Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (13): 2802-2811.doi: 10.12307/2025.099
Previous Articles Next Articles
Platelet-rich plasma intervenes in chondrocyte autophagy and apoptosis for treatment of osteoarthritis
Wang Yaomin1, 2, Zhang Kefan1, 2, Wang Dening1, Ren Qiang1, Li Jian1, Shi Hui1
- 1Binzhou Medical University Hospital, Binzhou 256600, Shandong Province, China; 2Binzhou Medical University, Binzhou 264003, Shandong Province, China
-
Received:2024-03-30Accepted:2024-05-27Online:2025-05-08Published:2024-09-12 -
Contact:Shi Hui, MD, Chief physician, Binzhou Medical University Hospital, Binzhou 256600, Shandong Province, China -
About author:Wang Yaomin, Master candidate, Binzhou Medical University Hospital, Binzhou 256600, Shandong Province, China; Binzhou Medical University, Binzhou 264003, Shandong Province, China. Zhang Kefan, Master candidate, Binzhou Medical University Hospital, Binzhou 256600, Shandong Province, China; Binzhou Medical University, Binzhou 264003, Shandong Province, China. Wang Yaomin and Zhang Kefan contributed equally to this article. -
Supported by:Shandong Provincial Clinical Key Specialized Discipline Construction Project, No. SLCZDZK-0302 (to SH); Binzhou Medical University Xu Rongxiang Regenerative Medicine Development Plan Project, No. BY2019XRX04 (to RQ); Shandong Province Medical and Health Technology Development Plan, No. 2017WS040 (to RQ); Shandong Province Medical and Health Technology Project, No. 202304070630 (to LJ)
CLC Number:
Cite this article
Wang Yaomin, Zhang Kefan, Wang Dening, Ren Qiang, Li Jian, Shi Hui. Platelet-rich plasma intervenes in chondrocyte autophagy and apoptosis for treatment of osteoarthritis[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(13): 2802-2811.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
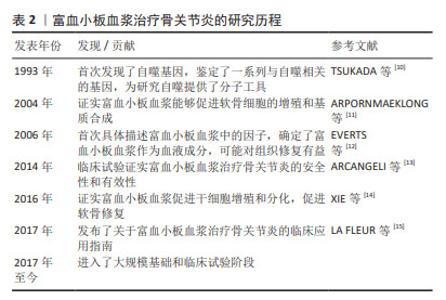
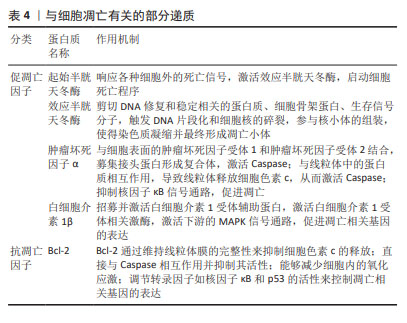
在骨关节炎进展过程中,涉及到的细胞因子包括凋亡蛋白(Caspase-2,8,3,7、Bcl-2、Bax、肿瘤坏死因子α)、自噬标志物(LC3Ⅱ/Ⅰ、Beclin、P62/SQSTM1、HSC70)等。另外,哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)通路、核因子κB(nuclear factor kappa-B,NF-κB)信号通路、线粒体应激通路及死亡受体通路对机体免疫功能产生重要的影响。富血小板血浆通过调节Caspase、Bcl-2、Bax、LC3-Ⅱ/Ⅰ、P62、Beclin1等因子,有效抑制mTOR通路和NF-κB通路,从而延缓骨关节炎进展,促进软骨组织修复。目前富血小板血浆治疗骨关节炎仍是研究者讨论的热点。富血小板血浆、细胞自噬及凋亡与骨关节炎的研究历程见表2。"
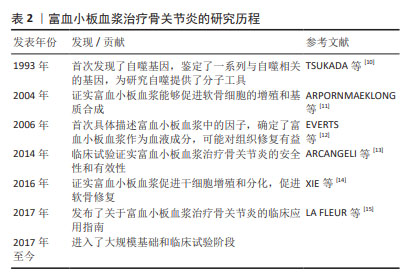

2.1 富血小板血浆干预细胞自噬对骨关节炎的影响 2.1.1 富血小板血浆通过LC3介导的途径增强细胞微自噬 LC3Ⅱ/Ⅰ是哺乳动物自噬相关基因8蛋白的同源物,其可以在前自噬泡和自噬泡膜的表层找到,并参与自噬体的重建,目前已被认为是监测自噬小体形成的关键因素。LC3Ⅱ/Ⅰ会泛素化结合蛋白SQSTM1,可以有效激活和增强自噬机制,共同促进自噬体的延伸闭合至成熟,从而加速自噬过程。通常情况下,软骨细胞维持着较低水平的自噬能力,有助于清除衰老的细胞器和大分子,而LC3的过表达会诱导自噬活动增强,降解软骨细胞受损的细胞器,供细胞回收利用[16]。研究表明,LC3Ⅱ的浓度越高,自噬的程度就越强烈,而LC3Ⅱ/Ⅰ比例的变化可以反映出自噬的程度。 YANG等[17]将富血小板血浆释放物与大鼠软骨细胞共培养发现,自噬细胞标志物LC3Ⅱ/Ⅰ的表达显著升高,这表明富血小板血浆可以诱导骨关节炎软骨细胞的自噬。作者推测,软骨细胞接受LC3Ⅱ/Ⅰ有效刺激后,更易借助细胞自噬进行自我保护,引起软骨细胞对炎症的抵抗,这或许可为延缓骨关节炎进展提供基因修饰性治疗。HUANG等[18]用糖皮质激素诱导大鼠股骨头坏死模型,关节腔注射富血小板裂解物,结果显示与正常组相比,mTOR表达显著下调且LC3Ⅱ/Ⅰ表达上调,证实了富血小板裂解物在LC3介导的自噬调控中有重要作用,这一机制有望进一步拓宽富血小板血浆在骨关节炎中发挥软骨保护作用的理解。 2.1.2 富血小板血浆促进细胞巨自噬 (1) Beclin1:是一种由自噬相关基因编码的蛋白质,是哺乳动物自噬相关基因6蛋白的同源物,并且在蛋白质复合物中通过UVRAG蛋白与Vps34相互作用[19],从而促进自噬的形成和发展。Beclin1作为一种多效的细胞因子,在包括阿尔茨海默症和血管性痴呆症在内的多种神经系统疾病中扮演关键角色;此外,它还在诸如骨质疏松症和痛风性关节炎等炎性疾病的发生和发展中起着重要作用,为这些疾病的治疗提供了新的方向和选择。 Beclin1在骨关节炎患者中的表达水平会显著影响细胞自噬和细胞凋亡,进而影响细胞活力,这表明Beclin1可能成为骨关节炎治疗的潜在靶标和研究焦点,尽管如此,关于富血小板血浆通过影响Beclin1来促进软骨细胞修复的研究仍相对较少。SHAFIK等[20]研究表明,富血小板血浆通过抑制自噬对胶原诱导关节炎有显著改善作用。另外SHEELA等[21]研究表明,富血小板血浆在5%-20%的浓度范围内可以有效促进牙髓细胞的迁移、增殖和成骨分化,细胞内Beclin1的表达显著升高。这些研究为富血小板血浆在骨关节炎治疗中的应用提供了新的视角和理论基础。 (2) P62/SQSTM1:充当一种多功能支架蛋白,不仅负责识别和传递信号,还促进细胞自我修复,进而调控细胞内的泛素化过程。作为关键衔接分子,其专门识别并指引特定细胞器和蛋白质聚集体至自噬体进行降解[22],其表达水平已被证实是预测早期骨关节炎的一个独立指标。 研究发现P62的表达量与自噬活性之间存在显著的负相关性[23]。在实验性骨关节炎模型小鼠的膝关节中,P62表达显著上调[24],因此,针对P62/SQSTM1的靶向抑制可能是延缓骨关节炎进展和促进软骨修复的有效策略。富血小板血浆以自噬依赖性发挥软骨保护作用[17]。尽管许多研究人员已经发现富血小板血浆治疗膝骨关节炎有积极作用,但其确切的作用机制尚存在争议。未来,富血小板血浆可能以基因治疗的方式特异性地作用于P62,从而改善骨关节炎的症状。然而临床效果可能因富血小板血浆的配比、每种成分的浓度以及治疗的频率、休息时间等因素的不同而有所差异[25]。 自噬修饰试剂雷帕霉素(Rapamycin)是mTOR的典型抑制剂和强自噬诱导剂,ZHANG等[26]学者探索发现自噬激活剂雷帕霉素可减少SQSTM1/P62聚集,减少泛素化蛋白质积累,并改善小鼠运动神经元的自噬缺陷通量。LERNER等[27]发现雷帕霉素处理的成纤维细胞中P62/SQSTM1蛋白水平降低,而 mRNA水平升高。雷帕霉素作为临床批准的自噬修饰药物,其对动物或人软骨细胞的作用机制尚不明确,临床疗效仍需大量基础和临床试验数据证实。 2.1.3 富血小板血浆与分子伴侣介导的细胞自噬的关系 热休克蛋白是一类在生物体受到高温或其他应激原刺激时被合成的蛋白质,是存在于细胞器中的进化保守分子[28]。热休克蛋白通过稳定细胞内蛋白和维持细胞内稳态来提高细胞存活率等方面发挥保护作用[29]。CHEN等[30]发现相较于未接受热休克处理的细胞,热休克(在41 ℃下维持1 h)处理的间充质干细胞成软骨分化和成熟更为迅速,Ⅱ型胶原和聚集聚糖的表达更高,在间充质干细胞分化过程中,热休克蛋白70水平上调,Ⅰ型和Ⅹ型胶原蛋白表达增强。总的来说,周期性热休克可触发间充质干细胞向软骨细胞分化。目前尚未有明确的研究直接探讨富血小板血浆对热休克蛋白的具体作用。然而,考虑到富血小板血浆中含有的生长因子和蛋白质可能对细胞功能产生影响,以及热休克蛋白在细胞应激响应和生存中的关键作用,这一领域值得进一步研究。未来可能的研究方向包括探索富血小板血浆释放物如何影响热休克蛋白的表达和功能,以及如何影响细胞的生理状态和疾病进程。 文章总结了参与骨关节炎发生发展过程中的部分自噬因子的作用机制,见表3。"
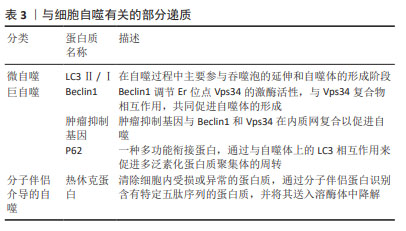
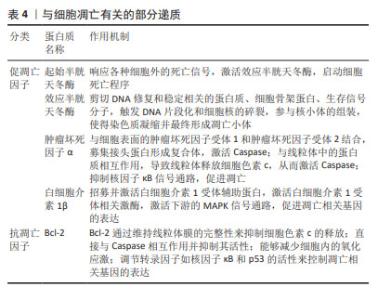
研究发现,富血小板血浆中的多种因子能够影响骨关节炎软骨细胞的自噬水平,转化生长因子β和胰岛素样生长因子1能够促进软骨细胞的自噬,而血管内皮生长因子则可能抑制自噬。因此,不能一味地认为各种浓度的富血小板血浆均具有促进自噬作用,探究各个因子在不同条件下的作用至关重要。上述骨关节炎中的自噬相关基因与软骨细胞浸润免疫密切相关,中国学者对富血小板血浆在浸润免疫方面的最新研究集中于创面修复以及妇科疾病诊治方面,近年来逐渐研究富血小板血浆调节软骨细胞浸润免疫和干预分子伴侣介导的自噬治疗骨关节炎,希望能在软骨修复和再生方面取得重大突破。 2.2 富血小板血浆干预细胞凋亡对骨关节炎的影响 2.2.1 富血小板血浆抑制促凋亡因子的表达 (1) Caspase:是骨关节炎软骨细胞凋亡的关键分子,它们在调节细胞凋亡和细胞非凋亡功能中扮演着重要角色,包括细胞增殖、分化和迁移。Caspase分为两类:一类是通过细胞凋亡信号通路激活的起始半胱天冬酶(Caspase-2、8、9和10),另一类是效应半胱天冬酶(Caspase-3、6和7),后者在不断扩大的级联反应中负责执行细胞凋亡的过程[31-33]。起始半胱天冬酶具有与基序相互作用的长前结构域,例如上游接头蛋白中存在的死亡效应结构域或半胱天冬酶募集结构域。而效应半胱天冬酶具有较短的前结构域,这些结构域在经过上游引发子半胱天冬酶的切割后被激活[34]。因此,Caspase信号通路的改变或其组成部分的变异可能会影响软骨的稳定性,进而促进骨关节炎的发展。 WU等[35]选用人输尿管的尿路上皮内膜细胞,缺氧培养3 h后,细胞活力显著降低,细胞色素c、Caspase-3表达显著增加,早期细胞凋亡率显著提高,而富血小板血浆处理后上述指标均改善。PATEL等[36]选用白化豚鼠动物模型代表自发的进行性体质量诱导的膝骨关节炎,每周接受3次富血小板血浆注射,结果表明多剂量富血小板血浆注射3-6个月时具有抗凋亡及软骨保护的作用,同时富血小板血浆干预后第3,6个月均可观察到Caspase-3的显著下降,这表明注射富血小板血浆可能直接或间接导致半胱天冬酶的表达水平下降进而抑制细胞凋亡。研究结果表明,抑制Caspase-3所引发的微环境变化,可以显著改善骨关节炎的功能,为膝骨关节炎的治疗提供了新的思路和方法。 (2)肿瘤坏死因子和白细胞介素1β:是骨关节炎的主要促炎因子,肿瘤坏死因子α是肿瘤坏死因子超家族中的19个配体之一[37],广泛存在于肿瘤微环境中,被视为参与骨关节炎生理病理过程的关键炎性因子。肿瘤坏死因子α能够诱导多种基质金属蛋白酶和其他炎症生物标志物的产生,从而加剧炎症反应[38]。白细胞介素1β能独立地诱导炎症反应和分解代谢作用,并能与其他递质协同作用,破坏关节软骨和关节的其他结构。骨关节炎患者的滑液、滑膜、软骨和软骨下骨层的白细胞介素1β水平普遍升高。此外,白细胞介素1β也影响基质金属蛋白酶,这些酶对软骨成分均具有破坏作用[39]。由于白细胞介素1β和肿瘤坏死因子α的作用,软骨细胞往往衰老的更快,并诱导细胞凋亡,这些因素共同推动了骨关节炎的病理进程[40]。 富血小板血浆的应用显著降低了肿瘤坏死因子α和白细胞介素1β的表达水平,并有效减轻了骨关节炎兔软骨的变性程度[41]。SAIF等[42]对类风湿关节炎患者注射富血小板血浆,对照组关节内注射类固醇,经过对比得出富血小板血浆是一种安全有效的治疗方法,其对炎症细胞因子白细胞介素1β、肿瘤坏死因子α具有下调作用,并抑制细胞凋亡。富血小板血浆来源外泌体能够有效抑制骨关节炎患者体内由白细胞介素1β引发的慢性炎症反应,并且可以有效阻止软骨细胞凋亡和变性,从而起到保护作用[43]。ZHOU等[44]回顾性研究99例膝骨关节炎患者,其中试验组接受了关节内注射富血小板血浆联合玻璃酸钠,而对照组仅注射玻璃酸钠,结果显示试验组的临床疗效改善程度显著高于对照组,肿瘤坏死因子α和白细胞介素1β的表达水平显著低于对照组。 2.2.2 富血小板血浆促进抗凋亡因子的表达 Bcl-2是凋亡核心机制的关键组成部分,其主要功能是抑制细胞凋亡。研究表明,通过检测t(14;18)基因的结构域,可以确认Bcl-2基因的存在,是评估细胞凋亡状态的关键因子[45]。Bcl-2蛋白拥有4个保守结构域,即BH1、BH2、BH3和BH4,这些结构域使得Bcl-2能够整合到线粒体外膜、内质网和核膜中,从而发挥抗凋亡的作用。 Bcl-2通过调节线粒体膜通透性来控制线粒体凋亡信号传导。TAO等[46]通过肌肉注射甲泼尼龙以诱导大鼠股骨头坏死模型,探讨富血小板血浆来源外泌体在抗细胞凋亡、促进成骨细胞生长方面的潜在作用。实验发现,富血小板血浆来源外泌体能够有效抑制细胞色素c的释放,提高抗凋亡蛋白Bcl-2水平,同时也能够抑制Caspase-3的激活,因此具有良好的抗凋亡效应,这种抗凋亡机制主要是由富血小板血浆来源外泌体激活Akt/Bcl-2信号通路所调节的。WANG等[47]建立了地塞米松诱导的大鼠骨髓间充质干细胞模型,发现地塞米松诱导后细胞凋亡率和凋亡相关蛋白Caspase-3水平升高,抗凋亡相关蛋白Bcl-2降低,而加入富血小板血浆后减弱了地塞米松的影响,Bcl-2表达水平升高,细胞凋亡率显著下降。 综上所述,富血小板血浆会显著抑制细胞凋亡。然而,富血小板血浆的成分比较复杂,其具体作用机制有待进一步研究。在未来的研究中,可以进一步建立动物模型探讨富血小板血浆内细胞因子对Bcl-2家族相关蛋白的影响,这也为富血小板血浆在其他肌肉骨骼疾病中的应用奠定了基础。 文章总结了参与骨关节炎发生发展过程中的部分凋亡因子的作用机制,见表4。"
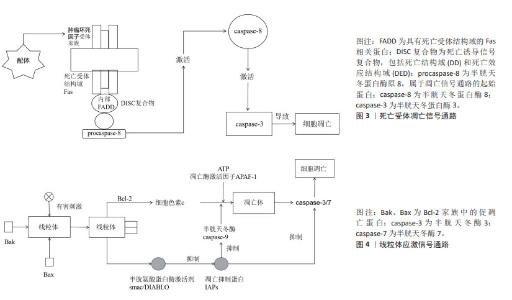
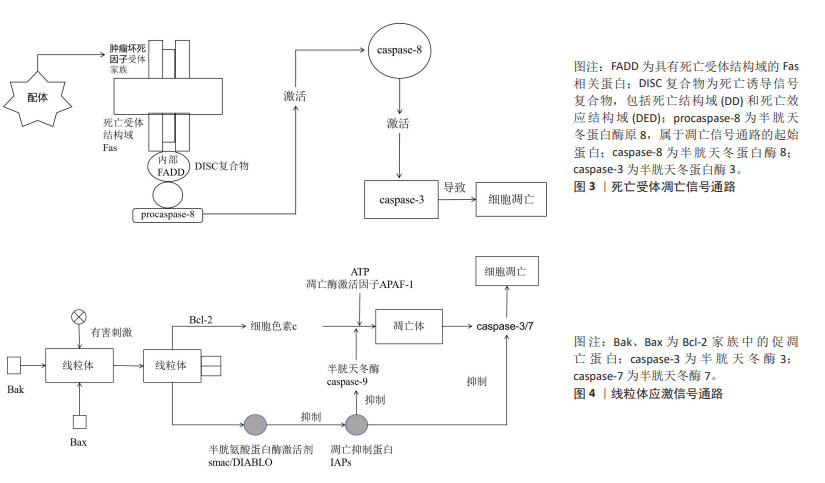
在骨关节炎的治疗策略中,传统的口服和外用止痛药物、运动疗法及物理治疗往往效果有限,作为一种创新的疗法,富血小板血浆引起了广泛关注,但中国学者对富血小板血浆的研究主要集中于骨关节炎愈合的短期疗效和长期预后,而对于其具体的作用机制尚缺乏深入理解。多个细胞凋亡基因及细胞因子是关键启动分子,可以作为治疗骨关节炎的重要靶点,尽管国外的研究结果多基于体外或体内临床前研究,将其应用到临床实践仍需要大量的基础及临床试验数据支撑,目前文献中的结论仍然不尽一致。因此,对于富血小板血浆治疗骨关节炎的研究需要进一步加强对以上基因及相关蛋白的了解,以便在未来的研究中进一步探讨富血小板血浆治疗骨关节炎的作用机制,优化治疗方案,为骨关节炎患者提供更加有效的治疗手段。 2.3 富血小板血浆干预信号转导通路对骨关节炎的影响 2.3.1 mTOR 作为哺乳动物的一种关键的基因激酶,在自我修复进程中扮演着至关重要的角色,可以促进细胞的增殖、改善新陈代谢以及增强免疫力。mTOR功能紊乱可能会导致多种病变,例如衰老、阿尔茨海默病、高血压以及恶性肿瘤[48]。mTOR的功能主要在于改善细胞的功能,通过改变外界刺激、改善周围环境以及改变细胞内部环境实现的。当能量需求增加时,AKT或者丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)就会被激活,这将阻断mTOR信号通路,进而阻止unc-51样自噬激活激酶1的磷酸化,最终阻止自噬的发生[49]。研究发现,抑制磷脂酰肌醇3-激酶(phosphoinositide 3-kinase,PI3K)/蛋白激酶B(protein kinase B,AKT)/哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)信号通路可以有效增强关节软骨细胞的自我修复能力,从而缓解骨关节炎的炎症反应[50]。另一项研究表明,短期内使用糖皮质激素会增加人软骨细胞的自噬,但长期使用会导致细胞凋亡。治疗前给予雷帕霉素,与仅使用地塞米松的细胞相比,细胞活力和自噬增加[51]。最新的临床前研究表明,在mTOR抑制剂治疗或mTOR基因敲除的动物模型中,骨关节炎的严重程度显著降低,尽管已经取得了这些进展,但是雷帕霉素及其衍生物在骨关节炎临床研究中的疾病修饰作用尚未得到充分验证。 JIAO等[52]证实了富血小板血浆能够通过激活AKT/mTOR通路来保护心脏免受脂多糖诱导的损伤。CAO等[53]研究表明,在缺氧条件下,富血小板血浆能够通过减少细胞凋亡、增加细胞活力、促进细胞迁移和细胞增殖来保护前交叉韧带成纤维细胞,富血小板血浆的这种保护作用与PI3K/Akt/mTOR通路相关。因此,富血小板血浆作用于mTOR信号通路的作用或可成为骨关节炎的潜在治疗靶点,未来仍需大量的临床试验来验证mTOR抑制剂的不良反应、基因缺失的安全性和有效性,以期为骨关节炎患者寻求有潜力和前途的治疗方式。 2.3.2 NF-κB NF-κB家族由5种重要的成员组织:RelA/p65、RelB、c-Rel、p50以及p52。NF-κB信号转导的激活能够促使多种酶的活化,如基质金属蛋白酶、ADAMTS4以及ADAMTS5,这些酶的活化可以促进骨关节炎的发展,导致关节软骨逐渐降解[54]。在骨关节炎关节软骨细胞中,肿瘤坏死因子α、白细胞介素1β、白细胞介素6的激活可以促进基质金属蛋白酶的生成[55],从而提高NF-κB的传递效率[56]。NF-κB可通过诱导前列腺素E2、一氧化氮合酶、一氧化氮和环氧合酶2的产生来加剧关节损伤,从而促进组织炎症、分解代谢因子的合成和关节软骨细胞的凋亡。NF-κB信号转导通路已经成为骨关节炎治疗的一个重要靶点。 QI等[57]在兔大面积半月板缺损模型中揭示了富血小板血浆通过NF-κB信号通路发挥抗炎作用。另一项研究表明,富血小板血浆疗法可能通过调节线粒体凋亡和NF-κB通路相关的炎症反应,从而减轻滑液诱导的损伤[58]。ZHAO等[59]在兔软骨细胞中加入多柔比星诱导炎症,激活NF-κB通路,IκBα磷酸化和NF-κB易位到细胞核,富血小板血浆以剂量依赖性方式抑制了这些变化,表明富血小板血浆通过阻断NF-κB通路抑制炎症反应。这些结果可能为富血小板血浆在骨关节炎治疗中的应用提供更多支持性证据。 随着对NF-κB信号通路抑制剂的研发,如环氧合酶2异构体、糖皮质激素等,新型试剂(肿瘤坏死因子α受体抑制剂、白细胞介素1β受体拮抗剂、IKK抑制剂和NF-κB核易位抑制剂)的临床试验正在进行中,但临床试验结果仍存在争议。已有研究表明,在癌症耐药性的背景下,NF-κB信号通路抑制剂研究偏向于联合疗法、个性化医疗和特异性抑制剂的开发,非特异性抑制剂有可能影响药物的代谢和效果,同时其最佳的治疗剂量仍需进一步深入研究[60]。另外一项研究显示,由于系统性NF-κB阻断可能导致非肿瘤靶向毒性,目前临床上尚无有用的NF-κB抑制剂专门用于治疗肿瘤患者,未来应重点关注靶向致癌NF-κB信号的上游调节因子或下游效应新分子,以及靶向单个NF-κB亚基的药物[61]。此外,另一项研究提出NF-κB抑制剂在多种疾病的发病机制中扮演着关键角色,包括神经退行性疾病如阿尔茨海默病,但目前无法平衡抑制炎症和保持生理免疫反应之间的微妙界限,仍有不希望发生的不良反应[62]。雷公藤内酯是NF-κB活化的抑制剂,通过炎症信号通路调节免疫细胞的功能和细胞因子的表达,以及维持氧化还原平衡和肠道微生物群稳态来发挥治疗作用[63]。但它在不同动物的体内实验和临床试验中显示出了剂量和时间依赖性的毒性,对肝、肾、生殖系统、心、脾、肺和胃肠道均具有明显毒副作用,目前仍在评估其在膝骨关节炎患者中的安全性和有效性。 2.3.3 死亡受体通路 细胞外凋亡信号主要通过死亡受体结合配体来感知。已有研究表明,死亡受体包括Fas(CD95/APO-1)、TNFR和TRAIL受体1和2,属于肿瘤坏死因子受体超家族,其特征是存在死亡结构域、胞质结构域和富含半胱氨酸的胞外结构域。当受体接受凋亡信号时,TRAIL与其功能性细胞表面死亡受体连接或诱导死亡受体三聚化或聚集使衔接蛋白Fas相关死亡结构域募集到受体的细胞质区域,引起pro-Caspase-8、Caspase-8、Caspase-7以及Caspase-3的自我催化,从而有效地触发细胞凋亡[64]。然而,在某些细胞类型中,活性Caspase-8的凋亡信号不足以激活下游Caspase酶,例如Caspase-3和Caspase-7,在这些细胞中,被Caspase-8(tBid)切割的Bid易位到线粒体并激活线粒体凋亡途径[65],因此,TRAIL/死亡受体/FADD/Caspase-8信号转导通常会触发凋亡细胞死亡,见图3。 Caspase-3的表达在富血小板血浆的介导下明显减少,而Bcl-2的表达则有所增加,这两种因素都会阻碍细胞色素c的释放,并且会对Caspase-3的激活产生影响,最终导致底物的分离,使得死亡受体通路介导的细胞凋亡变得不可逆转。 2.3.4 线粒体应激通路 线粒体外膜渗透是线粒体应激通路的核心,它允许线粒体膜间隙中的蛋白进入细胞质,例如凋亡诱导因子和内切酶,进而引发凋亡,直接改变了原有的抗凋亡蛋白的结构,导致凋亡发生[65]。Bcl-2家族蛋白在线粒体外膜渗透的上游发挥作用,Bcl-2、Bcl-XL等凋亡蛋白能够阻碍细胞色素c的产生,但是Bax、Bcl-2以及BH3等同源拮抗剂/杀伤剂/死亡激动剂却能够活化这一过程,有助于细胞色素c从线粒体中释放。细胞色素c与胞质中的单体凋亡蛋白酶激活因子1结合,促进凋亡蛋白酶激活因子1寡聚形成凋亡小体。凋亡小体与半胱天冬蛋白酶原Caspase-9结合。激活的Caspase-9可以切割Caspase-3和Caspase-7,促进细胞凋亡[66],见图4。 在某些情况下,Bcl-2和Bax之间的平衡被打乱,会导致线粒体的功能发生改变。当Bax水平过高,促凋亡作用增强,Bax介导的线粒体外膜渗透增多,从而引起下游半胱天冬酶的级联化学反应,导致细胞死亡。一项最新的研究表明,富血小板血浆能够有效减少Bax基因的表达,并有效阻止内源性凋亡[8]。屈一鸣等[9]采取改良Hulth法制备左膝骨关节炎模型,肌肉注射富血小板血浆,分离和培养软骨细胞,结果显示实验组细胞凋亡率会大幅度下降,同时,Bax蛋白表达也会相应减少,Bcl-2蛋白表达则会增加,这些改变都会阻止细胞色素c的产生,最终有效阻止细胞凋亡。 在骨关节炎的发生发展中,信号转导通路的异常激活或抑制起着关键作用。然而,针对信号通路的研究相对匮乏,具有双重作用机制,且其调节水平不易控制,仍需要进一步研究。除mTOR信号通路外,其他信号通路如JAK/STAT通路和线粒体蛋白FUNDC2通路的研究尚显不足,这些信号通路为富血小板血浆在生物医学治疗骨关节炎领域提供了新的视角。期待在未来的研究中进一步探讨信号转导通路在骨关节炎中的作用机制,并开发针对信号通路的治疗方法,为骨关节炎患者提供更有效的治疗手段。"
| [1] YAO Q, WU X, TAO C, et al. Osteoarthritis: pathogenic signaling pathways and therapeutic targets. Signal Transduct Target Ther. 2023; 8(1):56. [2] GU YT, CHEN J, MENG ZL, et al. Research progress on osteoarthritis treatment mechanisms. Biomed Pharmacother. 2017;93:1246-1252. [3] QIAO X, YAN L, FENG Y, et al. Efficacy and safety of corticosteroids, hyaluronic acid, and PRP and combination therapy for knee osteoarthritis: a systematic review and network meta-analysis. BMC Musculoskelet Disord. 2023;24(1):926. [4] AMIRI MA, FARSHIDFAR N, MIRON RJ, et al. The Potential Therapeutic Effects of Platelet-Derived Biomaterials on Osteoporosis: A Comprehensive Review of Current Evidence. Int J Biomater. 2023;2023: 9980349. [5] SHAHID M, KUNDRA R. Platelet-rich plasma (PRP) for knee disorders. EFORT Open Rev. 2017;2(1):28-34. [6] EVERTS P, ONISHI K, JAYARAM P, et al. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int J Mol Sci. 2020;21(20):7794. [7] LV X, ZHAO T, DAI Y, et al. New insights into the interplay between autophagy and cartilage degeneration in osteoarthritis. Front Cell Dev Biol. 2022;10:1089668. [8] MOUSSA M, LAJEUNESSE D, HILAL G, et al. Platelet rich plasma (PRP) induces chondroprotection via increasing autophagy, anti-inflammatory markers, and decreasing apoptosis in human osteoarthritic cartilage. Exp Cell Res. 2017;352(1):146-156. [9] 屈一鸣,邵高海,张铭华,等.自体富血小板血浆对骨关节炎模型兔软骨细胞凋亡及NLRP3/IL-1β通路的影响[J].中国病理生理杂志, 2020,36(6):1089-1095. [10] TSUKADA M, OHSUMI Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993; 333(1-2):169-174. [11] ARPORNMAEKLONG P, KOCHEL M, DEPPRICH R,et al. Influence of platelet-rich plasma (PRP) on osteogenic differentiation of rat bone marrow stromal cells. An in vitro study. Int J Oral Maxillofac Surg. 2004;33(1):60-70. [12] EVERTS PA, KNAPE JT, WEIBRICH G, et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006;38(2):174-187. [13] ARCANGELI ML, BARDIN F, FRONTERA V, et al. Function of Jam-B/Jam-C interaction in homing and mobilization of human and mouse hematopoietic stem and progenitor cells. Stem Cells. 2014;32(4):1043-1054. [14] XIE Q, MA Q, JI B, et al. Incremental value of SPECT/CT in detection of Meckel’s diverticulum in a 10-year-old child. Springerplus. 2016; 5(1):1270. [15] LA FLEUR P, ARGÁEZ C. Platelet-Rich Plasma Injections for Wound Healing and Tissue Rejuvenation: A Review of Clinical Effectiveness, Cost-Effectiveness and Guidelines. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health, 2017. [16] DIKIC I, ELAZAR Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19(6):349-364. [17] YANG F, HU H, YIN W, et al. Autophagy Is Independent of the Chondroprotection Induced by Platelet-Rich Plasma Releasate. Biomed Res Int. 2018;2018:9726703. [18] HUANG Z, WANG Q, ZHANG T, et al. Hyper-activated platelet lysates prevent glucocorticoid-associated femoral head necrosis by regulating autophagy. Biomed Pharmacother. 2021;139:111711. [19] DONG C, HUI P, WU Z, et al. CircRNA LOC729852 promotes bladder cancer progression by regulating macrophage polarization and recruitment via the miR-769-5p/IL-10 axis. J Cell Mol Med. 2024; 28(7):e18225. [20] SHAFIK NM, EL-ESAWY RO, MOHAMED DA, et al. Regenerative effects of glycyrrhizin and/or platelet rich plasma on type-II collagen induced arthritis: Targeting autophay machinery markers, inflammation and oxidative stress. Arch Biochem Biophys. 2019;675:108095. [21] SHEELA S, ALGHALBAN FM, KHALIL KA, et al. Synthesis and Biocompatibility Evaluation of PCL Electrospun Membranes Coated with MTA/HA for Potential Application in Dental Pulp Capping. Polymers (Basel). 2022;14(22):4862. [22] JEONG SJ, ZHANG X, RODRIGUEZ-VELEZ A, et al. p62/SQSTM1 and Selective Autophagy in Cardiometabolic Diseases. Antioxid Redox Signal. 2019;31(6):458-471. [23] LI L, SHEN C, NAKAMURA E, et al. SQSTM1 is a pathogenic target of 5q copy number gains in kidney cancer. Cancer Cell. 2013;24(6): 738-750. [24] LI H, MIAO D, ZHU Q, et al. MicroRNA-17-5p contributes to osteoarthritis progression by binding p62/SQSTM1. Exp Ther Med. 2018;15(2):1789-1794. [25] CHEN N, LI M, YANG J, et al. Slow-sculpting graphene oxide/alginate gel loaded with platelet-rich plasma to promote wound healing in rats. Front Bioeng Biotechnol. 2024;12:1334087. [26] ZHANG X, CHEN S, SONG L, et al. MTOR-independent, autophagic enhancer trehalose prolongs motor neuron survival and ameliorates the autophagic flux defect in a mouse model of amyotrophic lateral sclerosis. Autophagy. 2014;10(4):588-602. [27] LERNER C, BITTO A, PULLIAM D, et al. Reduced mammalian target of rapamycin activity facilitates mitochondrial retrograde signaling and increases life span in normal human fibroblasts. Aging Cell. 2013; 12(6):966-977. [28] ZUO D, SUBJECK J, WANG XY. Unfolding the Role of Large Heat Shock Proteins: New Insights and Therapeutic Implications. Front Immunol. 2016;7:75. [29] SHENDE P, BHANDARKAR S, PRABHAKAR B. Heat Shock Proteins and their Protective Roles in Stem Cell Biology. Stem Cell Rev Rep. 2019;15(5):637-651. [30] CHEN J, LI C, WANG S. Periodic heat shock accelerated the chondrogenic differentiation of human mesenchymal stem cells in pellet culture. PLoS One. 2014;9(3):e91561. [31] GIUNTA S, CASTORINA A, MARZAGALLI R, et al. Ameliorative effects of PACAP against cartilage degeneration. Morphological, immunohistochemical and biochemical evidence from in vivo and in vitro models of rat osteoarthritis. Int J Mol Sci. 2015;16(3): 5922-5944. [32] BROCKMUELLER A, BUHRMANN C, SHAYAN P, et al. Calebin A modulates inflammatory and autophagy signals for the prevention and treatment of osteoarthritis. Front Immunol. 2024;15:1363947. [33] MUSUMECI G, CASTROGIOVANNI P, LORETO C, et al. Post-traumatic caspase-3 expression in the adjacent areas of growth plate injury site: a morphological study. Int J Mol Sci. 2013;14(8):15767-15784. [34] DING K, SONG C, HU H, et al. The Role of NLRP3 Inflammasome in Diabetic Cardiomyopathy and Its Therapeutic Implications. Oxid Med Cell Longev. 2022;2022:3790721. [35] WU ZS, LUO HL, CHUANG YC, et al. Platelet Lysate Therapy Attenuates Hypoxia Induced Apoptosis in Human Uroepithelial SV-HUC-1 Cells through Regulating the Oxidative Stress and Mitochondrial-Mediated Intrinsic Apoptotic Pathway. Biomedicines. 2023;11(3):935. [36] PATEL S, MISHRA NP, CHOUHAN DK, et al. Chondroprotective effects of multiple PRP injections in osteoarthritis by apoptosis regulation and increased aggrecan synthesis- Immunohistochemistry based Guinea pig study. J Clin Orthop Trauma. 2022;25:101762. [37] KOMURA M, WANG C, ITO S, et al. Simultaneous Expression of CD70 and POSTN in Cancer-Associated Fibroblasts Predicts Worse Survival of Colorectal Cancer Patients. Int J Mol Sci. 2024;25(5):2537. [38] BUCKLAND J. Osteoarthritis: positive feedback between ADAMTS-7 and TNF in OA. Nat Rev Rheumatol. 2013;9(10):566. [39] MUKHERJEE A, DAS B. The role of inflammatory mediators and matrix metalloproteinases (MMPs) in the progression of osteoarthritis. Biomater Biosyst. 2024;13:100090. [40] UMOH IO, DOS REIS HJ, DE OLIVEIRA ACP. Molecular Mechanisms Linking Osteoarthritis and Alzheimer’s Disease: Shared Pathways, Mechanisms and Breakthrough Prospects. Int J Mol Sci. 2024;25(5): 3044. [41] YIN WJ, XU HT, SHENG JG, et al. Advantages of Pure Platelet-Rich Plasma Compared with Leukocyte- and Platelet-Rich Plasma in Treating Rabbit Knee Osteoarthritis. Med Sci Monit. 2016;22: 1280-1290. [42] SAIF DS, HEGAZY NN, ZAHRAN ES. Evaluating the Efficacy of Intra-articular Injections of Platelet Rich Plasma (PRP) in Rheumatoid Arthritis Patients and its Impact on Inflammatory Cytokines, Disease Activity and Quality of Life. Curr Rheumatol Rev. 2021;17(2): 232-241. [43] VAN BUUL GM, KOEVOET WL, KOPS N, et al. Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. Am J Sports Med. 2011;39(11):2362-2370. [44] ZHOU B, FENG H, LEI B, et al. Effect of autologous platelet-rich plasma combined with sodium hyaluronate on clinical efficacy and serum inflammatory factors in patients with knee osteoarthritis. Am J Transl Res. 2022;14(12):8724-8732. [45] MUSUMECI G, CASTROGIOVANNI P, TROVATO FM, et al. Biomarkers of Chondrocyte Apoptosis and Autophagy in Osteoarthritis. Int J Mol Sci. 2015;16(9):20560-20575. [46] TAO SC, YUAN T, RUI BY, et al. Exosomes derived from human platelet-rich plasma prevent apoptosis induced by glucocorticoid-associated endoplasmic reticulum stress in rat osteonecrosis of the femoral head via the Akt/Bad/Bcl-2 signal pathway. Theranostics. 2017;7(3): 733-750. [47] WANG Y, LUAN S, YUAN Z, et al. Therapeutic effect of platelet-rich plasma on glucocorticoid-induced rat bone marrow mesenchymal stem cells in vitro. BMC Musculoskelet Disord. 2022;23(1):151. [48] TOMAZ DA SILVA P, ZHANG Y, Theodorakis E, et al. Cellular energy regulates mRNA degradation in a codon-specific manner. Mol Syst Biol. 2024;20(5):506-520. [49] PINTO AP, DA ROCHA AL, MARAFON BB, et al. Impact of Different Physical Exercises on the Expression of Autophagy Markers in Mice. Int J Mol Sci. 2021;22(5):2635. [50] XUE JF, SHI ZM, ZOU J, et al. Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy of articular chondrocytes and attenuates inflammatory response in rats with osteoarthritis. Biomed Pharmacother. 2017;89:1252-1261. [51] XIAO L, LIN S, XU W, et al. Downregulation of Sox8 mediates monosodium urate crystal-induced autophagic impairment of cartilage in gout arthritis. Cell Death Discov. 2023;9(1):95. [52] JIAO Y, ZHANG Q, ZHANG J, et al. Platelet-rich plasma ameliorates lipopolysaccharide-induced cardiac injury by inflammation and ferroptosis regulation. Front Pharmacol. 2022;13:1026641. [53] CAO Y, LI Y, FU SC, et al. Platelet-rich plasma pretreatment protects anterior cruciate ligament fibroblasts correlated with PI3K-Akt-mTOR pathway under hypoxia condition. J Orthop Translat. 2022;34: 102-112. [54] WU Z, LIANG Y, KHAN A, et al. Is occupational noise associated with arthritis? Cross-sectional evidence from US population. BMC Public Health. 2024;24(1):371. [55] CHEN S, WU X, LAI Y, et al. Kindlin-2 inhibits Nlrp3 inflammasome activation in nucleus pulposus to maintain homeostasis of the intervertebral disc. Bone Res. 2022;10(1):5. [56] LIU F, ZHAO Y, PEI Y, et al. Role of the NF-kB signalling pathway in heterotopic ossification: biological and therapeutic significance. Cell Commun Signal. 2024;22(1):159. [57] QI Y, TANG R, SHI Z, et al. Wnt5a/Platelet-rich plasma synergistically inhibits IL-1β-induced inflammatory activity through NF-κB signaling pathway and prevents cartilage damage and promotes meniscus regeneration. J Tissue Eng Regen Med. 2021;15(7):612-624. [58] INTERNATIONAL BR. Retracted: PRP from Personal Blood Relieves Joint Fluid-Inducing Synovial Injury through NF-κB Pathway and Mitochondrial Apoptosis in Human Synovial Fibroblast Cells. Biomed Res Int. 2023;2023:9875202. [59] ZHAO H, ZHU W, MAO W, et al. Platelet-rich plasma inhibits Adriamycin-induced inflammation via blocking the NF-κB pathway in articular chondrocytes. Mol Med. 2021;27(1):66. [60] LI Y, ZHAO B, PENG J, et al. Inhibition of NF-κB signaling unveils novel strategies to overcome drug resistance in cancers. Drug Resist Updat. 2024;73:101042. [61] VERZELLA D, CORNICE J, ARBORETTO P, et al. The NF-κB Pharmacopeia: Novel Strategies to Subdue an Intractable Target. Biomedicines. 2022; 10(9):2233. [62] SIVAMARUTHI BS, RAGHANI N, CHORAWALA M, et al. NF-κB Pathway and Its Inhibitors: A Promising Frontier in the Management of Alzheimer’s Disease. Biomedicines. 2023;11(9):2587. [63] CHENG Y, ZHAO Y, ZHENG Y. Therapeutic potential of triptolide in autoimmune diseases and strategies to reduce its toxicity. Chin Med. 2021;16(1):114. [64] OH YT, SUN SY. Regulation of Cancer Metastasis by TRAIL/Death Receptor Signaling. Biomolecules. 2021;11(4):499. [65] XU Z, YIN W, ZHANG Y, et al. Comparative evaluation of leukocyte- and platelet-rich plasma and pure platelet-rich plasma for cartilage regeneration. Sci Rep. 2017;7:43301. [66] SPIERINGS D, MCSTAY G, SALEH M, et al. Connected to death: the (unexpurgated) mitochondrial pathway of apoptosis. Science. 2005; 310(5745):66-67. |
| [1] | Ma Chi, Wang Ning, Chen Yong, Wei Zhihan, Liu Fengji, Piao Chengzhe. Application of 3D-printing patient-specific instruments combined with customized locking plate in opening wedge high tibial osteotomy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1863-1869. |
| [2] | Yu Shuai, Liu Jiawei, Zhu Bin, Pan Tan, Li Xinglong, Sun Guangfeng, Yu Haiyang, Ding Ya, Wang Hongliang. Hot issues and application prospects of small molecule drugs in treatment of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1913-1922. |
| [3] | Zhao Jiyu, Wang Shaowei. Forkhead box transcription factor O1 signaling pathway in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1923-1930. |
| [4] | Sun Yundi, Cheng Lulu, Wan Haili, Chang Ying, Xiong Wenjuan, Xia Yuan. Effect of neuromuscular exercise for knee osteoarthritis pain and function: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1945-1952. |
| [5] | Deng Keqi, Li Guangdi, Goswami Ashutosh, Liu Xingyu, He Xiaoyong. Screening and validation of Hub genes for iron overload in osteoarthritis based on bioinformatics [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1972-1980. |
| [6] | Li Huayuan, Li Chun, Liu Junwei, Wang Ting, Li Long, Wu Yongli. Effect of warm acupuncture on PINK1/Parkin pathway in the skeletal muscle of rats with chronic fatigue syndrome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1618-1625. |
| [7] | Zhou Panpan, Cui Yinglin, Zhang Wentao, Wang Shurui, Chen Jiahui, Yang Tong . Role of cellular autophagy in cerebral ischemic injury and the regulatory mechanism of traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1650-1658. |
| [8] | Wang Qiuyue, Jin Pan, Pu Rui . Exercise intervention and the role of pyroptosis in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1667-1675. |
| [9] | Zhu Hanmin, Wang Song, Xiao Wenlin, Zhang Wenjing, Zhou Xi, He Ye, Li Wei, . Mitophagy regulates bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1676-1683. |
| [10] | Chen Yueping, Chen Feng, Peng Qinglin, Chen Huiyi, Dong Panfeng . Based on UHPLC-QE-MS, network pharmacology, and molecular dynamics simulation to explore the mechanism of Panax notoginseng in treating osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1751-1760. |
| [11] | Yin Lu, Jiang Chuanfeng, Chen Junjie, Yi Ming, Wang Zihe, Shi Houyin, Wang Guoyou, Shen Huarui. Effect of Complanatoside A on the apoptosis of articular chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1541-1547. |
| [12] | Chen Shuai, Jin Jie, Han Huawei, Tian Ningsheng, Li Zhiwei . Causal relationship between circulating inflammatory cytokines and bone mineral density based on two-sample Mendelian randomization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1556-1564. |
| [13] | Wang Peiguang, Zhang Xiaowen, Mai Meisi, Li Luqian, Huang Hao. Generalized equation estimation of the therapeutic effect of floating needle therapy combined with acupoint embedding on different stages of human knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1565-1571. |
| [14] | Jin Kai, Tang Ting, Li Meile, Xie Yuan. Effects of conditioned medium and exosomes of human umbilical cord mesenchymal stem cells on proliferation, migration, invasion, and apoptosis of hepatocellular carcinoma cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1350-1355. |
| [15] | Liu Qi, Li Linzhen, Li Yusheng, Jiao Hongzhuo, Yang Cheng, Zhang Juntao. Icariin-containing serum promotes chondrocyte proliferation and chondrogenic differentiation of stem cells in the co-culture system of three kinds of cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1371-1379. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||