Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (11): 2268-2276.doi: 10.12307/2025.344
Previous Articles Next Articles
Neurotrophin-3 receptor switching promotes neural functional recovery in rats after spinal cord injury
Cong Yan, Yu Jian, Sun Zhide, Kang Dawei
- Department of Emergency, Affiliated Hospital of Chengde Medical University, Chengde 067000, Hebei Province, China
-
Received:2024-02-24Accepted:2024-04-03Online:2025-04-18Published:2024-08-10 -
Contact:Yu Jian, Master, Professor, Chief physician, Department of Emergency, Affiliated Hospital of Chengde Medical University, Chengde 067000, Hebei Province, China -
About author:Cong Yan, Master, Attending physician, Department of Emergency, Affiliated Hospital of Chengde Medical University, Chengde 067000, Hebei Province, China -
Supported by:Hebei Provincial Natural Science Foundation (Youth Fund), No. H2021406027 (to CY)
CLC Number:
Cite this article
Cong Yan, Yu Jian, Sun Zhide, Kang Dawei. Neurotrophin-3 receptor switching promotes neural functional recovery in rats after spinal cord injury[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(11): 2268-2276.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
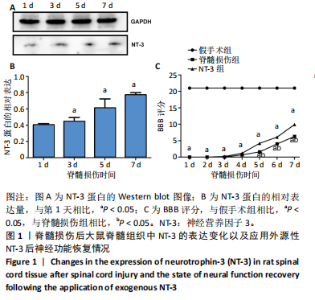
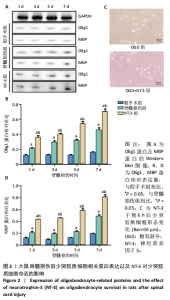
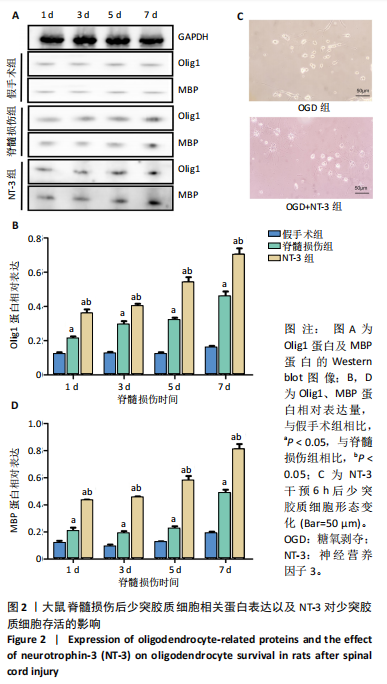
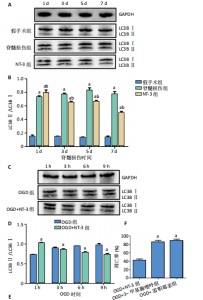
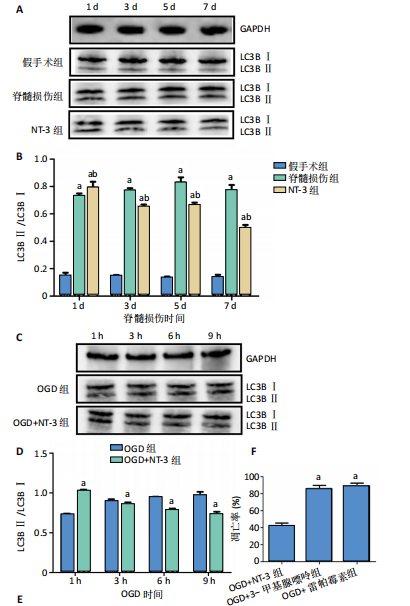
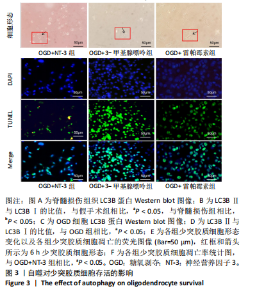
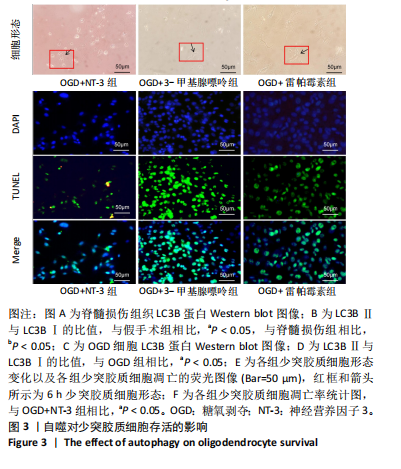
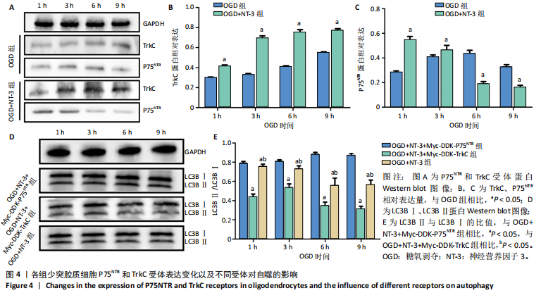
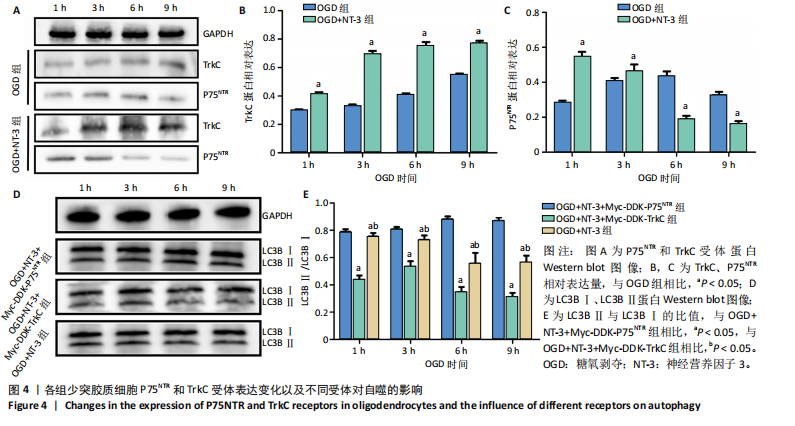
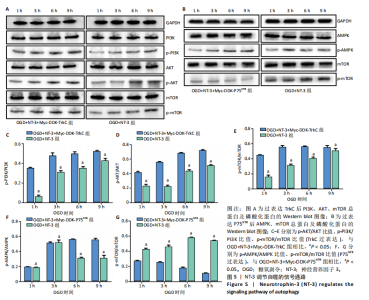
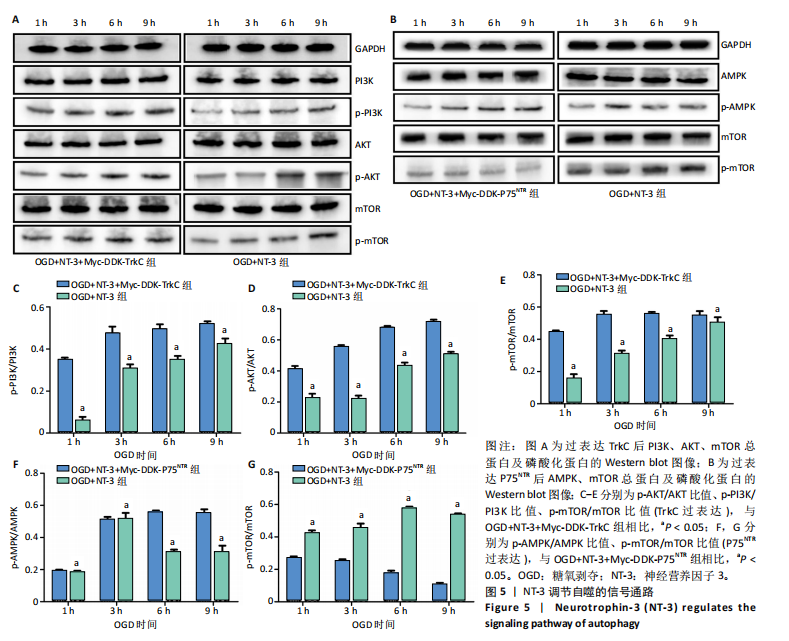
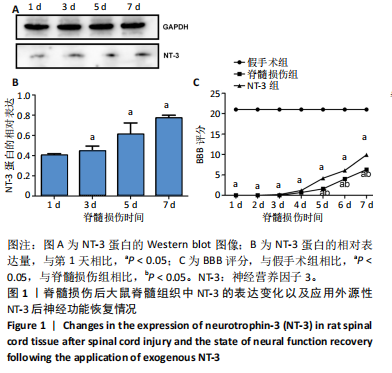
2.1 实验动物数量分析 动物实验中计划参与实验的SD大鼠24只,假手术组、脊髓损伤组、NT-3组,每组8只。由于创伤或术后感染共死亡6只。大鼠死亡后不计入数据统计,将重新造模补充相应的大鼠。 2.2 NT-3有利于大鼠脊髓损伤后神经功能恢复 大鼠脊髓损伤后,受损节段脊髓组织中NT-3的表达呈现持续增多的趋势,说明神经元可能通过自分泌的方式分泌NT-3进行自我修复(图1A,B)。通过蛛网膜下腔注射的方式,使人工合成的NT-3能够到达脊髓损伤区域。应用BBB评分评估脊髓损伤组及NT-3组大鼠1-7 d神经功能恢复情况,发现应用NT-3治疗的大鼠脊髓损伤后神经功能恢复较快(图1C)。 2.3 NT-3能够促进少突胶质细胞分化、髓鞘重塑以及存活 脊髓组织中主要细胞构成是神经元和胶质细胞,由于少突胶质细胞是脊髓髓鞘形成的关键细胞,直接影响神经传导功能,所以少突胶质细胞是促进神经功能恢复的重要细胞。通过Western blot检测NT-3组脊髓组织中Olig1蛋白及MBP蛋白表达,发现在NT-3的干预下这两种蛋白高表达(图2A,B,D),这意味着NT-3具有促进少突胶质细胞分化及髓鞘化的功能。 少突胶质细胞想要发挥促进神经功能恢复作用,必须先解决如何在损伤的脊髓组织中存活的问题。实验发现NT-3能够维持少突胶质细胞存活,在显微镜下观察发现OGD组和OGD+NT-3组少突胶质细胞形态在6 h后发生了显著变化,应用NT-3的少突胶质细胞仍有突触,但OGD组大部分少突胶质细胞突触回缩并出现细胞脱落(图2C)。 2.4 NT-3能够通过双向调节自噬维持少突胶质细胞存活 应用Western blot检测各组大鼠脊髓组织和各组少突胶质细胞中自噬蛋白LC3B表达。通过比较各时间点LC3BⅡ/LC3BⅠ灰度值的比值,发现应用NT-3后自噬呈现先增加后减弱的变化趋势(图3A-D)。根据细胞实验,NT-3能够在细胞损伤的最初3 h内促进自噬,之后便表现出抑制自噬的作用。通过观察6 h时OGD+NT-3组、OGD+3-甲基腺嘌呤组、OGD+雷帕霉素组少突胶质细胞形态,发现单一的促进或抑制自噬都不能维持少突胶质细胞存活,但这种自噬水平先增加后减弱的双向调节方式更有利于少突胶质细胞存活,在6 h时OGD+3-甲基腺嘌呤组、OGD+雷帕霉素组少突胶质细胞均失去了正常的细胞形态(图3E红框区域)。应用TUNEL检测凋亡水平也证实了这一点,发现单一的促进或抑制自噬均使细胞凋亡率上升(图3F)。 2.5 NT-3通过受体切换调节少突胶质细胞自噬 P75NTR和TrkC受体是NT-3可结合的受体,通过Western blot检测少突胶质细胞中这两种受体的表达,发现OGD+NT-3组在3 h后可检测到P75NTR减少,而TrkC受体在此时间段表达增多(图4A-C),这种受体切换可能影响着NT-3对自噬的调节。在OGD建模后分别转染P75NTR及TrkC受体质粒,发现转染TrkC质粒的少突胶质细胞自噬蛋白LC3BⅡ表达减少,而转染P75NTR质粒的少突胶质细胞自噬蛋白LC3BⅡ表达增多(图4D,E)。 2.6 NT-3经P75NTR/AMPK/mTOR促进自噬,经TrkC/PI3K/AKT/mTOR抑制自噬 为进一步验证P75NTR和TrkC受体具体激活哪些信号通路来调控自噬。在已知的调控自噬信号通路中寻找变化明显的磷酸化蛋白。实验发现转染TrkC质粒后,PI3K、AKT、mTOR磷酸化蛋白表达均增多(图5A,C,D,E)。转染P75NTR质粒后,AMPK磷酸化增强,mTOR磷酸化减少(图5B,F,G)。结合已知的促进或抑制自噬的信号通路,可以证实P75NTR经AMPK信号通路抑制mTOR磷酸化促进自噬,而TrkC受体则可以通过激活PI3K、AKT信号通路促进mTOR磷酸化抑制自噬。"
| [1] DORRIAN RM, BERRYMAN CF, LAUTO A, et al. Electrical stimulation for the treatment of spinal cord injuries: A review of the cellular and molecular mechanisms that drive functional improvements. Front Cell Neurosci. 2023;17:1095259. [2] PUKOS N, MARION CM, ARNOLD WD, et al. Chronic demyelination and myelin repair after spinal cord injury in mice: A potential link for glutamatergic axon activity. Glia. 2023;71(9):2096-2116. [3] ALIZADEH A, KARIMI-ABDOLREZAEE S. Microenvironmental regulation of oligodendrocyte replacement and remyelination in spinal cord injury. J Physiol. 2016;594(13):3539-3552. [4] FAN H, TANG HB, SHAN LQ, et al. Quercetin prevents necroptosis of oligodendrocytes by inhibiting macrophages/microglia polarization to M1 phenotype after spinal cord injury in rats. J Neuroinflammation. 2019;16(1):206. [5] LI N, YAO M, LIU J, et al. Vitamin D Promotes Remyelination by Suppressing c-Myc and Inducing Oligodendrocyte Precursor Cell Differentiation after Traumatic Spinal Cord Injury. Int J Biol Sci. 2022; 18(14):5391-5404. [6] KEEFE KM, SHEIKH IS, SMITH GM. Targeting Neurotrophins to Specific Populations of Neurons: NGF, BDNF, and NT-3 and Their Relevance for Treatment of Spinal Cord Injury. Int J Mol Sci. 2017;18(3):548. [7] THOMAS AM, SEIDLITS SK, GOODMAN AG, et al. Sonic hedgehog and neurotrophin-3 increase oligodendrocyte numbers and myelination after spinal cord injury. Integr Biol (Camb). 2014;6(7):694-705. [8] SMITH DR, DUMONT CM, PARK J, et al. Polycistronic Delivery of IL-10 and NT-3 Promotes Oligodendrocyte Myelination and Functional Recovery in a Mouse Spinal Cord Injury Model. Tissue Eng Part A. 2020;26(11-12):672-682. [9] YAN H, WOOD PM. NT-3 weakly stimulates proliferation of adult rat O1(-)O4(+) oligodendrocyte-lineage cells and increases oligodendrocyte myelination in vitro. J Neurosci Res. 2000;62(3):329-335. [10] KUMAR S, KAHN MA, DINH L, et al. NT-3-mediated TrkC receptor activation promotes proliferation and cell survival of rodent progenitor oligodendrocyte cells in vitro and in vivo. J Neurosci Res. 1998;54(6):754-765. [11] AHUJA CS, MARTIN AR, FEHLINGS M. Recent advances in managing a spinal cord injury secondary to trauma. F1000Res. 2016;5:F1000 Faculty Rev-1017. [12] FAN B, WEI Z, YAO X, et al. Microenvironment Imbalance of Spinal Cord Injury. Cell Transplant. 2018;27(6):853-866. [13] RONG Y, LIU W, WANG J, et al. Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy. Cell Death Dis. 2019;10(5):340. [14] LIU S, SARKAR C, DINIZO M, et al. Disrupted autophagy after spinal cord injury is associated with ER stress and neuronal cell death. Cell Death Dis. 2015;6(1):e1582. [15] XU C, MAO L, TIAN H, et al. MICAL1 (molecule interacting with CasL 1) protects oligodendrocyte cells from oxidative injury through regulating apoptosis, autophagy in spinal cord injury. Neurosci Lett. 2021;750:135712. [16] WALKER CL, WALKER MJ, LIU NK, et al. Systemic bisperoxovanadium activates Akt/mTOR, reduces autophagy, and enhances recovery following cervical spinal cord injury. PLoS One. 2012;7(1):e30012. [17] LI W, YAO S, LI H, et al. Curcumin promotes functional recovery and inhibits neuronal apoptosis after spinal cord injury through the modulation of autophagy. J Spinal Cord Med. 2021;44(1):37-45. [18] KAKOTY V, K C S, DUBEY SK, et al. Epigenetic regulation and autophagy modulation debilitates insulin resistance associated Alzheimer’s disease condition in rats. Metab Brain Dis. 2022;37(4):927-944. [19] RICHNER M, ULRICHSEN M, ELMEGAARD SL, et al. Peripheral nerve injury modulates neurotrophin signaling in the peripheral and central nervous system. Mol Neurobiol. 2014;50(3):945-970. [20] KIM MS, KIM GM, CHOI YJ, et al. TrkC promotes survival and growth of leukemia cells through Akt-mTOR-dependent up-regulation of PLK-1 and Twist-1. Mol Cells. 2013;36(2):177-184. [21] LI P, SHI J, HE Q, et al. Streptococcus pneumoniae induces autophagy through the inhibition of the PI3K-I/Akt/mTOR pathway and ROS hypergeneration in A549 cells. PLoS One. 2015;10(3):e0122753. [22] BARRETT GL. The p75 neurotrophin receptor and neuronal apoptosis. Prog Neurobiol. 2000;61(2):205-229. [23] COSTANTINI C, ROSSI F, FORMAGGIO E, et al. Characterization of the signaling pathway downstream p75 neurotrophin receptor involved in beta-amyloid peptide-dependent cell death. J Mol Neurosci. 2005; 25(2):141-156. [24] SUI X, KONG N, YE LI, et al. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer letters. 2014;344(2):174-179. [25] KARSY M, HAWRYLUK G. Modern Medical Management of Spinal Cord Injury. Curr Neurol Neurosci Rep. 2019;19(9):65. [26] ALMAD A, SAHINKAYA FR, MCTIGUE DM. Oligodendrocyte fate after spinal cord injury. Neurotherapeutics. 2011;8(2):262-273. [27] DUMONT RJ, OKONKWO DO, VERMA S, et al. Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol. 2001;24(5):254-264. [28] GONZALEZ A, MOYA-ALVARADO G, GONZALEZ-BILLAUT C, et al. Cellular and molecular mechanisms regulating neuronal growth by brain-derived neurotrophic factor. Cytoskeleton (Hoboken). 2016;73(10): 612-628. [29] GORDON T. The physiology of neural injury and regeneration: The role of neurotrophic factors. J Commun Disord. 2010;43(4):265-273. [30] BOYCE VS, MENDELL LM. Neurotrophic factors in spinal cord injury. Handb Exp Pharmacol. 2014;220:443-460. [31] BRADBURY EJ, KING VR, SIMMONS LJ, et al. NT-3, but not BDNF, prevents atrophy and death of axotomized spinal cord projection neurons. Eur J Neurosci. 1998;10(10):3058-3068. [32] DUNCAN GJ, MANESH SB, HILTON BJ, et al. The fate and function of oligodendrocyte progenitor cells after traumatic spinal cord injury. Glia. 2020;68(2):227-245. [33] ZHANG C, QIU M, FU H. Oligodendrocytes in central nervous system diseases: the effect of cytokine regulation. Neural Regen Res. 2024; 19(10):2132-2143. [34] MEKHAIL M, ALMAZAN G, TABRIZIAN M. Purine-crosslinked injectable chitosan sponges promote oligodendrocyte progenitor cells’ attachment and differentiation. Biomater Sci. 2015;3(2):279-287. [35] KANNO H, OZAWA H, SEKIGUCHI A, et al. Induction of autophagy and autophagic cell death in damaged neural tissue after acute spinal cord injury in mice. Spine (Phila Pa 1976). 2011;36(22):E1427-1434. [36] LI R, LI D, WU C, et al. Nerve growth factor activates autophagy in Schwann cells to enhance myelin debris clearance and to expedite nerve regeneration. Theranostics. 2020;10(4):1649-1677. [37] XU B, FANG J, WANG J, et al. Inhibition of autophagy and RIP1/RIP3/MLKL-mediated necroptosis by edaravone attenuates blood spinal cord barrier disruption following spinal cord injury. Biomed Pharmacother. 2023;165:115165. [38] TAVERNARAKIS N. Regulation and Roles of Autophagy in the Brain. Adv Exp Med Biol. 2020;1195:33. [39] Mirzahosseini G, Ishrat T. Modulation of p75 neurotrophin receptor mitigates brain damage following ischemic stroke in mice. Neural Regen Res. 2024;19(10):2093-2094. |
| [1] | Yin Lu, Jiang Chuanfeng, Chen Junjie, Yi Ming, Wang Zihe, Shi Houyin, Wang Guoyou, Shen Huarui. Effect of Complanatoside A on the apoptosis of articular chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1541-1547. |
| [2] | Li Huayuan, Li Chun, Liu Junwei, Wang Ting, Li Long, Wu Yongli. Effect of warm acupuncture on PINK1/Parkin pathway in the skeletal muscle of rats with chronic fatigue syndrome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1618-1625. |
| [3] | Zhou Panpan, Cui Yinglin, Zhang Wentao, Wang Shurui, Chen Jiahui, Yang Tong . Role of cellular autophagy in cerebral ischemic injury and the regulatory mechanism of traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1650-1658. |
| [4] | Zhu Hanmin, Wang Song, Xiao Wenlin, Zhang Wenjing, Zhou Xi, He Ye, Li Wei, . Mitophagy regulates bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1676-1683. |
| [5] | Jin Kai, Tang Ting, Li Meile, Xie Yuan. Effects of conditioned medium and exosomes of human umbilical cord mesenchymal stem cells on proliferation, migration, invasion, and apoptosis of hepatocellular carcinoma cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1350-1355. |
| [6] | Wan Lingling, Wu Mengying, Zhang Yujiao, Luo Qingqing. Inflammatory factor interferon-gamma affects migration and apoptosis of human vascular smooth muscle cells through pyroptosis pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1422-1428. |
| [7] | Zheng Rongfa, Mo Weibin, Huang Peng, Chen Junji, Liang Ting, Zi Fangyu, Li Guofeng. Effects of electroacupuncture on the expression of metabolic enzymes and autophagy genes in gastrocnemius muscle tissues of exercising rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1127-1136. |
| [8] | Liu Zhezhe, Yu Meiqing, Wang Tingting, Zhang Min, Li Baiyan. Troxerutin modulates nuclear factor-kappaB signaling pathway to inhibit brain injury and neuronal apoptosis in cerebral infarction rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1137-1143. |
| [9] | Chen Yuning, Jiang Ying, Liao Xiangyu, Chen Qiongjun, Xiong Liang, Liu Yue, Liu Tong. Buqi Huoxue Compounds intervene with the expression of related factors and autophagy related proteins in a rat model of cerebral ischemia/reperfusion [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1152-1158. |
| [10] | He Guanghui, Yuan Jie, Ke Yanqin, Qiu Xiaoting, Zhang Xiaoling. Hemin regulates mitochondrial pathway of oxidative stress in mouse chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1183-1191. |
| [11] | He Bo, Chen Wen, Ma Suilu, He Zhijun, Song Yuan, Li Jinpeng, Liu Tao, Wei Xiaotao, Wang Weiwei, Xie Jing . Pathogenesis and treatment progress of flap ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1230-1238. |
| [12] | Liu Lingyun, He Guixin, Qin Weibin, Song Hui, Zhang Liwen, Tang Weizhi, Yang Feifei, Zhu Ziyi, Ou Yangbin . Improvement of myocardial injury by traditional Chinese medicine: mitochondrial calcium homeostasis mediates macrophage autophagy and pyroptosis pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1276-1284. |
| [13] | Lan Shuangli, Xiang Feifan, Deng Guanghui, Xiao Yukun, Yang Yunkang, Liang Jie. Naringin inhibits iron deposition and cell apoptosis in bone tissue of osteoporotic rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 888-898. |
| [14] | Xu Tianjie, Fan Jiaxin, Guo Xiaoling, Jia Xiang, Zhao Xingwang, Liu kainan, Wang Qian. Metformin exerts a protective effect on articular cartilage in osteoarthritis rats by inhibiting the PI3K/AKT/mTOR pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1003-1012. |
| [15] | Xu Fei, Yan Jinqiang, Chai Shoudong. Mechanical stress regulates apoptosis in vascular smooth muscle cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1064-1072. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||