. Role of the Mitochondrial Protein Import Machinery and Protein Processing in Heart Disease. Front Cardiovasc Med. 2021; 8:749756. [40] VAISAR T, BABENKO I, HORVATH KV, et al. Relationships between HDL subpopulation proteome and HDL function in overweight/obese people with and without coronary heart disease. Atherosclerosis. 2024;397:118565. [41] GAMPP O, KAGAVATH H,RIEK R. NMR tools to detect protein allostery. Curr Opin Struct Biol. 2024;86:102792. [42] BARYSHEV A, LA FLEUR A, GROVES B, et al. Massively parallel measurement of protein-protein interactions by sequencing using MP3-seq. Nat Chem Biol. 2024;20(11):1514-1523. [43] KUGLER V, SCHWAIGHOFER S, FEICHTNER A, et al. Impact of protein and small molecule interactions on kinase conformations. Elife. 2024; 13:RP94755. [44] LESKINEN HL, UDVADIA AJ. Development and Validation of a Proximity Labeling Fusion Protein Construct to Identify the Protein-Protein Interactions of Transcription Factors. Methods Mol Biol. 2025;2848: 269-297. [45] MOON JY, CHAI JC, YU B, et al. Metabolomic Signatures of Sedentary Behavior and Cardiometabolic Traits in US Hispanics/Latinos: Results from HCHS/SOL. Med Sci Sports Exerc. 2023;55(10):1781-1791. [46] PINCKAERS PJM, KOUW IWK, HENDRIKS FK,et al. No differences in muscle protein synthesis rates following ingestion of wheat protein,milk protein,and their protein blend in healthy, young males. Br J Nutr. 2021;126(12):1832-1842. [47] KRITIKOS S, PAPANIKOLAOU K, DRAGANIDIS D, et al. soy protein supplementation on recovery kinetics following speed endurance training in competitive male soccer players: a randomized controlled trial. J Int Soc Sports Nutr. 2021;18(1):23. [48] HOLWERDA AM, TROMMELEN J, KOUW IWK, et al. Exercise Plus Presleep Protein Ingestion Increases Overnight Muscle Connective Tissue Protein Synthesis Rates in Healthy Older Men. Int J Sport Nutr Exerc Metab. 2021;31(3):217-226. [49] MOORE DR, CAMERA DM, ARETA JL, et al. Beyond muscle hypertrophy: why dietary protein is important for endurance athletes. Appl Physiol Nutr Metab. 2014;39(9):987-997. [50] KATO H, SUZUKI K, BANNAI M, et al. Protein Requirements Are Elevated in Endurance Athletes after Exercise as Determined by the Indicator Amino Acid Oxidation Method. PLoS One. 2016;11(6):e0157406. [51] TROMMELEN J, BETZ MW, VAN LOON LJC. The Muscle Protein Synthetic Response to Meal Ingestion Following Resistance-Type Exercise. Sports Med. 2019;49(2):185-197. [52] SHIMOMURA Y, KITAURA Y. Physiological and pathological roles of branched-chain amino acids in the regulation of protein and energy metabolism and neurological functions. Pharmacol Res. 2018;133: 215-217. [53] SAUNDERS B, ELLIOTT-SALE K, ARTIOLI GG, et al. β-alanine supplementation to improve exercise capacity and performance: a systematic review and meta-analysis. Br J Sports Med. 2017;51(8): 658-669. [54] KELLY B, PEARCE EL. Amino Assets: How Amino Acids Support Immunity. Cell Metab. 2020;32(2):154-175. [55] HENRIKSSON J. Effect of exercise on amino acid concentrations in skeletal muscle and plasma. J Exp Biol. 1991;160:149-165. [56] LEES MJ, WILSON OJ, WEBB EK, et al. Novel Essential Amino Acid Supplements Following Resistance Exercise Induce Aminoacidemia and Enhance Anabolic Signaling Irrespective of Age: A Proof-of-Concept Trial. Nutrients. 2020;12(7):2067. [57] JÄGER R, KERKSICK CM, CAMPBELL BI, et al. International Society of Sports Nutrition Position Stand: protein and exercise. J Int Soc Sports Nutr. 2017;14:20. [58] RODRIGUEZ DCP, WEBER KC, SUNDBERG B, et al. MAGPIE: An interactive tool for visualizing and analyzing protein-ligand interactions. Protein Sci. 2024;33(8):e502. [59] LI G, LI Z, LIU J. Amino acids regulating skeletal muscle metabolism: mechanisms of action,physical training dosage recommendations and adverse effects. Nutr Metab (Lond). 2024;21(1):41. [60] CHRISTENSEN HN, OXENDER DL. Transport of amino acids into and across cells. Am J Clin Nutr. 1960;8:131-136. [61] CAMPBELL NWC, PATEL SH, FERRANDI P, et al. Impact of essential amino acid intake,resistance exercise,and aging on the concentration of Achilles peritendinous amino acids and procollagen Iα1 in humans. Amino Acids. 2023;55(6):777-787. [62] YANG Z, YU Z, XIAO B. Coordinated Regulation of Myelination by Growth Factor and Amino-acid Signaling Pathways. Neurosci Bull. 2023; 39(3):453-465. [63] FANG M, BAUER CE. Regulation of stringent factor by branched-chain amino acids. Proc Natl Acad Sci U S A. 2018;115(25):6446-6451. [64] BONIFACIO MA, CASSANO A, VINCENTI A, et al. In Vitro Evaluation of the Effects of Hyaluronic Acid and an Aminoacidic Pool on Human Osteoblasts. Biomedicines. 2023;11(3):751. [65] LOMBARD V, GRUDININ S, LAINE E. Explaining Conformational Diversity in Protein Families through Molecular Motions. Sci Data. 2024;11(1): 752. [66] DONG Y, ZHANG X, MIAO R, et al. Branched-chain amino acids promotes the repair of exercise-induced muscle damage via enhancing macrophage polarization. Front Physiol. 2022;13:1037090. [67] KUMAR S, PANDEY G. Biofortification of pulses and legumes to enhance nutrition. Heliyon. 2020;6(3):e03682. [68] TKACZEWSKA J, JAMROZ E, KULAWIK P, et al. Evaluation of the potential use of a carp (Cyprinus carpio) skin gelatine hydrolysate as an antioxidant component. Food Funct. 2019;10(2):1038-1048. [69] ZHANG H, CHEN Y, GUO Y, et al. Label-free quantification proteomics reveals the active peptides from protein degradation during anaerobic fermentation of tea. LWT. 2021;(39):111950. [70] HA S, KIM I, TAKATA T, et al. Identification of ᴅ-amino acid-containing peptides in human serum. PLoS One. 2017;12(12):e0189972. [71] PRAET SFE, PURDAM CR, WELVAERT M, et al. Oral Supplementation of Specific Collagen Peptides Combined with Calf-Strengthening Exercises Enhances Function and Reduces Pain in Achilles Tendinopathy Patients. Nutrients. 2019;11(1):76. [72] APOSTOLOPOULOS V, BOJARSKA J, CHAI TT, et al. A Global Review on Short Peptides: Frontiers and Perspectives. Molecules. 2021;26(2):430. [73] YOO SH, LEE HS. Foldectures: 3D Molecular Architectures from Self-Assembly of Peptide Foldamers. Acc Chem Res. 2017;50(4):832-841. |
| [1] | Yu Shuai, Liu Jiawei, Zhu Bin, Pan Tan, Li Xinglong, Sun Guangfeng, Yu Haiyang, Ding Ya, Wang Hongliang. Hot issues and application prospects of small molecule drugs in treatment of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1913-1922. |
| [2] | Li Jun, Gong Jingjing, Sun Guobin, Guo Rui, Ding Yang, Qiang Lijuan, Zhang Xiaoli, Fang Zhanhai . miR-27a-3p promotes the proliferation of human hypertrophic scar fibroblasts by regulating mitogen-activated protein kinase signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1609-1617. |
| [3] | Zhao Jiacheng, Ren Shiqi, Zhu Qin, Liu Jiajia, Zhu Xiang, Yang Yang. Bioinformatics analysis of potential biomarkers for primary osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1741-1750. |
| [4] | Yin Lu, Jiang Chuanfeng, Chen Junjie, Yi Ming, Wang Zihe, Shi Houyin, Wang Guoyou, Shen Huarui. Effect of Complanatoside A on the apoptosis of articular chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1541-1547. |
| [5] | Aikepaer · Aierken, Chen Xiaotao, Wufanbieke · Baheti. Osteogenesis-induced exosomes derived from human periodontal ligament stem cells promote osteogenic differentiation of human periodontal ligament stem cells in an inflammatory microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1388-1394. |
| [6] | Zhang Haojun, Li Hongyi, Zhang Hui, Chen Haoran, Zhang Lizhong, Geng Jie, Hou Chuandong, Yu Qi, He Peifeng, Jia Jinpeng, Lu Xuechun. Identification and drug sensitivity analysis of key molecular markers in mesenchymal cell-derived osteosarcoma [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1448-1456. |
| [7] | Sun Yuting, Wu Jiayuan, Zhang Jian. Physical factors and action mechanisms affecting osteogenic/odontogenic differentiation of dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1531-1540. |
| [8] | Yu Ting, Lyu Dongmei, Deng Hao, Sun Tao, Cheng Qian. Icariin pretreatment enhances effect of human periodontal stem cells on M1-type macrophages [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1328-1335. |
| [9] | Zhao Ruihua, Chen Sixian, Guo Yang, Shi Lei, Wu Chengjie, Wu Mao, Yang Guanglu, Zhang Haoheng, Ma Yong. Wen-Shen-Tong-Du Decoction promoting spinal cord injury repair in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1118-1126. |
| [10] | Zheng Lin, Jin Wenjun, Luo Shanshan, Huang Rui, Wang Jie, Cheng Yuting, An Zheqing, Xiong Yue, Gong Zipeng, Liao Jian. Eucommia ulmoides promotes alveolar bone formation in ovariectomized rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1159-1167. |
| [11] | Zhang Debao, Wang Peng, Li Kun, Zhang Shaojie, Li Zhijun, Li Shuwen, Wu Yimin. Epidural fibrous scar formation in rabbits following autologous ligamentum flavum intervention [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1168-1175. |
| [12] | Ji Huihui, Jiang Xu, Zhang Zhimin, Xing Yunhong, Wang Liangliang, Li Na, Song Yuting, Luo Xuguang, Cui Huilin, Cao Ximei. SR9009 combined with indolepropionic acid alleviates inflammation in C2C12 myoblasts through the nuclear factor-kappa B signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1220-1229. |
| [13] | He Bo, Chen Wen, Ma Suilu, He Zhijun, Song Yuan, Li Jinpeng, Liu Tao, Wei Xiaotao, Wang Weiwei, Xie Jing . Pathogenesis and treatment progress of flap ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1230-1238. |
| [14] | Zhang Wenhua, Li Xun, Zhang Weichao, Li Xinying, Ma Guoao, Wang Xiaoqiang . Promoting myogenesis based on the SphK1/S1P/S1PR2 signaling pathway: a new perspective on improving skeletal muscle health through exercise [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1265-1275. |
| [15] | Qian Kun, Li Ziqing, Sun Shui . Endoplasmic reticulum stress in the occurrence and development of common degenerative bone diseases [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1285-1295. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||