Chinese Journal of Tissue Engineering Research ›› 2021, Vol. 25 ›› Issue (19): 3077-3082.doi: 10.3969/j.issn.2095-4344.3503
Previous Articles Next Articles
Application and effect of induced pluripotent stem cells in bone surgery tissue engineering
Xia Guoming, Xu Qiang, Liu Xuqiang, Yu Xiaolong, Dai Min
- Department of Orthopedics, First Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi province, China
-
Received:2020-03-11Revised:2020-03-19Accepted:2020-06-10Online:2021-07-09Published:2021-01-14 -
Contact:ai Min, Master, Professor, Department of Orthopedics, First Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi province, China -
About author:Xia Guoming, Physician, Department of Orthopedics, First Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi province, China
CLC Number:
Cite this article
Xia Guoming, Xu Qiang, Liu Xuqiang, Yu Xiaolong, Dai Min. Application and effect of induced pluripotent stem cells in bone surgery tissue engineering[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 3077-3082.
share this article

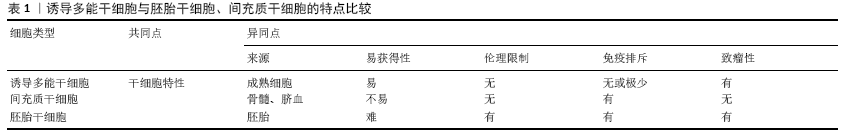
2.1 诱导多能干细胞 日本的TAKAHASHI及YAMANAKA[1] 于2006年在世界范围内首次通过反转录病毒载体将4个关键转录因子基因(Oct3/4,Sox2,Klf4,c-Myc)转入成年小鼠成纤维细胞中,使其重编程为具有胚胎干细胞特性的诱导多能干细胞。TAKAHASHI等[2]在2007年再次使用同样的4个基因组合成功地将人成纤维细胞重编程为诱导多能干细胞。与此同时,俞君英教授带领的团队成功利用诱导多能干细胞技术将人皮肤纤维母细胞诱导成几乎与胚胎干细胞完全一样的多能干细胞[3]。该团队后续还利用人淋巴母细胞重新编程为无EBV诱导的多能干细胞,以及从正常和赘生性骨髓、脐血单核细胞中高效生成无转基因的诱导多能干细胞[4-5]。目前利用多能性转录因子重编程的效率并不高,为了改善重编程不高的问题,TAKAHASHI团队[6]通过研究发现利用细胞周期调控因子和一些影响表观修饰的因子可以用来提高重编程效率。哈佛大学研究团队通过添加特殊化合物的方法使诱导多能干细胞重编程的成功率提高100倍。 以上研究成果都为诱导多能干细胞在组织再生工程领域的蓬勃发展奠基了坚实基础,同样为诱导多能干细胞技术运用于临床作出了巨大贡献。随着诱导多能干细胞编程的技术日益成熟,诱导多能干细胞俨然有机会成为医学领域的佼佼者,成为推动人类医学变革的关键因子。 2.2 诱导多能干细胞来源的细胞特性及治疗效果 2.2.1 诱导多能干细胞来源的细胞特性 诱导多能干细胞自问世以来就引起了生命科学基础研究和医学领域的广泛关注,成功取代了胚胎干细胞和间充质干细胞,成为干细胞领域的热门研究方向[7]。相对于间充质干细胞、胚胎干细胞等其他干细胞,诱导多能干细胞来源的细胞有其特有的优势,其优势之处:①诱导多能干细胞不使用卵细胞和胚胎等涉及生殖伦理的细胞和组织,而由体细胞反转录导入特定的转录因子(Oct3/4、Sox2、Klf4、c-Myc)产生,摆脱了胚胎干细胞无法回避的伦理障碍;②诱导多能干细胞具备来源广、相对易获得、个体特异性或某种疾病特异性的优点;③因诱导多能干细胞来源于自体细胞,使其在骨外科组织工程领域作为种子细胞而更具有同源性,与患者达到很好的匹配度,不太可能在移植后引起免疫排斥反应[8-9],见表1。 然而,据报道随着基因测序技术的发展,诱导多能干细胞基因组在表达不稳定性方面已经得到证实[10]。诱导多能干细胞的基因不稳定性会使其在组织工程运用中产生严重的安全隐患, 阻碍了基于诱导多能干细胞技术的骨外科组织再生工程领域的发展。 2.2.2 诱导多能干细胞来源的细胞治疗效果 在2014年,首次进行了基于诱导多能干细胞治疗的人体临床试验,患有年龄相关视网膜病变的日本女性植入了用其自身皮肤成纤维细胞重编程获取的诱导多能干细胞来源的视网膜色素上皮细胞片,最终得到了较满意的结果,术后无积液性出血或其他严重问题[11]。然而由于诱导多能干细胞基因组突变性致使第二次临床人体临床试验被迫停止[12-13]。有研究结果表明,将诱导多能干细胞衍生的角质形成细胞移植到全层皮肤伤口部位可加速上皮再形成并减少瘢痕形成[14]。综合基于诱导多能干细胞技术治疗疾病的临床研究显示,诱导多能干细胞来源的细胞治疗对目前很多无法治愈的疾病具有更理想的结果,而且在治疗过程中未出现明显的不良反应及并发症。 2.3 诱导多能干细胞在骨外科组织工程中的运用进展 2.3.1 诱导多能干细胞技术在肌腱组织工程中的运用进展 在临床治疗肌腱损伤的修复过程中,根据损伤部位及程度采取不同的手术方式,比如跟腱断裂,外科医生通常会选择断端缝合,而膝关节的十字韧带断裂则通过关节镜技术行肌腱移植。虽然这些外科干预缩短了肌腱的修复时长,但最后都有纤维组织填充形成瘢痕导致肌腱弹性减低、瘢痕粘连,最终影响肢体功能且易发生二次损伤。这无疑是诱导多能干细胞技术在肌腱再生工程领域研究及运用的内在推动力。 在肌腱修复领域,干细胞研究报道极少,且报道的研究几乎局限于体外及动物实验[15-16]。在一项关于人诱导多能干细胞来源的神经嵴干细胞促进大鼠髌腱缺损肌腱修复的实验研究显示:诱导多能干细胞来源的神经嵴干细胞处理的肌腱修复效果优于对照组;组织学和力学检查显示,诱导多能干细胞来源的神经嵴干细胞能显著促进肌腱的愈合,表现为基质合成和力学性能的改善[17]。另有研究显示,诱导多能干细胞的在肌腱损伤愈合过程中增加和重建血运具有很好的潜 力[18]。KouroUpis研究团队[19]基于诱导多能干细胞的生物人工前十字韧带移植物的生成,可有效修复前十字韧带,克服了传统异体肌腱或自体肌腱的局限及不足。有研究从马等大型动物获得诱导多能干细胞治疗修复损伤的肌腱,并获得实质性的成功[20]。诱导多能干细胞衍生的腱细胞样细胞促进小鼠损伤肌腱再生并减少瘢痕形成,为肌腱损伤提供有效的治疗方法[21]。与众多研究结果不同,后期的一项研究显示,与胚胎干细胞相比,马的诱导多能干细胞向肌腱分化能力更 差[22]。 虽然研究显示诱导多能干细胞修复肌腱具有较好的效果。但是诱导多能干细胞重编程不完全的问题和诱导多能干细胞还存在因重编程基因组不稳定产生致瘤性的安全问题未解决之前,仍不能运用于骨外科组织工程的人体试验及临床中。何时能替代间充质干细胞、胚胎干细胞在肌腱修复领域的角色,仍旧是个未知数,但是越来越多的实验性研究结果显示诱导多能干细胞在治疗肌腱损伤方面都起到良好的效果。 2.3.2 诱导多能干细胞在骨再生重建中的运用进展 骨缺损是目前骨科领域面临的巨大挑战之一[23-24],同种异体骨及自体骨移植是弥补骨缺损常用的手术治疗方案。随着组织工程技术的兴起,该技术也被尝试用于骨组织缺损的修复。间充质干细胞是当前骨组织工程最常用的种子细胞,随着供者年龄增长、骨质疏松以及风湿性关节炎等疾病发生,其更新及增殖能力逐渐下降,由此造成自体间充质干细胞的供给不足,无法满足大部分骨缺损患者的需要[25-27]。诱导多能干细胞替代间充质干细胞有可能成为骨组织工程的新型种子细胞。 诱导多能干细胞虽然有高分化特性,亦同时存在致瘤性缺陷,因此首先将其诱导为间充质干细胞后再考虑植入机体内,减少恶性肿瘤的发生概率[28-29]。YE的研究团队[30]把诱导多能干细胞种植到一种盘状亲水性的丝心蛋白支架中,在体外培养条件下获得良好的多向分化潜能,用于修复成年小鼠标准颅骨缺损,5周后检测结果显示在新生骨形成方面诱导多能干细胞移植组明显好于单纯支架组。TANG及LIU等团队[31-32]利用成人骨髓CD34+细胞来源的诱导多能干细胞及磷酸钙骨水泥支架构建骨组织修复材料,体外实验中各项成骨检测都表现出了极富研究前景的结果。KANG 等[33]利用腺苷诱导人诱导多能干细胞成骨的实验研究显示来源于人诱导多能干细胞的成骨细胞参与了骨缺损的愈合,而没有形成畸胎瘤,这项研究给诱导多能干细胞技术的运用避免致瘤性提供了一定的研究方向。2017年一项研究显示诱导多能干细胞的成骨能力等于或优于间充质干细胞,而且细胞来源对其重编程获得诱导多能干细胞的成骨潜力没有显著差异[34]。更近的一项研究表明:诱导多能干细胞复合三维支架系统可模拟天然干细胞生态位的生理复杂性,对骨骼组织的修复和再生非常有利[35]。 以上研究可以看出:①诱导多能干细胞的致瘤性可以通过改进治疗策略而避免;②诱导多能干细胞完全有定性分化为骨组织的能力(较间充质干细胞更强),用于修复骨缺损时有较好的结果;③诱导多能干细胞复合支架可以相比单纯诱导多能干细胞达到更好的疗效。总之,克服诱导多能干细胞不足及制备合适的生物支架,对诱导多能干细胞技术运用于骨组织再生重建具有极大帮助。未来十年或者更长时间,如何让诱导多能干细胞技术安全有效地运用于骨组织再生领域的仍是一项重大挑战及研究方向。 2.3.3 诱导多能干细胞在软骨修复领域的运用进展 软骨因本身无血管的组织特点使其损伤后自我修复能力差。该疾病患者往往因关节疼痛与功能障碍导致生存质量明显下降。骨性关节炎是世界上最常见的关节疾病,其发病因素就是关节软骨损伤。在60岁以上人群中骨性关节炎发病率男性为10%,女性为13%[36]。目前骨性关节炎使用药物治疗仍以缓解疼痛的对症治疗为主,当达到一定手术指征后,外科医生会考虑实施关节置换、关节融合等手术治疗。然而外科手术治疗创伤大、花费贵,并且有时难以达到令人满意的治疗效果[37]。因此,寻找针对骨性关节炎软骨损伤行之有效而又微创的治疗方法迫在眉睫。 现已证明自体软骨细胞移植在治疗关节软骨损伤尤其是膝关节软骨损伤更具疗效 [38-40]。然而自体软骨细胞来源不足,无法提供给大面积软骨缺损患者使用,而诱导多能干细胞作为软骨组织工程极具潜力的种子细胞能够解决自体软骨细胞来源不足的问题。对此人们作了很多体外及动物实验。UTO 的研究团队[41]在小鼠关节软骨缺损处移植入诱导多能干细胞和明胶的混合物,后期观察到损伤的软骨关节面再生;KO等[42]将诱导多能干细胞在海藻酸钠水凝胶中培养并诱导其定向软骨细胞分化,随后与海藻酸钠水凝胶复合物移植入关节软骨缺损的免疫缺陷小鼠模型中,12周后在缺损处观察到表面光滑、附着牢固的软骨样组织,成功地修复了关节软骨缺损。通过结合超纯化藻酸盐凝胶(UPAL凝胶)的三维培养和间充质干细胞样细胞(iPS-MSC)进行多步分化的方法将诱导多能干细胞分化为软骨细胞。超纯化藻酸盐凝胶培养物中的诱导多能干细胞来源间充质干细胞样细胞依次增强了软骨生成标记的表达,而没有上调成骨和脂肪生成标记的表达,并且在组织学上显示出均质的软骨生成[43]。2016年有研究结果显示诱导多能干细胞可有效修复骨性关节炎小鼠模型的软骨损伤[44]。虽然在体外软骨分化领域诱导多能干细胞还没有满意的结果,但诱导多能干细胞是软骨再生的可选择细胞来源[45]。此外,日本的YAMASHITA研究团队[46]将纯化的诱导多能干细胞源性透明软骨细胞微粒移植入严重联合免疫缺陷小鼠和免疫抑制迷你猪的关节软骨缺损处,新生软骨得以存活,并与宿主关节软骨相互融合,不但成功修复了关节软骨缺损,还没有异位组织和肿瘤形成,保证了诱导多能干细胞临床应用的安全性,并且迷你猪体内实验的结果还表明诱导多能干细胞可适用于大型动物,预示着人类关节软骨损伤同样可以被诱导多能干细胞修复。 尽管以诱导多能干细胞为种子细胞在组织再生工程中具有良好的临床应用前景,但实际运用到临床,仍需大量的实验性研究提供更有力的依据。 2.3.4 诱导多能干细胞在脊髓神经修复领域中的运用进展 脊髓损伤是一种破坏性的神经系统疾病,损害运动、感觉和自主神经通路。神经前体细胞移植可促进脊髓损伤后的功能恢复,但是神经前体细胞主要从胚胎干细胞或胎儿组织中获得,受到伦理学的限制。体外培养的运动神经元能够表现出电生理和突触活性、典型运动神经标记物的表达以及移植到脊髓损伤中的能力。迄今为止干细胞的异质性和治疗方案的多样性还很难把握,以及炎症、瘢痕等不良微环境的影响,仍难以实现干细胞治疗脊髓损伤的临床试验。然而诱导多能干细胞比其他干细胞具有无伦理限制和更低的免疫原性使其更具临床应用价值。因此挖掘诱导多能干细胞技术运用于脊髓损伤治疗势在必行。 YAMANAKA和他的同事建立了可以从体细胞产生诱导多能干细胞的方法,这一创新发展在脊髓损伤再生领域取得了快速进展。诱导多能干细胞复合组织工程支架治疗可以促进脊髓损伤组织重建,并可以促进横断脊髓模型运动功能的恢复[47]。与人骨髓间充质干细胞相比,诱导多能干细胞来源的神经祖细胞治疗脊髓损伤由于移植存活率高,对保护组织、减少胶质瘢痕形成和增加轴突萌生的影响,使运动功能恢复最快[48]。有研究小组成功地用诱导多能干细胞生产了神经前体细胞,并证明了移植后对脊髓损伤动物模型的有益作用[49]。 为克服诱导多能干细胞移植后的致瘤性等问题,选择体细胞的来源至关重要,在重编程因子转染过程中使用无整合系统,并彻底研究诱导多能干细胞来源神经前体细胞在质量管理方面的特性。 2.4 诱导多能干细胞技术在骨外科组织工程中研究及运用展望 至今为止仍然有许多疾病无法治愈或因资源匮乏无法得到及时救治。干细胞技术的目标是通过体外制造功能性生物结构来减轻供体器官的严重短缺,这让很多疾病缠身的患者看到了救治的希望。 值得注意的是诱导多能干细胞于2006年问世,其因众多优势得到广泛关注,大有取代间充质干细胞成为骨外科组织工程最有希望的种子细胞,可以认为诱导多能干细胞技术进一步推动了骨外科组织工程的研究发展。众所周知,组织工程包括种子细胞、支架、体内微环境3要素,除了对种子细胞的研究外,人们对支架的选择同样投入大量的研究,根据目前的研究趋势,制备更优的生物支架材料与选择组织工程种子细胞同样重要,一种可注射、温敏性的纳米级别的生物支架被提上了研究日程,这种生物支架制备需要用到3D打印等快速成型技术,3D打印技术如何行之有效与诱导多能干细胞技术结合运用于骨外科组织工程研究中,将是该领域的研究热点。"
| [1] TAKAHASHI K, YAMANAKA S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663-676. [2] TAKAHASHI K, TANABE K, OHNUKI M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007; 131(5):861-872. [3] YU J, VODYANIK MA, SMUGA-OTTO K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858): 1917-1920. [4] RAJESH D, DICKERSON SJ, YU J, et al. Human lymphoblastoid B-cell lines reprogrammed to EBV-free induced pluripotent stem cells. Blood. 2011;118(7):1797-1800. [5] HU K, YU J, SUKNUNTHA K, et al. Efficient generation of transgene-free induced pluripotent stem cells from normal and neoplastic bone marrow and cord blood mononuclear cells. Blood. 2011;117(14):e109-119. [6] TAKAHASHI K, YAMANAKA S. A decade of transcription factor-mediated reprogramming to pluripotency. Nat Rev Mol Cell Biol. 2016;17(3): 183-193. [7] VAN LAAKE LW, QIAN L, CHENG P, et al. Reporter-based isolation of induced pluripotent stem cell- and embryonic stem cell-derived cardiac progenitors reveals limited gene expression variance. Circ Res. 2010;107(3):340-347. [8] ARAKI R, UDA M, HOKI Y, et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494(7435):100-104. [9] GUHA P, MORGAN JW, MOSTOSLAVSKY G, et al. Lack of Immune Response to Differentiated Cells Derived from Syngeneic Induced Pluripotent Stem Cells. Cell Stem Cell. 2017;21(1):144-148. [10] YOSHIHARA M, HAYASHIZAKI Y, MURAKAWA Y. Genomic Instability of iPSCs: Challenges Towards Their Clinical Applications. Stem Cell Rev Rep. 2017;13(1):7-16. [11] CYRANOSKI D. Japanese woman is first recipient of next generation stem cells. Nature. 2014;12. [12] CHAKRADHAR S. An eye to the future: Researchers debate best path for stem cell-derived therapies. Nat Med. 2016;22(2):116-119. [13] GARBER K. RIKEN suspends first clinical trial involving induced pluripotent stem cells. Nat Biotechnol. 2015;33(9):890-891. [14] YAN Y, JIANG J, ZHANG M, et al. Effect of iPSCs-derived keratinocytes on healing of full-thickness skin wounds in mice. Exp Cell Res. 2019;385(1): 111627. [15] CHEN X, SONG XH, YIN Z, et al. Stepwise differentiation of human embryonic stem cells promotes tendon regeneration by secreting fetal tendon matrix and differentiation factors. Stem Cells. 2009;27(6): 1276-1287. [16] NI M, LUI PP, RUI YF, et al. Tendon-derived stem cells (TDSCs) promote tendon repair in a rat patellar tendon window defect model. J Orthop Res. 2012;30(4):613-619. [17] XU W, WANG Y, LIU E, et al. Human iPSC-derived neural crest stem cells promote tendon repair in a rat patellar tendon window defect model. Tissue Eng Part A. 2013;19(21-22):2439-2451. [18] BOHÁČ M, CSÖBÖNYEIOVÁ M, KUPCOVÁ I, et al. Stem cell regenerative potential for plastic and reconstructive surgery. Cell Tissue Bank. 2016; 17(4):735-744. [19] KOUROUPIS D, KYRKOU A, TRIANTAFYLLIDI E, et al. Generation of stem cell-based bioartificial anterior cruciate ligament (ACL) grafts for effective ACL rupture repair. Stem Cell Res. 2016;17(2):448-457. [20] NAGY K, SUNG HK, ZHANG P, et al. Induced pluripotent stem cell lines derived from equine fibroblasts. Stem Cell Rev Rep. 2011;7(3):693-702. [21] KOMURA S, SATAKE T, GOTO A, et al. Induced pluripotent stem cell-derived tenocyte-like cells promote the regeneration of injured tendons in mice. Sci Rep. 2020;10(1):3992. [22] BAVIN EP, SMITH O, BAIRD AE, et al. Equine Induced Pluripotent Stem Cells have a Reduced Tendon Differentiation Capacity Compared to Embryonic Stem Cells. Front Vet Sci. 2015;2:55. [23] BAUER TW, MUSCHLER GF. Bone graft materials. An overview of the basic science. Clin Orthop Relat Res. 2000;(371):10-27. [24] LAURENCIN C, KHAN Y, EL-AMIN SF. Bone graft substitutes. Expert Rev Med Devices. 2006;3(1):49-57. [25] MENDES SC, TIBBE JM, VEENHOF M, et al. Bone tissue-engineered implants using human bone marrow stromal cells: effect of culture conditions and donor age. Tissue Eng. 2002;8(6):911-920. [26] STENDERUP K, JUSTESEN J, CLAUSEN C, et al. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33(6):919-926. [27] RODRÍGUEZ JP, MONTECINOS L, RÍOS S, et al. Mesenchymal stem cells from osteoporotic patients produce a type I collagen-deficient extracellular matrix favoring adipogenic differentiation. J Cell Biochem. 2000;79(4):557-565. [28] YAMANAKA S. Induction of pluripotent stem cells from mouse fibroblasts by four transcription factors. Cell Prolif. 2008;41 Suppl 1 (Suppl 1):51-56. [29] JUNG Y, BAUER G, NOLTA JA. Concise review: Induced pluripotent stem cell-derived mesenchymal stem cells: progress toward safe clinical products. Stem Cells. 2012;30(1):42-47. [30] YE JH, XU YJ, GAO J, et al. Critical-size calvarial bone defects healing in a mouse model with silk scaffolds and SATB2-modified iPSCs. Biomaterials. 2011;32(22):5065-5076. [31] TANG M, CHEN W, LIU J, et al. Human induced pluripotent stem cell-derived mesenchymal stem cell seeding on calcium phosphate scaffold for bone regeneration. Tissue Eng Part A. 2014 ;20(7-8):1295-1305. [32] LIU J, CHEN W, ZHAO Z, et al. Reprogramming of mesenchymal stem cells derived from iPSCs seeded on biofunctionalized calcium phosphate scaffold for bone engineering. Biomaterials. 2013;34(32):7862-7872. [33] KANG H, SHIH YR, NAKASAKI M, et al. Small molecule-driven direct conversion of human pluripotent stem cells into functional osteoblasts. Sci Adv. 2016 ;2(8):e1600691. [34] BASTAMI F, NAZEMAN P, MOSLEMI H, et al. Induced pluripotent stem cells as a new getaway for bone tissue engineering: A systematic review. Cell Prolif. 2017;50(2):e12321. [35] RANA D, KUMAR S, WEBSTER TJ, et al. Impact of Induced Pluripotent Stem Cells in Bone Repair and Regeneration. Curr Osteoporos Rep. 2019;17(4):226-234. [36] MA VY, CHAN L, CARRUTHERS KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil. 2014;95(5):986-995. [37] LIN W, CHEN M, HU C, et al. Endowing iPSC-Derived MSCs with Angiogenic and Keratinogenic Differentiation Potential: A Promising Cell Source for Skin Tissue Engineering. Biomed Res Int. 2018;2018: 8459503. [38] OCHS BG, MÜLLER-HORVAT C, ALBRECHT D, et al. Remodeling of articular cartilage and subchondral bone after bone grafting and matrix-associated autologous chondrocyte implantation for osteochondritis dissecans of the knee. Am J Sports Med. 2011;39(4):764-773. [39] VISTE A, PIPERNO M, DESMARCHELIER R, et al. Autologous chondrocyte implantation for traumatic full-thickness cartilage defects of the knee in 14 patients: 6-year functional outcomes. Orthop Traumatol Surg Res. 2012;98(7):737-743. [40] ZHANG Z, ZHONG X, JI H, et al. Matrix-induced autologous chondrocyte implantation for the treatment of chondral defects of the knees in Chinese patients. Drug Des Devel Ther. 2014;8:2439-2448. [41] UTO S, NISHIZAWA S, TAKASAWA Y, et al. Bone and cartilage repair by transplantation of induced pluripotent stem cells in murine joint defect model. Biomed Res. 2013;34(6):281-288. [42] KO JY, KIM KI, PARK S, et al. In vitro chondrogenesis and in vivo repair of osteochondral defect with human induced pluripotent stem cells. Biomaterials. 2014;35(11):3571-3581. [43] HONTANI K, ONODERA T, TERASHIMA M, et al. Chondrogenic differentiation of mouse induced pluripotent stem cells using the three-dimensional culture with ultra-purified alginate gel. J Biomed Mater Res A. 2019;107(5):1086-1093. [44] ZHU Y, WU X, LIANG Y, et al. Repair of cartilage defects in osteoarthritis rats with induced pluripotent stem cell derived chondrocytes. BMC Biotechnol. 2016;16(1):78. [45] DIEDERICHS S, GABLER J, AUTENRIETH J, et al. Differential Regulation of SOX9 Protein During Chondrogenesis of Induced Pluripotent Stem Cells Versus Mesenchymal Stromal Cells: A Shortcoming for Cartilage Formation. Stem Cells Dev. 2016;25(8):598-609. [46] YAMASHITA A, MORIOKA M, YAHARA Y, et al. Generation of scaffoldless hyaline cartilaginous tissue from human iPSCs. Stem Cell Reports. 2015; 4(3):404-418. [47] ZHOU X, SHI G, FAN B, et al. Polycaprolactone electrospun fiber scaffold loaded with iPSCs-NSCs and ASCs as a novel tissue engineering scaffold for the treatment of spinal cord injury. Int J Nanomedicine. 2018;13:6265-6277. [48] KUBINOVA S, MURALI R, SYKOVA E, et al. A Comparative Study of Three Different Types of Stem Cells for Treatment of Rat Spinal Cord Injury. Cell Transplant. 2017;26(4):585-603. [49] NAGOSHI N, OKANO H. iPSC-derived neural precursor cells: potential for cell transplantation therapy in spinal cord injury. Cell Mol Life Sci. 2018;75(6):989-1000. [50] HUANG Y, ZHANG XF, GAO G, et al. 3D bioprinting and the current applications in tissue engineering. Biotechnol J. 2017;12(8):1600734. |
| [1] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [2] | Min Youjiang, Yao Haihua, Sun Jie, Zhou Xuan, Yu Hang, Sun Qianpu, Hong Ensi. Effect of “three-tong acupuncture” on brain function of patients with spinal cord injury based on magnetic resonance technology [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-8. |
| [3] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [4] | Hu Kai, Qiao Xiaohong, Zhang Yonghong, Wang Dong, Qin Sihe. Treatment of displaced intra-articular calcaneal fractures with cannulated screws and plates: a meta-analysis of 15 randomized controlled trials [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1465-1470. |
| [5] | Huang Dengcheng, Wang Zhike, Cao Xuewei. Comparison of the short-term efficacy of extracorporeal shock wave therapy for middle-aged and elderly knee osteoarthritis: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1471-1476. |
| [6] | Xu Feng, Kang Hui, Wei Tanjun, Xi Jintao. Biomechanical analysis of different fixation methods of pedicle screws for thoracolumbar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1313-1317. |
| [7] | Jiang Yong, Luo Yi, Ding Yongli, Zhou Yong, Min Li, Tang Fan, Zhang Wenli, Duan Hong, Tu Chongqi. Von Mises stress on the influence of pelvic stability by precise sacral resection and clinical validation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1318-1323. |
| [8] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [9] | Zhang Yu, Tian Shaoqi, Zeng Guobo, Hu Chuan. Risk factors for myocardial infarction following primary total joint arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1340-1345. |
| [10] | Wei Wei, Li Jian, Huang Linhai, Lan Mindong, Lu Xianwei, Huang Shaodong. Factors affecting fall fear in the first movement of elderly patients after total knee or hip arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1351-1355. |
| [11] | Wang Jinjun, Deng Zengfa, Liu Kang, He Zhiyong, Yu Xinping, Liang Jianji, Li Chen, Guo Zhouyang. Hemostatic effect and safety of intravenous drip of tranexamic acid combined with topical application of cocktail containing tranexamic acid in total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1356-1361. |
| [12] | Xiao Guoqing, Liu Xuanze, Yan Yuhao, Zhong Xihong. Influencing factors of knee flexion limitation after total knee arthroplasty with posterior stabilized prostheses [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1362-1367. |
| [13] | Huang Zexiao, Yang Mei, Lin Shiwei, He Heyu. Correlation between the level of serum n-3 polyunsaturated fatty acids and quadriceps weakness in the early stage after total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1375-1380. |
| [14] | Zhang Chong, Liu Zhiang, Yao Shuaihui, Gao Junsheng, Jiang Yan, Zhang Lu. Safety and effectiveness of topical application of tranexamic acid to reduce drainage of elderly femoral neck fractures after total hip arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1381-1386. |
| [15] | Wang Haiying, Lü Bing, Li Hui, Wang Shunyi. Posterior lumbar interbody fusion for degenerative lumbar spondylolisthesis: prediction of functional prognosis of patients based on spinopelvic parameters [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1393-1397. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||