Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (30): 6449-6456.doi: 10.12307/2025.907
Previous Articles Next Articles
Differences of calorie restriction and time-restricted feeding on metabolic indices and gut microbiota of mice
Cui Yuena1, Chen Xiaoyu1, Liang Meiting2, Chen Wujin1, He Yi1, 2, Dilinur·Ekpa1, Du Manxi1, Zhu Yuqiu1, Abuduwupuer·Haibier1, #br# Sun Yuping1, 3#br#
- 1Xinjiang Medical University, Urumqi 830054, Xinjiang Uyghur Autonomous Region, China; 2Xinjiang Second Medical College, Karamay 834099, Xinjiang Uyghur Autonomous Region, China; 3Xinjiang Key Laboratory of Molecular Biology for Endemic Diseases, Urumqi 830054, Xinjiang Uyghur Autonomous Region, China
-
Received:2024-10-14Accepted:2024-11-22Online:2025-10-28Published:2025-03-28 -
Contact:Sun Yuping, MD, Professor, Xinjiang Medical University, Urumqi 830054, Xinjiang Uyghur Autonomous Region, China; Xinjiang Key Laboratory of Molecular Biology for Endemic Diseases, Urumqi 830054, Xinjiang Uyghur Autonomous Region, China -
About author:Cui Yuena, MS, Xinjiang Medical University, Urumqi 830054, Xinjiang Uyghur Autonomous Region, China -
Supported by:Natural Science Foundation of Xinjiang Uygur Autonomous Region (Youth Science Fund), No. 2022D01C211 (to CXY)
CLC Number:
Cite this article
Cui Yuena, Chen Xiaoyu, Liang Meiting, Chen Wujin, He Yi, Dilinur·Ekpa, Du Manxi, Zhu Yuqiu, Abuduwupuer·Haibier, Sun Yuping. Differences of calorie restriction and time-restricted feeding on metabolic indices and gut microbiota of mice[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6449-6456.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
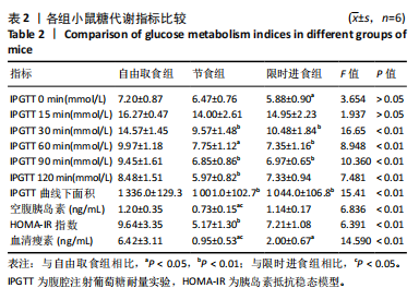
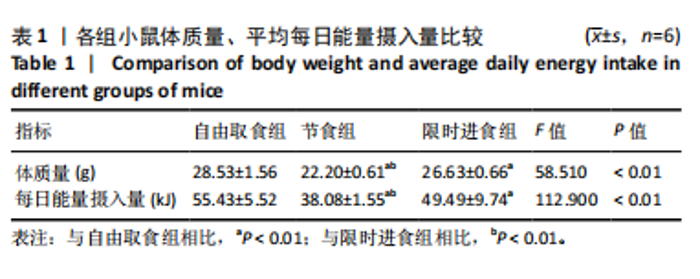
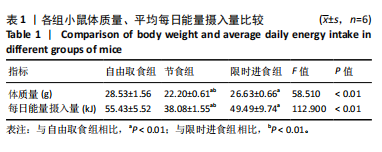
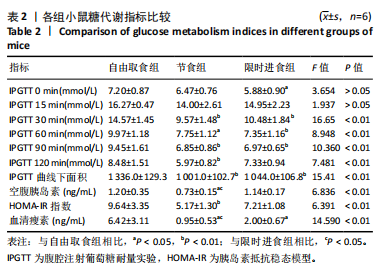
2.2.2 各组小鼠糖代谢指标比较 与自由取食组相比,节食组干预后每个时间点的血糖浓度均降低,除了0 min和15 min时刻,差异均有显著性意义(P < 0.05);限时进食组每个时间点的血糖浓度均降低,除了15 min和120 min时刻,差异均有显著性意义(P < 0.05)。节食组和限时进食组腹腔注射葡萄糖耐量实验曲线下面积与自由取食组相比显著降低(P < 0.01)。节食组小鼠空腹胰岛素水平和HOMA-IR指数与自由取食组相比均降低(P < 0.05),但限时进食组与自由取食组相比差异无显著性意义(P > 0.05)。此外,与自由取食组相比,节食组和限时进食组血清瘦素水平均降低(P < 0.05)。与限时进食组相比,节食组空腹胰岛素和血清瘦素水平降低(P < 0.05),其他指标差异均无显著性意义(P > 0.05),见表2。以上结果表明,节食和限时进食干预后小鼠糖代谢均有所改善,但限时进食效果不如节食。"
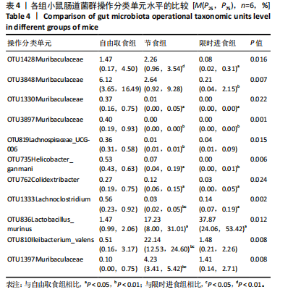
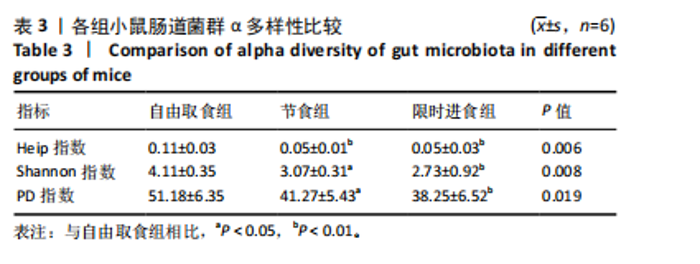
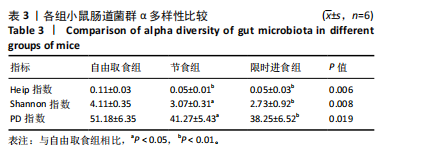
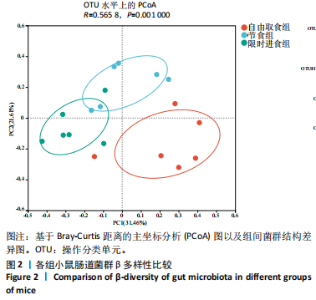
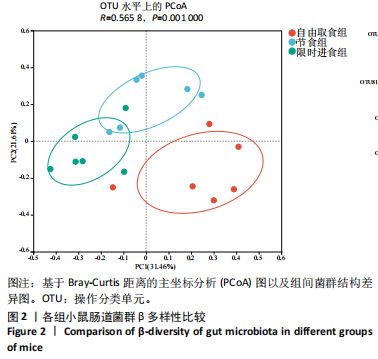
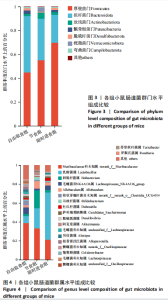
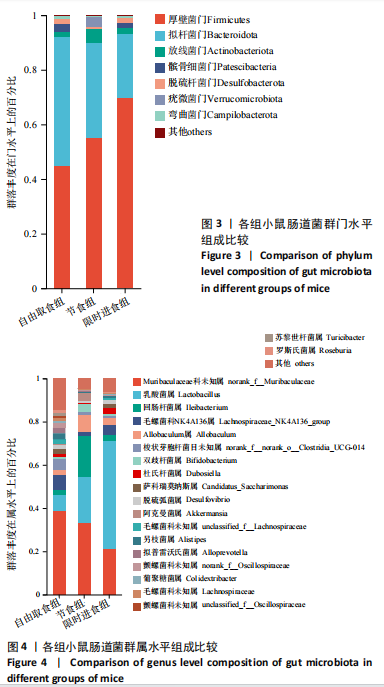
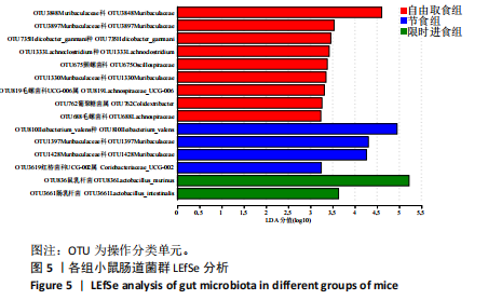
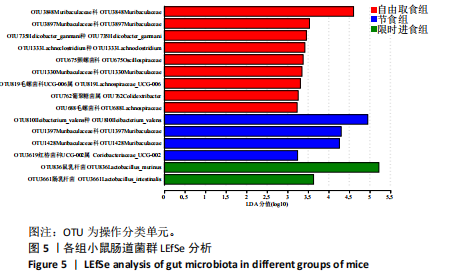
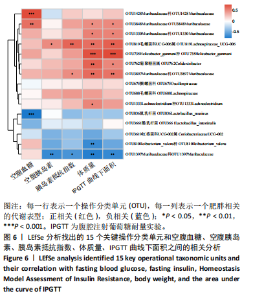
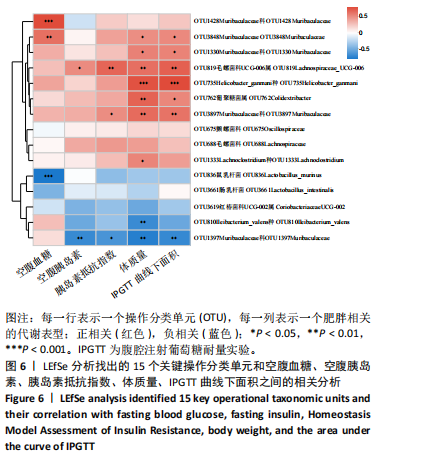
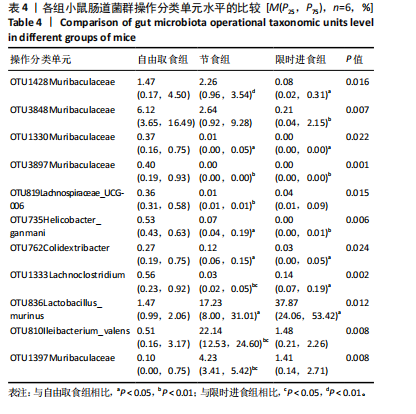
2.3.2 β多样性比较 限时进食组小鼠的肠道菌群趋向于节食组,与自由取食组在PC1轴上显著分开,该方向能解释整体菌群变异度的31.46%;而节食组小鼠的肠道菌群则在PC2轴上与自由取食组小鼠的肠道菌群存在显著差异,该方向能解释整体菌群变异度的21.61%,见图2,提示节食和限时进食小鼠的肠道菌群整体结构发生改变。 2.3.3 门水平组成比较 对丰度在1%以上的7个优势门进行分析,发现与自由取食组相比,节食组放线菌的相对丰度增加,弯曲菌门的相对丰度减少;限时进食组厚壁菌门的相对丰度增加,拟杆菌、弯曲菌门的相对丰度减少;与节食组相比,限时进食组厚壁菌门、髌骨细菌门、脱硫杆菌门的相对丰度增加,差异均有显著性意义(P < 0.05),见图3。 2.3.4 属水平组成比较 对丰度在1%以上的21个优势属进行分析,发现与自由取食组相比,节食组回肠杆菌属、双歧杆菌属丰度升高,毛螺菌科_NK4A136、梭状芽胞杆菌目_UCG-014、毛螺菌科的未知属、颤螺菌科的未知属、另枝菌属、螺杆菌属、葡聚糖菌属、苏黎世杆菌、罗斯氏菌丰度降低;限时进食组乳酸菌属丰度升高,Muribaculaceae科的未知属、梭状芽胞杆菌目_UCG-014、颤螺菌科的未知属、另枝菌属、毛螺菌科的未知属、葡聚糖菌属、苏黎世杆菌属、罗斯氏菌属丰度降低。与节食组相比,限时进食组乳酸菌属、萨科瑞莫纳斯属、脱硫弧菌属丰度显著升高,回肠杆菌属、双歧杆菌属丰度降低,以上差异均有显著性意义(P < 0.05),见图4。 2.3.5 肠道菌群LEfSe分析找出关键操作分类单元 通过LEfSe分析在操作分类单元水平上发现15个可能是节食和限时进食干预的关键操作分类单元,这些操作分类单元分别属于Muribaculaceae(5个操作分类单元)、毛螺菌科(3个操作分类单元,1个属于Lachnoclostridium,1个属于毛螺菌科_UCG-006)、乳杆菌属(2个操作分类单元,其中1个属于鼠乳杆菌,1个属于肠乳杆菌)、颤螺菌科(2个操作分类单元,其中1个属于葡聚糖菌属)、Helicobacter_ganmani(1个操作分类单元)、红蝽菌科_UCG-002(1个操作分类单元)、Ileibacterium_valens(1个操作分类单元)。LDA得分最高的为OTU836鼠乳杆菌,数值为5.22,其次是OTU810 Ileibacterium_valens,数值为4.95,见图5。 2.3.6 关键操作分类单元与代谢表型关联分析 将15个关键操作分类单元与小鼠代谢表型进行Spearman相关分析,发现11个操作分类单元与小鼠代谢表型相关,其中8个呈正相关,3个呈负相关(P < 0.05)。呈正相关的操作分类单元中OTU735 Helicobacter_ganmani,OTU762葡聚糖菌属,OTU819 毛螺菌科_UCG-006及Muribaculaceae科的OTU1330、OTU3848和OTU3897与体质量和腹腔注射葡萄糖耐量实验曲线下面积呈正相关,Muribaculaceae科的OTU1428和OTU3848与空腹血糖呈正相关OTU1333 Lachnoclostridium仅与体质量呈正相关,OTU810 Ileibacterium_valens仅与体质量呈负相关,OTU836鼠乳杆菌与空腹血糖呈负相关;11个指标中最值得关注的是OTU819毛螺菌科_UCG-006,与体质量、腹腔注射葡萄糖耐量实验曲线下面积、HOMA-IR、空腹胰岛素均呈正相关,而OTU1397 Muribaculaceae与以上指标均呈负相关,见图6。 2.3.7 关键操作分类单元丰度变化 在与代谢表型呈正相关的8个操作分类单元中,节食组和限时进食组中5个操作分类单元(Muribaculaceae科的OTU1330和OTU3897、OTU735 Helicobacter_ganmani,OTU762葡聚糖菌属、OTU1333 Lachnoclostridium)丰度占比均低于自由取食组,OTU819毛螺菌科_UCG-006的丰度占比仅在节食组显著降低,Muribaculaceae科的OTU1428和OTU3848丰度占比仅在限时进食组显著降低。与节食组相比,OTU1333 Lachnoclostridium的丰度占比在限时进食组中升高,OTU1428 Muribaculaceae的丰度占比在限时进食组中降低。在与代谢表型呈负相关的3个操作分类单元中,OTU836鼠乳杆菌在节食组和限时进食组丰度占比均显著升高,分别高达17.23% (8.00%,31.01%),37.87% (24.06%,53.42%),OTU810 Ileibacterium_valens仅在节食组中显著升高,丰度占比22.14%(12.53%,24.60%),OTU1397 Muribaculaceae仅在节食组中显著升高。与节食组相比,OTU810 Ileibacterium_valens、OTU1397 Muribaculaceae的丰度占比在限时进食组中降低,以上差异均有显著性意义(P < 0.05),见表4。"
| [1] LONGO VD, ANDERSON RM. Nutrition, longevity and disease: From molecular mechanisms to interventions. Cell. 2022;185(9):1455-1470. [2] DI FRANCESCO A, DI GERMANIO C, BERNIER M, et al. A time to fast. Science. 2018;362(6416):770-775. [3] YAMASHITA S, IMAI S, MOMO K, et al. Investigation of the real-world situation and risk factors associated with olanzapine prescribed to diabetes patients by using a japanese claims database. Biol Pharm Bull. 2021;44(8):1151-1155. [4] DUREGON E, POMATTO-WATSON LCDD, BERNIER M, et al. Intermittent fasting: from calories to time restriction. Geroscience. 2021;43(3): 1083-1092. [5] KORD-VARKANEH H, SALEHI-SAHLABADI A, TINSLEY GM, et al. Effects of time-restricted feeding (16/8) combined with a low-sugar diet on the management of non-alcoholic fatty liver disease: A randomized controlled trial. Nutrition. 2023;105:111847. [6] 梁磊,念馨.肠道菌群:未来2型糖尿病治疗的新靶点[J].实用医学杂志,2022,38(3):261-265. [7] 张静,王肖枭,周怡,等.肠道菌群与疾病相关性的研究进展[J].基础医学与临床,2020,40(2):243-247. [8] ADAK A, KHAN MR. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. 2019;76(3):473-493. [9] WU J, YANG L, LI S, et al. Metabolomics Insights into the Modulatory Effects of Long-Term Low Calorie Intake in Mice. J Proteome Res. 2016;15(7):2299-2308. [10] ZHANG L, XUE X, ZHAI R, et al. Timing of Calorie Restriction in Mice Impacts Host Metabolic Phenotype with Correlative Changes in Gut Microbiota. mSystems. 2019;4(6):e00348-19. [11] PIECZYŃSKA-ZAJĄC JM, MALINOWSKA A, ŁAGOWSKA K, et al. The effects of time-restricted eating and Ramadan fasting on gut microbiota composition: a systematic review of human and animal studies. Nutr Rev. 2024;82(6):777-793. [12] 潘凤伟.节食小鼠肠道中优势乳杆菌的分离鉴定及其在衰老相关炎症中的作用[D].上海:上海交通大学,2018. [13] SUN J, SHE Y, FANG P, et al. Time-restricted feeding prevents metabolic diseases through the regulation of galanin/GALR1 expression in the hypothalamus of mice. Eat Weight Disord. 2022;27(4):1415-1425. [14] TSITSOU S, ZACHARODIMOS N, POULIA KA, et al. Effects of Time-Restricted Feeding and Ramadan Fasting on Body Weight, Body Composition, Glucose Responses, and Insulin Resistance: A Systematic Review of Randomized Controlled Trials. Nutrients. 2022;14(22):4778. [15] MAIFELD A, BARTOLOMAEUS H, LÖBER U, et al. Fasting alters the gut microbiome reducing blood pressure and body weight in metabolic syndrome patients. Nat Commun. 2021;12(1):1970. [16] LI L, SU Y, LI F, et al. The effects of daily fasting hours on shaping gut microbiota in mice. BMC Microbiol. 2020;20(1):65. [17] GOH KL, CHAN WK, SHIOTA S, et al. Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter. 2011;16 Suppl 1(0 1):1-9. [18] LI X, LV H, SHI F, et al. The potential therapeutic effects of hydroxypropyl cellulose on acute murine colitis induced by DSS. Carbohydr Polym. 2022;289:119430. [19] GUO J, WANG P, CUI Y, et al. Protective Effects of Hydroxyphenyl Propionic Acids on Lipid Metabolism and Gut Microbiota in Mice Fed a High-Fat Diet. Nutrients. 2023;15(4):1043. [20] GUO M, XING D, WANG J, et al. Potent Intestinal Mucosal Barrier Enhancement of Nostoc commune Vaucher Polysaccharide Supplementation Ameliorates Acute Ulcerative Colitis in Mice Mediated by Gut Microbiota. Nutrients. 2023;15(13):3054. [21] CHEN H, ZHAO H, QI X, et al. Lactobacillus plantarum HF02 alleviates lipid accumulation and intestinal microbiota dysbiosis in high-fat diet-induced obese mice. J Sci Food Agric. 2023;103(9):4625-4637. [22] ZHANG F, ZHOU Y, CHEN H, et al. Curcumin Alleviates DSS-Induced Anxiety-Like Behaviors via the Microbial-Brain-Gut Axis. Oxid Med Cell Longev. 2022;2022:6244757. [23] HAO H, ZHANG X, TONG L, et al. Effect of Extracellular Vesicles Derived From Lactobacillus plantarum Q7 on Gut Microbiota and Ulcerative Colitis in Mice. Front Immunol. 2021;12:777147. [24] WANG J, HAN L, LIU Z, et al. Genus unclassified_Muribaculaceae and microbiota-derived butyrate and indole-3-propionic acid are involved in benzene-induced hematopoietic injury in mice. Chemosphere. 2023;313:137499. [25] SHAO J, LI Z, GAO Y, et al. Construction of a “Bacteria-Metabolites” Co-Expression Network to Clarify the Anti-Ulcerative Colitis Effect of Flavonoids of Sophora flavescens Aiton by Regulating the “Host-Microbe” Interaction. Front Pharmacol. 2021;12:710052. [26] LIU Y, ZHOU M, YANG M, et al. Pulsatilla chinensis Saponins Ameliorate Inflammation and DSS-Induced Ulcerative Colitis in Rats by Regulating the Composition and Diversity of Intestinal Flora. Front Cell Infect Microbiol. 2021;11:728929. [27] MO J, NI J, ZHANG M, et al. Mulberry Anthocyanins Ameliorate DSS-Induced Ulcerative Colitis by Improving Intestinal Barrier Function and Modulating Gut Microbiota. Antioxidants (Basel). 2022;11(9):1674. [28] YUAN G, TAN M, CHEN X. Punicic acid ameliorates obesity and liver steatosis by regulating gut microbiota composition in mice. Food Funct. 2021;12(17):7897-7908. [29] ZHAO D, CAO J, JIN H, et al. Beneficial impacts of fermented celery (Apium graveolens L.) juice on obesity prevention and gut microbiota modulation in high-fat diet fed mice. Food Funct. 2021;12(19):9151-9164. [30] SONG H, SHEN X, CHU Q, et al. Vaccinium bracteatum Thunb. fruit extract reduces high-fat diet-induced obesity with modulation of the gut microbiota in obese mice. J Food Biochem. 2021;45(7):e13808. [31] SLATTERY C, COTTER PD, O’TOOLE PW. Analysis of Health Benefits Conferred by Lactobacillus Species from Kefir. Nutrients. 2019;11(6):1252. [32] DEMPSEY E, CORR SC. Lactobacillus spp. for Gastrointestinal Health: Current and Future Perspectives. Front Immunol. 2022;13:840245. [33] 陈晓瑜,陈峰,张晨虹.一株节食小鼠肠道优势鼠乳杆菌加速DSS致慢性结肠炎恢复[J].基因组学与应用生物学,2021,40(2):855-866. [34] RANGAN P, CHOI I, WEI M, et al. Fasting-Mimicking Diet Modulates Microbiota and Promotes Intestinal Regeneration to Reduce Inflammatory Bowel Disease Pathology. Cell Rep. 2019;26(10):2704-2719.e6. [35] QIU J, CHEN L, ZHANG L, et al. Xie Zhuo Tiao Zhi formula modulates intestinal microbiota and liver purine metabolism to suppress hepatic steatosis and pyroptosis in NAFLD therapy. Phytomedicine. 2023; 121:155111. [36] LU X, QI C, ZHENG J, et al. The Antidepressant Effect of Deoiled Sunflower Seeds on Chronic Unpredictable Mild Stress in Mice Through Regulation of Microbiota-Gut-Brain Axis. Front Nutr. 2022;9:908297. [37] JI S, HAN S, YU L, et al. Jia Wei Xiao Yao San ameliorates chronic stress-induced depression-like behaviors in mice by regulating the gut microbiome and brain metabolome in relation to purine metabolism. Phytomedicine. 2022;98:153940. |
| [1] |
Zhao Wensheng, Li Xiaolin, Peng Changhua, Deng Jia, Sheng Hao, Chen Hongwei, Zhang Chaoju, He Chuan.
Gut microbiota and osteoporotic fractures #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1296-1304.
|
| [2] |
Sun Guanghan, Xie Zhencong, Sun Mi, Xu Yang, Guo Dong.
Therapeutic effect and mechanism by which Trichosanthis Fructus-Allii Macrostemonis Bulbus regulates gut microbiota in a rat model of coronary heart disease #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 917-927.
|
| [3] | Zeng Hao, Zou Shunyi, Li Zhengpeng, Chai Yuan, Huang Yourong, Zhang Xiaoyun. Intestinal flora regulates bone metabolism: a visual analysis of literature from the Web of Science Core Collection [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5652-5661. |
| [4] | Liu Kexin, Ma Chao, Liu Kai, Hao Maochen, Wang Xingru, Meng Lingting, Dong Mei, Wang Jianzhong . Screening and validation of glucose metabolism genes in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(20): 4181-4189. |
| [5] | Liang Zhou, Zhang Chi, Pan Chengzhen, Yang Bo, Pu Zhanglin, Liu Hua, Peng Jinhui, Wen Lichun, Ling Guanhan, Chen Feng. Anti-osteoporotic mechanisms of kaempferol based on gut microbiota and comprehensive targeted metabolomics [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(20): 4190-4204. |
| [6] | Wang Zikun, Li Shudong, Gao Shuang, Fan Shuhao, Li Cheng, Meng Chunyang. Relationship between intervertebral disc degeneration and 473 gut microbiotas: what can be learned from big data information in the FinnGen database [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(20): 4369-4378. |
| [7] | Yu Hanglin, Tian Haodong, Wen Shiyuan, Huang Li, Liu Haowei, Li Hansen, Wang Peisong, Peng Li. Changes in glucose metabolism and intestinal flora in patients with type 2 diabetes mellitus after high-intensity intermittent exercise [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(2): 286-293. |
| [8] | Guo Surong, Cao Shisheng, Mu Xingtong, Yang Qing, Zhang Juan. Compound 3k for osteoarthritis: mechanism of modulating oxidative stress pathway to improve chondrocyte glycolysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(2): 363-370. |
| [9] | Fang Zhijie, Ma Qiangping, Dong Wantao, Wu Junyuan, Lu Yunlin. Genetic causal relationship between gut microbiota and osteoporosis: analysis of 211 gut microbiota from the UK database [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(18): 3941-3947. |
| [10] | Zhang Qiuping, Xu Qian, Tian Huajun, Chu Yudan, He Junliang, Ma Guoqiang, Qiu Jun. The gut microbiota characteristics of athletes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(14): 3051-3060. |
| [11] | Chai Jinlian, Li Shudong, Li Wei, Du Haitao, Dong Limin, Liang Xuezhen, Wang Ping. Gut microbiota and drug-associated osteonecrosis: a two‑sample Mendelian randomization study [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(27): 4325-4331. |
| [12] | Liu Yulu, Jia Weiwei, Dai Yaling, Xu Wenshan, Ding Yanyi, Liang Shengxiang, Liu Weilin, Chen Lidian. Effects of time-specific AMP-activated protein kinase alpha1/2 gene knockout on hippocampal energy metabolism and synaptic plasticity in mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(20): 3230-3235. |
| [13] |
Li Ying, Lin Wentao, Weng Xiquan.
Effects of different exercise intensities on visfatin level and glucose metabolism in type 2 diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(26): 4196-4200. |
| [14] | Deng Ying, Zeng Ling-huan, Li Wei, Wu Yi. Insulin promotes the microvessel formation in fat grafts [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(18): 3318-3324. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||