Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (13): 2713-2719.doi: 10.12307/2025.074
Previous Articles Next Articles
Beta-hydroxybutyric acid improves energy dysfunction of mouse hippocampal neuron HT22 cells induced by amyloid-β protein 1-42
Ye Yucai1, Fu Chaojing1, Li Yan2, Li Xinru1, Chai Shifan1, Cai Hongyan3, Wang Zhaojun1
- Wang Zhaojun, PhD, Associate professor, Department of Physiology, School of Basic Medicine, Shanxi Medical University, Key Laboratory of Cell Physiology, Ministry of Education, Taiyuan 030000, Shanxi Province, China
-
Received:2024-03-07Accepted:2024-05-07Online:2025-05-08Published:2024-09-11 -
Contact:Wang Zhaojun, PhD, Associate professor, Department of Physiology, School of Basic Medicine, Shanxi Medical University, Key Laboratory of Cell Physiology, Ministry of Education, Taiyuan 030000, Shanxi Province, China -
About author:Ye Yucai, Master candidate, Department of Physiology, School of Basic Medicine, Shanxi Medical University, Key Laboratory of Cell Physiology, Ministry of Education, Taiyuan 030000, Shanxi Province, China -
Supported by:National Natural Science Foundation of China (General Project), No. 82171428 (to CHY); Shanxi Province Basic Research Natural Science Research (General Project), No. 20210302123306 (to WZJ)
CLC Number:
Cite this article
Ye Yucai, Fu Chaojing, Li Yan, Li Xinru, Chai Shifan, Cai Hongyan, Wang Zhaojun. Beta-hydroxybutyric acid improves energy dysfunction of mouse hippocampal neuron HT22 cells induced by amyloid-β protein 1-42[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(13): 2713-2719.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
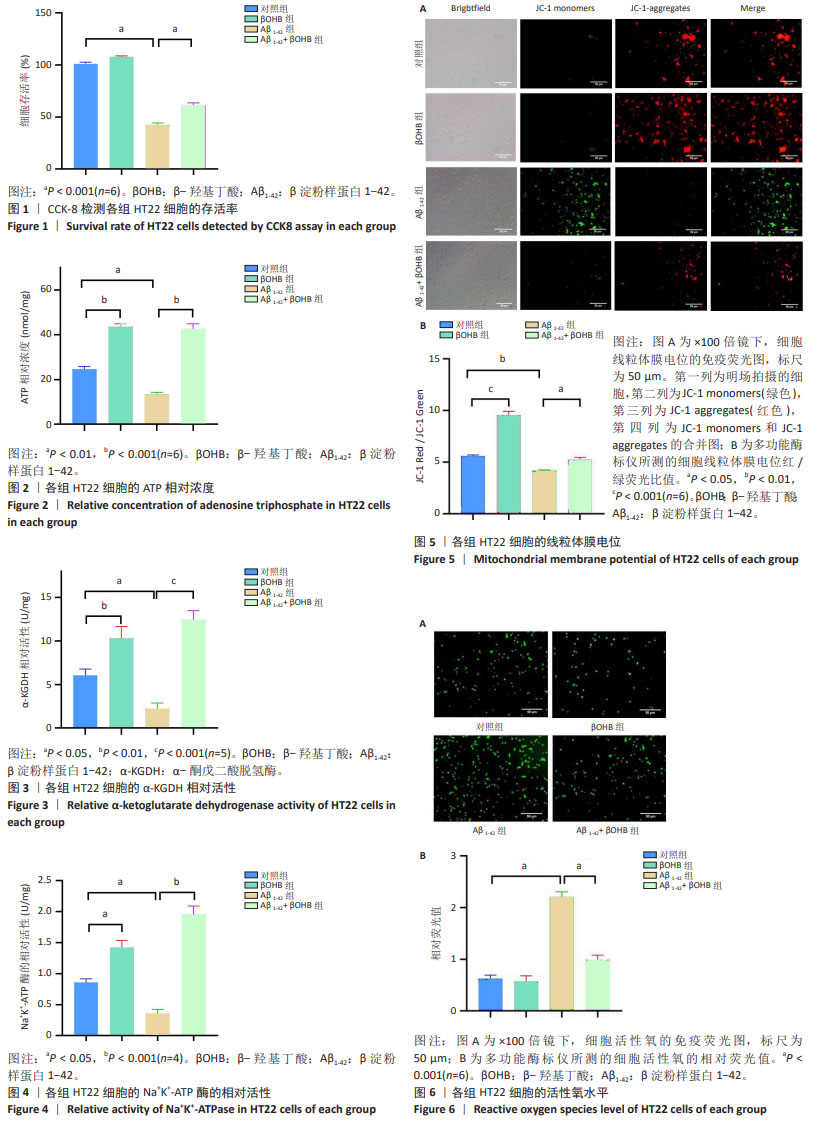
2.1 β-羟基丁酸改善Aβ1-42诱导的HT22细胞的存活率 对照组、β-羟基丁酸组、Aβ1-42组、Aβ1-42+β-羟基丁酸组的细胞存活率分别为(101.080±1.738)%,(107.955±0.898)%,(42.375±1.916)%,(61.320±2.427)%。与对照组相比,β-羟基丁酸组的细胞存活率无统计学差异(P > 0.05),Aβ1-42组的细胞存活率明显下降(P < 0.001),提示Aβ1-42对HT22细胞具有损伤作用;与Aβ1-42组相比,Aβ1-42+β-羟基丁酸组的细胞存活率明显升高(P < 0.001),提示β-羟基丁酸提高了Aβ1-42诱导的HT22细胞的存活率,见图1。 2.2 β-羟基丁酸增加Aβ1-42诱导HT22细胞的ATP水平,改善能量缺陷 对照组、β-羟基丁酸组、Aβ1-42组、Aβ1-42+β-羟基丁酸组细胞的ATP相对浓度(nmol/mg)分别为24.644±1.179,43.547±1.423,13.369±0.069,42.653±2.294。与对照组相比,β-羟基丁酸组的ATP相对浓度明显升高(P < 0.001),表明β-羟基丁酸促进ATP生成。与对照组相比,Aβ1-42组的ATP相对浓度明显下降(P < 0.01);与Aβ1-42组相比,Aβ1-42+β-羟基丁酸组的ATP相对浓度明显升高(P < 0.001),表明β-羟基丁酸增加了Aβ1-42诱导的HT22细胞的ATP水平,改善能量缺陷,见图2。 2.3 β-羟基丁酸增加Aβ1-42诱导HT22细胞的α-KGDH活性,促进ATP生成 对照组、β-羟基丁酸组、Aβ1-42组、Aβ1-42+β-羟基丁酸组细胞的α-KGDH的相对活性(U/mg)分别为6.035±0.749,10.298±1.372,2.245±0.622,12.429±1.068。与对照组相比,β-羟基丁酸组的α-KGDH相对活性明显升高(P < 0.01),表明β-羟基丁酸有利于ATP的生成。与对照组相比,Aβ1-42组的α-KGDH相对活性明显下降(P < 0.05);与Aβ1-42组相比,Aβ1-42+β-羟基丁酸组的α-KGDH相对活性明显升高(P < 0.001),提示β-羟基丁酸增加了Aβ1-42诱导的HT22细胞的α-KGDH活性,促进ATP生成,见图3。 2.4 β-羟基丁酸增加Aβ1-42诱导HT22细胞的Na+K+-ATP酶活性,促进ATP分解产能 对照组、β-羟基丁酸组、Aβ1-42组、Aβ1-42+β-羟基丁酸组细胞Na+K+-ATP酶的相对活性(U/mg)分别为0.859±0.058,1.420±0.118,0.356±0.068,1.960±0.131。与对照组相比,β-羟基丁酸组的Na+K+-ATP酶相对活性升高(P < 0.05),表明β-羟基丁酸有利于ATP分解产能。与对照组相比,Aβ1-42组Na+K+-ATP酶的相对活性下降(P < 0.05);与Aβ1-42组相比,Aβ1-42+β-羟基丁酸组Na+K+-ATP酶的相对活性明显升高(P < 0.001),提示β-羟基丁酸增加了Aβ1-42诱导的HT22细胞的Na+K+-ATP酶活性,促进ATP分解产能,有利于满足神经功能的高能量需求,见图4。 2.5 β-羟基丁酸增加Aβ1-42诱导HT22细胞的线粒体膜电位,改善线粒体生物能量功能 在倒置荧光显微镜×100倍镜下观察线粒体膜电位,与对照组相比,β-羟基丁酸组的红色荧光明显增加,提示β-羟基丁酸促进线粒体膜电位升高。与对照组相比,Aβ1-42组的红色荧光明显减少,绿色荧光明显增加;与Aβ1-42组相比,Aβ1-42+β-羟基丁酸组的绿色荧光明显减少,红色荧光增加,提示Aβ1-42诱导线粒体膜电位受损,β-羟基丁酸提高了Aβ1-42诱导的HT22细胞的线粒体膜电位,见图5A。 使用多功能酶标仪测量JC-1 Red/JC-1 Green值,对照组、β-羟基丁酸组、Aβ1-42组、Aβ1-42+β-羟基丁酸组细胞的JC-1 Red/JC-1 Green值分别为5.576±0.131,9.546±0.387,4.179±0.081,5.238±0.228。与对照组相比,β-羟基丁酸组的JC-1 Red/JC-1 Green值明显升高(P < 0.001),表明β-羟基丁酸改善线粒体膜电位。与对照组相比,Aβ1-42组的JC-1 Red/JC-1 Green值明显下降(P < 0.01);与Aβ1-42组相比,Aβ1-42+β-羟基丁酸组的JC-1 Red/JC-1 Green值明显升高(P < 0.05),见图5B,提示β-羟基丁酸提高了Aβ1-42诱导的HT22细胞的线粒体膜电位,改善线粒体生物能量功能。 2.6 β-羟基丁酸降低Aβ1-42诱导HT22细胞的活性氧水平,改善氧化应激 在倒置荧光显微镜的×100倍镜下观察活性氧水平,与对照组相比,Aβ1-42组的绿色荧光明显增加;与Aβ1-42组相比,Aβ1-42+β-羟基丁酸组的绿色荧光明显减少,提示Aβ1-42诱导活性氧增加,β-羟基丁酸降低了Aβ1-42诱导的HT22细胞的活性氧水平,见图6A。 使用多功能酶标仪测量细胞活性氧荧光值,对照组、β-羟基丁酸组、Aβ1-42组、Aβ1-42+β-羟基丁酸组细胞的活性氧相对荧光值(AU)分别为0.623±0.071,0.572±0.108,2.208±0.098,0.992±0.088。与对照组相比,β-羟基丁酸组的活性氧水平无统计学差异(P > 0.05),Aβ1-42组的活性氧水平明显增加(P < 0.001),提示Aβ1-42对HT22细胞具有氧化损伤作用;与Aβ1-42组相比,Aβ1-42+β-羟基丁酸组的活性氧水平明显降低(P < 0.001),见图6B,提示β-羟基丁酸降低Aβ1-42诱导的HT22细胞的活性氧水平,有利于改善氧化应激。"
| [1] BELFIORE R, RODIN A, FERREIRA E, et al. Temporal and regional progression of Alzheimer’s disease-like pathology in 3xTg-AD mice. Aging Cell. 2019;18(1):e12873. [2] PANG K, JIANG R, ZHANG W, et al. An App knock-in rat model for Alzheimer’s disease exhibiting Aβ and tau pathologies, neuronal death and cognitive impairments. Cell Res. 2022;32(2):157-175. [3] BALUSU S, HORRÉ K, THRUPP N, et al. MEG3 activates necroptosis in human neuron xenografts modeling Alzheimer’s disease. Science. 2023;381(6663):1176-1182. [4] HOWARTH C, GLEESON P, ATTWELL D. Updated energy budgets for neural computation in the neocortex and cerebellum. J Cereb Blood Flow Metab. 2012;32(7):1222-1232. [5] CUNNANE SC, TRUSHINA E, MORLAND C, et al. Brain energy rescue: an emerging therapeutic concept for neurodegenerative disorders of ageing. Nat Rev Drug Discov. 2020;19(9):609-633. [6] ROLFE DF, BROWN GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77(3): 731-758. [7] TAKAHASHI S. Neuroprotective Function of High Glycolytic Activity in Astrocytes: Common Roles in Stroke and Neurodegenerative Diseases. Int J Mol Sci. 2021;22(12):6568. [8] PAN RY, HE L, ZHANG J, et al. Positive feedback regulation of microglial glucose metabolism by histone H4 lysine 12 lactylation in Alzheimer’s disease. Cell Metab. 2022;34(4):634-648.e6. [9] SAITO ER, MILLER JB, HARARI O, et al. Alzheimer’s disease alters oligodendrocytic glycolytic and ketolytic gene expression. Alzheimers Dement. 2021;17(9):1474-1486. [10] ZHANG SS, ZHU L, PENG Y, et al. Long-term running exercise improves cognitive function and promotes microglial glucose metabolism and morphological plasticity in the hippocampus of APP/PS1 mice. J Neuroinflammation. 2022;19(1):34. [11] LI S, SHENG ZH. Energy matters: presynaptic metabolism and the maintenance of synaptic transmission. Nat Rev Neurosci. 2022;23(1):4-22. [12] TZIORAS M, MCGEACHAN RI, DURRANT CS, et al. Synaptic degeneration in Alzheimer disease. Nat Rev Neurol. 2023;19(1):19-38. [13] ASHLEIGH T, SWERDLOW RH, BEAL MF. The role of mitochondrial dysfunction in Alzheimer’s disease pathogenesis. Alzheimers Dement. 2023;19(1):333-342. [14] VENKATARAMAN AV, MANSUR A, RIZZO G, et al. Widespread cell stress and mitochondrial dysfunction occur in patients with early Alzheimer’s disease. Sci Transl Med. 2022;14(658):eabk1051. [15] HUANG CW, RUST NC, WU HF, et al. Low glucose induced Alzheimer’s disease-like biochemical changes in human induced pluripotent stem cell-derived neurons is due to dysregulated O-GlcNAcylation. Alzheimers Dement. 2023;19(11):4872-4885. [16] AN Y, VARMA VR, VARMA S, et al. Evidence for brain glucose dysregulation in Alzheimer’s disease. Alzheimers Dement. 2018;14(3): 318-329. [17] KÜNTZELMANN A, GUENTHER T, HABERKORN U, et al. Impaired cerebral glucose metabolism in prodromal Alzheimer’s disease differs by regional intensity normalization. Neurosci Lett. 2013;534:12-17. [18] WANG W, ZHAO F, MA X, et al. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: recent advances. Mol Neurodegener. 2020;15(1):30. [19] SAMANTA S, AKHTER F, ROY A, et al. New cyclophilin D inhibitor rescues mitochondrial and cognitive function in Alzheimer’s disease. Brain. 2023:awad432. doi: 10.1093/brain/awad [20] BUTTERFIELD DA, HALLIWELL B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci. 2019;20(3): 148-160. [21] PUCHALSKA P, CRAWFORD PA. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017;25(2):262-284. [22] CUNNANE SC, COURCHESNE-LOYER A, ST-PIERRE V, et al. Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer’s disease. Ann N Y Acad Sci. 2016;1367(1):12-20. [23] MUSA-VELOSO K, LIKHODII SS, CUNNANE SC. Breath acetone is a reliable indicator of ketosis in adults consuming ketogenic meals. Am J Clin Nutr. 2002;76(1):65-70. [24] MUSA-VELOSO K, LIKHODII SS, RARAMA E, et al. Breath acetone predicts plasma ketone bodies in children with epilepsy on a ketogenic diet. Nutrition. 2006;22(1):1-8. [25] CROTEAU E, CASTELLANO CA, FORTIER M, et al. A cross-sectional comparison of brain glucose and ketone metabolism in cognitively healthy older adults, mild cognitive impairment and early Alzheimer’s disease. Exp Gerontol. 2018;107:18-26. [26] CASTELLANO CA, NUGENT S, PAQUET N, et al. Lower brain 18F-fluorodeoxyglucose uptake but normal 11C-acetoacetate metabolism in mild Alzheimer’s disease dementia. J Alzheimers Dis. 2015;43(4):1343-1353. [27] JENSEN NJ, WODSCHOW HZ, NILSSON M, et al. Effects of Ketone Bodies on Brain Metabolism and Function in Neurodegenerative Diseases. Int J Mol Sci. 2020;21(22):8767. [28] DING F, YAO J, RETTBERG JR, et al. Early decline in glucose transport and metabolism precedes shift to ketogenic system in female aging and Alzheimer’s mouse brain: implication for bioenergetic intervention. PLoS One. 2013;8(11):e79977. [29] FUKAO T, SONG XQ, MITCHELL GA, et al. Enzymes of ketone body utilization in human tissues: protein and messenger RNA levels of succinyl-coenzyme A (CoA):3-ketoacid CoA transferase and mitochondrial and cytosolic acetoacetyl-CoA thiolases. Pediatr Res. 1997;42(4):498-502. [30] PELLERIN L, BERGERSEN LH, HALESTRAP AP, et al. Cellular and subcellular distribution of monocarboxylate transporters in cultured brain cells and in the adult brain. J Neurosci Res. 2005;79(1-2): 55-64. [31] PIERRE K, PELLERIN L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem. 2005;94(1):1-14. [32] CUNNANE SC, COURCHESNE-LOYER A, VANDENBERGHE C, et al. Can Ketones Help Rescue Brain Fuel Supply in Later Life? Implications for Cognitive Health during Aging and the Treatment of Alzheimer’s Disease. Front Mol Neurosci. 2016;9:53. [33] ZHOU Y, SUN L, WANG H. Ketogenic Diet for Neonatal Hypoxic-Ischemic Encephalopathy. ACS Chem Neurosci. 2023;14(1):1-8. [34] 韩维娜,徐晓庆,史晋宁,等.胰高血糖素样肽1受体激动剂治疗阿尔茨海默病的潜在靶点预测及验证[J].中国组织工程研究,2024, 28(16):2568-2573. [35] DE LEON MJ, FERRIS SH, GEORGE AE, et al. Positron emission tomographic studies of aging and Alzheimer disease. AJNR Am J Neuroradiol. 1983;4(3):568-571. [36] FARIA-PEREIRA A, MORAIS VA. Synapses: The Brain’s Energy-Demanding Sites. Int J Mol Sci. 2022;23(7):3627. [37] ROJAS-MORALES P, TAPIA E, PEDRAZA-CHAVERRI J. β-Hydroxybutyrate: A signaling metabolite in starvation response? Cell Signal. 2016;28(8): 917-923. [38] THEVENET J, DE MARCHI U, DOMINGO JS, et al. Medium-chain fatty acids inhibit mitochondrial metabolism in astrocytes promoting astrocyte-neuron lactate and ketone body shuttle systems. FASEB J. 2016;30(5):1913-1926. [39] TRIGO D, VITÓRIA JJ, DA CRUZ E SILVA OAB. Novel therapeutic strategies targeting mitochondria as a gateway in neurodegeneration. Neural Regen Res. 2023;18(5):991-995. [40] HUANG WC, PENG Z, MURDOCK MH, et al. Lateral mammillary body neurons in mouse brain are disproportionately vulnerable in Alzheimer’s disease. Sci Transl Med. 2023;15(692):eabq1019. [41] BLAUSTEIN MP, LARICCIA V, KHANANSHVILI D, et al. Multipurpose Na+ ions mediate excitation and cellular homeostasis: Evolution of the concept of Na+ pumps and Na+/Ca2+ exchangers. Cell Calcium. 2020;87:102166. [42] JORGENSEN PL, HAKANSSON KO, KARLISH SJ. Structure and mechanism of Na,K-ATPase: functional sites and their interactions. Annu Rev Physiol. 2003;65:817-849. [43] HUANG CW, RUST NC, WU HF, et al. Altered O-GlcNAcylation and mitochondrial dysfunction, a molecular link between brain glucose dysregulation and sporadic Alzheimer’s disease. Neural Regen Res. 2023;18(4):779-783. [44] XIANG X, WIND K, WIEDEMANN T, et al. Microglial activation states drive glucose uptake and FDG-PET alterations in neurodegenerative diseases. Sci Transl Med. 2021;13(615):eabe5640. [45] FERNANDEZ-PEREZ EJ, MUÑOZ B, BASCUÑAN DA, et al. Synaptic dysregulation and hyperexcitability induced by intracellular amyloid beta oligomers. Aging Cell. 2021;20(9):e13455. [46] ZOTT B, SIMON MM, HONG W, et al. A vicious cycle of β amyloid-dependent neuronal hyperactivation. Science. 2019;365(6453): 559-565. [47] ANDERSEN JV, WESTI EW, JAKOBSEN E, et al. Astrocyte metabolism of the medium-chain fatty acids octanoic acid and decanoic acid promotes GABA synthesis in neurons via elevated glutamine supply. Mol Brain. 2021;14(1):132. |
| [1] | Wang Mi, Ma Shujie, Liu Yang, Qi Rui. Identification and validation of characterized gene NFE2L2 for ferroptosis in ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1466-1474. |
| [2] | Xie Liugang, Cui Shuke, Guo Nannan, Li Aoyu, Zhang Jingrui. Research hotspots and frontiers of stem cells for Alzheimer’s disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1475-1485. |
| [3] |
Li Tian, Ren Yuhua, Gao Yanping, Su Qiang.
Mechanism of agomelatine alleviating anxiety- and depression-like behaviors in APP/PS1 transgenic mice #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1176-1182.
|
| [4] | Su Qin, Jia Siwei, Guo Minfang, Meng Tao, Li Yanbing, Mu Bingtao, Song Lijuan, Ma Cungen, Yu Jiezhong. Lycium barbarum polysaccharide intervenes in SH-SY5Y cell injury induced by beta-amyloid protein 1-42: protective effect of mitochondrial autophagy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(31): 6688-6696. |
| [5] | He Ningjuan, Li Li, Wang Su, Yang Jianshe, Lei Siyun, Wang Yang. Effects of aerobic or resistance exercise on hippocampal ras/Drebrin dendritic spine plasticity in a mouse model of Alzheimer’s disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5528-5535. |
| [6] | Shen Zilong, Wu Mingjie, Chen Xiaojing, Zhou Xibin, Zhou Chunxiang. An experimental method for simultaneously extracting the dura mater and deep cervical lymph nodes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5543-5548. |
| [7] | Nan Songhua, Peng Chaojie, Cui Yinglin. Mitochondrial dysfunction and brain aging: a bibliometrics analysis based on the Web of Science Core Collection database [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5642-5651. |
| [8] | Jiang Qianping, Yang Dan, , , Wan Shilei, Xu Dandan, , , , Cao Lu, , Zhou Jing, , , . Role of O-linked N-acetylglucosamine glycosylation in neurodegenerative diseases and its clinical application prospects [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5704-5712. |
| [9] | Lin Huijie, Huang Yun, Huang Zhihua, Jiang Lixia. Hot topics on exosomes as drug delivery system in central nervous system diseases [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(23): 5013-5021. |
| [10] | Wang Zeqian, Duan Yanzhe, Wu Yige, Ma Dong, Huang Jianjun, Yan Yuqing, Song Lijuan. Inhibitory effect of hydroxy safflower yellow A on neuronal pyroptosis after glucose-oxygen deprivation/reglucose-reoxygenation treatment [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(19): 4044-4051. |
| [11] | Liu Dandan, Qin Hewei. Mechanism of action and progress of mitophagy, ferroptosis, cuproptosis, and disulfidptosis in Alzheimer’s disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(19): 4132-4144. |
| [12] | Chen Guilin, Tang Qiqiang. Cuproptosis-related genes in natural killer cells of Alzheimer’s disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(19): 4172-4180. |
| [13] | Zhang Songjiang, Li Longyang, Zhou Chunguang. Relationship between alpha7 nicotinic acetylcholine receptor and Alzheimer’s disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(18): 3915-3924. |
| [14] | Li Longyang, Zhang Songjiang, Zhao Xianmin, Zhou Chunguang, Gao Jianfeng. Electroacupuncture intervention on the proliferation and differentiation of hippocampal neurons and oligodendrocytes in Alzheimer’s disease model mice [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1029-1035. |
| [15] | Wen Huaneng, Lin Run, Wang Yixiao, Wang Bingshui, Liu Lu, Liu Chuanyao, Cai Canxin, Cui Shaoyang, Xu Mingzhu. Effects of electroacupuncture with “Zhi San Zhen” on Notch signaling pathway and synaptic plasticity in 5xFAD mice [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(32): 5148-5153. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||